Abstract
Objective
To determine how much influenza contributes to severe acute respiratory illness (SARI), a leading cause of death in children, among people of all ages in Bangladesh.
Methods
Physicians obtained nasal and throat swabs to test for influenza virus from patients who were hospitalized within 7 days of the onset of severe acute respiratory infection (SARI) or who consulted as outpatients for influenza-like illness (ILI). A community health care utilization survey was conducted to determine the proportion of hospital catchment area residents who sought care at study hospitals and calculate the incidence of influenza using this denominator.
Findings
The estimated incidence of SARI associated with influenza in children < 5 years old was 6.7 (95% confidence interval, CI: 0–18.3); 4.4 (95% CI: 0–13.4) and 6.5 per 1000 person–years (95% CI: 0–8.3/1000) during the 2008, 2009 and 2010 influenza seasons, respectively. The incidence of SARI in people aged ≥ 5 years was 1.1 (95% CI: 0.4–2.0) and 1.3 (95% CI: 0.5–2.2) per 10 000 person–years during 2009 and 2010, respectively. The incidence of medically attended, laboratory-confirmed seasonal influenza in outpatients with ILI was 10 (95% CI: 8–14), 6.6 (95% CI: 5–9) and 17 per 100 person–years (95% CI: 13–22) during the 2008, 2009 and 2010 influenza seasons, respectively.
Conclusion
Influenza-like illness is a frequent cause of consultation in the outpatient setting in Bangladesh. Children aged less than 5 years are hospitalized for influenza in greater proportions than children in other age groups.
ملخص
الغرض
تحديد مقدار مسؤولية الأنفلونزا في حدوث المرض التنفسي الحاد الوخيم، وهو سبب رئيسي في وفيات الأطفال، في جميع فئات العمر في بنغلاديش.
الطريقة
جمع الأطباء مسحات من الأنف والحلق لفحص وجود فيروس الأنفلونزا فيها، وذلك من مرضى أدخلوا المستشفى للعلاج وخلال 7 أيام منذ بدء المرض التنفسي الحاد الوخيم، أو من مرضى خارجيين أجروا مشاورة طبية حول الأمراض الشبيهة بالأنفلونزا. وأجرى الباحثون مسحاً لتحديد نسبة ساكني منطقة الخدمة الطبية الذين التمسوا الرعاية في المستشفى موقع الدراسة، ولحساب معدل وقوع الأنفلونزا مستخدمين هذا القاسم.
النتائج
بلغ المعدل التقديري لوقوع المرض التنفسي الحاد الوخيم المرتبط بالأنفلونزا في الأطفال أقل من عمر خمس سنوات 6.7 (فاصلة الثقة 95%: 0-18.3)، و 4.4 (فاصلة الثقة 95%: 0-13.4)، و 6.5 وذلك لكل 1000 شخص سنوياً (فاصلة الثقة 95%: 0-8.3/1000) وذلك خلال مواسم الإصابة بالأنفلونزا في الأعوام 2008، 2009، 2010 بالترتيب. وبلغ معدل وقوع المرض التنفسي الحاد الوخيم في من هم أكبر من أو يساوي عمر خمس سنوات 1.1 (فاصلة الثقة 95%: 0.4-2.0)، و 1.3 (فاصلة الثقة 95%: 0.5-2.2) لكل 10000 شخص سنوياً خلال العامين 2009، 2010 بالترتيب. وبلغ معدل وقوع الأنفلونزا الموسمية في المرضى الخارجيين الذين كشف عليهم طبياً وتم التأكد مختبرياً من إصابتهم ولديهم أمراض شبيهة بالأنفلونزا 10 (فاصلة الثقة 95%: 8-14)، و 6.6 (فاصلة الثقة 95%: 5-9)، و 17 لكل 100 شخص سنوياً (فاصلة الثقة 95%: 13-22) وذلك أثناء مواسم الإصابة بالأنفلونزا في الأعوام 2008، و 2009، و 2010، بالترتيب.
الاستنتاج
تعد الأمراض الشبيهة بالأنفلونزا سبباً متكرراً لطلب المشورة الطبية في العيادات الخارجية في بنغلاديش. والأطفال في عمر أقل من خمس سنوات الذي يجري علاجهم في المستشفيات يشكلون النسبة الأكبر من الأطفال في الفئات العمرية الأخرى.
Resumen
Objetivo
Determinar en qué medida contribuye la gripe a la enfermedad respiratoria aguda grave (ERAG), una de las principales causas de muerte infantil, en personas de todas las edades en Bangladesh.
Métodos
Los médicos tomaron muestras de exudado nasal y faríngeo para realizar la prueba del virus de la gripe en pacientes que estuvieron hospitalizados en los 7 días posteriores al inicio de la infección respiratoria agua grave (IRAG) o que acudieron a consulta como pacientes ambulatorios por síndrome gripal (SG). Se llevó a cabo un estudio sobre el uso de la asistencia sanitaria comunitaria para determinar la proporción de residentes del área de cobertura del hospital que solicitaron asistencia médica en hospitales pertenecientes al estudio y se calculó la incidencia de la gripe con este denominador.
Resultados
La incidencia estimada de la IRAG asociada con la gripe en niños menores de 5 años fue del 6,7 (95% de intervalo de confianza, IC: 0–18,3); 4,4 (95% IC: 0–13,4) y 6,5 por 1000 años–persona (95% IC: 0–8,3/1000) durante las temporadas de gripe de 2008, 2009 y 2010, respectivamente. La incidencia de la IRAG en las personas con una edad igual o superior a 5 años fue del 1,1 (95% IC: 0,4– 2,0) y 1,3 (95% IC: 0,5–2,2) por 10 000 años–persona durante 2009 y 2010, respectivamente. La incidencia de la gripe de temporada tratada médicamente y confirmada en laboratorio en pacientes ambulatorios con SG fue de 10 (95% IC: 8-14); 6,6 (95% IC: 5-9) y 17 por 100 años–persona (95% IC: 13-22/1000) durante las temporadas de gripe de 2008, 2009 y 2010, respectivamente.
Conclusión
El síndrome gripal es una causa frecuente de consulta en los centros ambulatorios en Bangladesh. La proporción de niños menores de 5 años hospitalizados por gripe es mayor que la de niños en otros grupos de edad.
Résumé
Objectif
Déterminer l’impact de la grippe sur le syndrome respiratoire aigu sévère (SRAS), une cause majeure de la mortalité chez les enfants, chez les personnes de tous les âges au Bangladesh.
Méthodes
Les médecins ont obtenu des écouvillons de prélèvement de nez et de gorge afin de tester le virus de la grippe chez des patients qui avaient été hospitalisés dans les 7 jours suivants l’apparition de l’infection respiratoire aiguë sévère (SRAS) ou qui avaient eu une consultation déambulatoire pour un syndrome de type grippal (STG). Une enquête sur l’utilisation des soins de santé communautaires a été effectuée afin de définir la proportion des riverains de la circonscription hospitalière, qui avaient reçu des soins dans les hôpitaux universitaires, et afin de calculer l’incidence de la grippe à l’aide de ce dénominateur.
Résultats
L’estimation de l’incidence du SRAS associé à la grippe chez les enfants de moins de 5 ans était de 6,7 (intervalle de confiance de 95%, IC: 0–18.3); de 4,4 (IC de 95%: 0–13.4) et de 6,5 pour 1 000 personnes-années (IC de 95%: 0–8.3/1000) lors des saisons de la grippe de 2008, 2009 et 2010, respectivement. L’incidence du SRAS chez les personnes âgées de plus de 5 ans était de 1,1 (IC de 95%: 0.4–2.0) et 1,3 (IC de 95%: 0.5–2.2) pour 10 000 personnes-années en 2009 et 2010, respectivement. L’incidence de la grippe saisonnière traitée médicalement et confirmée en laboratoire chez les patients en consultation ambulatoire souffrant du STG était de 10 (IC de 95%: 8–14), 6,6 (IC de 95%: 5–9) et de 17 pour 100 personnes-années (IC de 95%: 13–22) lors des saisons de la grippe de 2008, 2009 et 2010, respectivement.
Conclusion
Le syndrome de type grippal est une cause fréquente de consultation dans la configuration de la consultation ambulatoire au Bangladesh. Les enfants de moins de 5 ans sont hospitalisés pour la grippe dans des proportions supérieures aux enfants des autres groupes d’âge.
Резюме
Цель
Определить, в какой степени грипп способствует заражению лиц всех возрастов в Бангладеш тяжелой острой респираторной инфекцией (ТОРИ), которая является основной причиной детской смертности.
Методы
Врачами были взяты мазки из носа и зева для анализа на вирус гриппа у пациентов, которые были госпитализированы в течение семи дней от начала заболевания тяжелой острой респираторной инфекцией (ТОРИ) или консультировались у врача в амбулаторном режиме по поводу гриппоподобной болезни (ГПБ). Было проведено исследование пользования медико-санитарной помощью на уровне общин для определения доли жителей участка, обслуживаемого больницей, которые обращались за помощью в обследуемые медицинские учреждения, и расчета заболеваемости гриппом с использованием указанного показателя в качестве знаменателя.
Результаты
Оценочные показатели заболеваемости ТОРИ, коррелируемой с гриппом, у детей в возрасте до 5 лет, составили 6,7 (95% доверительный интервал, ДИ: 0–18,3); 4,4 (95% ДИ: 0–13,4) и 6,5 (95% ДИ: 0–8,3/1000) на 1000 человеко-лет за эпидемиологические сезоны гриппа 2008, 2009 и 2010 годов, соответственно. Заболеваемость ТОРИ у лиц в возрасте от 5 лет и старше составила 1,1 (95% ДИ: 0,4–2,0) и 1,3 (95% ДИ: 0,5–2,2) на 10 тыс. человеко-лет за 2009 и 2010 годы, соответственно. Заболеваемость лабораторно подтвержденным гриппом, потребовавшим обращения к врачу, у амбулаторных больных с ГПБ составила 10 (95% ДИ: 8–14), 6,6 (95% ДИ: 5–9) и 17 (95% ДИ: 13–22) на 100 человеко-лет за эпидемиологические сезоны гриппа 2008, 2009 и 2010 годов, соответственно.
Вывод
Гриппоподобная болезнь – распространенная причина обращения к врачу в амбулаторном режиме в Бангладеш. Доля детей в возрасте до 5 лет, госпитализируемых по поводу гриппа, выше, чем доля госпитализируемых детей других возрастных групп.
摘要
目的
确定流感在多大程度上导致孟加拉国所有年龄段人群中的严重急性呼吸系统疾病(SARI),SARI是导致儿童死亡的主要原因。
方法
医师从发生严重急性呼吸道感染7天内住院的病人或因流行性感冒样疾病就医的门诊病人获得咽喉抹片,进行流感病毒检测。对社区医疗保健利用情况进行了调查,从而确定处于医院服务区内的居民到医院寻求医疗服务的比例,并以此为标准计算流感发病率。
结果
2008、2009和2010年流感季节,估计5岁以下儿童中与流感相关的严重急性呼吸系统疾病的发病率分别为每年每千人中6.7例 (95%可信区间,0-18.3)、4.4例(95%可信区间,0-13.4)和6.5例 (95%可信区间,0-8.3/1000)。2009和2010流感季节5岁及以上人群严重急性呼吸系统疾病的发病率分别为每年每万人中1.1例(95%可信区间,0.4-2.0)和1.3例(95%可信区间,0.5-2.2)。此外,2008、2009和2010年流感季节患有流行性感冒样疾病的门诊病人中,就医且经实验室确认的季节性流感的发病率分别为每年每百人中10例(95%可信区间,8-14),6.6例(95%可信区间,5-9)和17例(95%可信区间,13-22)。
结论
流行性感冒样疾病是孟加拉国门诊就医的常见病。与其他年龄段的儿童相比,5岁以下儿童因流感住院的比例更大。
Introduction
Influenza is believed to be an important cause of morbidity and mortality worldwide but data about its burden in low-income tropical countries like Bangladesh are rarely available. Data on incidence as well as on risk factors are valuable in prioritizing influenza prevention and control efforts in the light of competing health interventions.1,2 Data documenting a high incidence of influenza in the United States of America and Europe has helped guide investments in influenza control and prevention in high-income countries.3–7 However, there is little information about the incidence of influenza in low-income tropical countries.8,9
According to health authorities, the 2009 pandemic influenza A (H1N1) virus caused approximately 6000 deaths in Bangladesh10 and cost Dhaka 6.1 million United States dollars (US$) in direct medical costs to the patient.11 Infrequent hand washing and poor respiratory hygiene (e.g. covering mouth when coughing),12 limited access to care, lack of awareness of antiviral treatment and its availability, a huge shortage of influenza vaccines early in the pandemic, and a high prevalence of malnutrition13 may have worsened the pandemic in low-income countries such as Bangladesh.14 In general, the rate of hospitalization associated with seasonal and pandemic influenza in low-income tropical and subtropical countries remains largely unknown.
In 2004, the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) initiated influenza surveillance in a population-based site among children aged less than 5 years in urban Dhaka. The incidence of laboratory-confirmed influenza infection in the site during 2004–2007 was estimated at 10.2 per 100 person–years among children aged less than 5 years seeking care at ambulatory clinics.15 Beginning in April 2007, the Government of Bangladesh and ICDDR,B initiated national hospital-based influenza surveillance with a network of 12 surveillance hospitals throughout the country to provide nationally-representative data from all age groups. Although this national surveillance system helped to establish the seasonality of influenza, which typically occurs during the monsoon season from May to September,16 the incidence of influenza could not be estimated because people in hospital catchment areas sought care not just in hospitals but in other facilities as well and data on the population at risk, needed for the denominator, were not available. In the present study, we used health utilization patterns in the catchment areas of four selected sentinel hospitals to estimate the incidence of both outpatient and hospital visits for seasonal and pandemic influenza illness during three influenza endemic periods during 2008–2010.
Methods
Enhanced hospital-based surveillance
Investigators selected one private and three government hospitals, each in a different region of Bangladesh. The four hospitals, which were participating in the 12-hospital Government of Bangladesh/ICDDR,B national hospital-based influenza surveillance programme, were Jahurul Islam Medical College Hospital in Kishorgonj; Comilla Medical College Hospital in Comilla; Shahid Ziaur Rahman Medical College Hospital in Bogra, and Sher-e-Bangla Medical College Hospital in Barisal. Beginning in May 2008, surveillance physicians identified patients with influenza-like illness (ILI, defined as sudden onset of subjective fever, cough or sore throat) in the outpatient departments at sentinel sites during two randomly selected days every month. Investigators only selected patients who resided in the subdistricts belonging to the hospital’s main catchment areas (i.e. where the majority of hospital patrons lived), as determined by reviewing hospital log books. Beginning in January 2009, also during two randomly selected days a month, surveillance physicians identified all cases of severe acute respiratory illness (SARI) among children less than 5 years old in sentinel site paediatric wards. To identify these children, physicians applied the definition of severe pneumonia:17 cough or difficulty breathing and at least one danger sign (i.e. chest indrawing, stridor while calm, history of convulsions, inability to drink, lethargy or unconsciousness, and/or intractable vomiting).18 Beginning in January 2009, surveillance physicians identified all patients aged 5 years or older admitted to medical wards who met the SARI case definition of subjective fever within the past 21 days and cough or sore throat.
Each physician collected clinical information using a structured questionnaire and obtained a nasal and throat swab from every eligible patient who presented within 7 days of symptom onset. Each participant’s nasal and throat swabs were pooled in viral transport media and transported to the ICDDR,B laboratory in a cool box at 2–8 °C. Specimens were stored at ≤ −70 °C until tested for the presence of influenza viruses by real-time reverse transcription polymerase chain reaction.19 Influenza A viruses were subtyped using H1, H3 and H5 primers provided by the Influenza Division at the United States Centers for Disease Control and Prevention.20 These tests were anticipated to have a sensitivity of 95% and specificity of 98%.21
Hospital catchment assessment
From 1 July 2008 and 6 January, ICDDR,B conducted a health care utilization survey of the four hospital catchment areas to determine the size of the population served by each hospital. To calculate the proportion of people with sudden fever, cough and sore throat (ILI) who visited or were admitted to sentinel sites out of all people with similar symptoms in the community, we estimated that we needed a sample consisting of 282 individuals with respiratory illness for the two-month period before the survey for a design effect of 2.5. To identify these 282 individuals, we needed to enrol approximately 32 households from each of 30 unions (i.e. an administrative unit of Bangladesh with about 28 000 inhabitants) at every one of the 4 sentinel sites, if we assumed that each household had a mean of 5 dwellers, that 15% of household members had had a respiratory infection during the 2 months preceding the survey, and that 10% of these individuals had sought care in a catchment area hospital (i.e. 288 = 32 × 30 × 5 × 4 × 0.15 × 0.10). We randomly sampled 30 unions from each hospital catchment area using a sampling strategy that accounted for the number of people living in each union (i.e. population proportionate to size sampling). Investigators programmed a spreadsheet to generate random spatial coordinates within the boundaries of the selected unions. Using global positioning systems, teams travelled to the randomly selected coordinates and approached the nearest dwelling for enrolment.
An interviewer in each site collected information on demographics (e.g. age, sex), pre-existing conditions (e.g. diagnosed lung or heart disease), history of respiratory illness (e.g. sudden onset fever, cough, sore throat, shortness of breath during the preceding week, 2 months and 1 year), and care-seeking behaviour (e.g. ambulatory care sought at sentinel site 2 months or less before the interview or hospitalization 1 year or less before the interview) for all household members. The interviewer then skipped the next two closest households and approached the third dwelling for enrolment until information was collected from 32 households in each of the 30 unions.
Data analysis
The investigators used census data to estimate the size of the age-specific populations living in the catchment areas during 2001 and applied a 1.5% annual growth rate to estimate their population in 2008 and 2009.22 The annual growth rate was estimated using crude birth rates and the national crude death rate, on the assumption that net migration was minimal.23 We estimated the rate of influenza among people with ILI by dividing the number of patients who presented to health services at sentinel sites for ILI and whose samples tested positive for influenza viruses by the census population of each catchment area and adjusting the results for the proportion of days during which active surveillance for ILI had been conducted (i.e. two days a month). We also adjusted for the proportion of the census population seeking care from other health-care providers (as per the health care utilization survey).
To estimate the rates of SARI associated with influenza (henceforth referred to as influenza-associated SARI) during the endemic period16 we multiplied the proportion of persons seeking care at an ambulatory clinic who were later admitted to wards (i.e. h/o, where h represents ILI patients admitted to hospital from the outpatient department with influenza, and o represents ILI patients in the outpatient department with confirmed influenza) by the estimated rate of ILI associated with influenza (henceforth referred to as influenza-associated ILI). We estimated the rate of influenza-associated ILI in the community by dividing the number of persons with samples positive for influenza viruses seeking care for ILI at the sentinel site (i.e. o) by the census population of the catchment area and adjusting (i.e. dividing) by the proportion of days during which active surveillance for ILI was conducted (i.e. two days a month during the year, or 2 × 12/365). We also adjusted (i.e. divided) by the proportion of the census population seeking care at facilities other than sentinel hospitals (i.e. s/c where s represents the number of patients with ILI who sought care at the sentinel site during the 2 months that preceded the health care utilization survey, and c represents the number of patients living in the catchment area who sought care from any provider for subjective fever, cough or sore throat during 2 months before the survey.
We used nonparametric bootstrapping methods to estimate the 95% confidence intervals (CIs) of the incidence rates for ILI and influenza-associated SARI .24 Investigators calculated the rates of influenza-associated SARI only for 2009 and 2010, when the hospitals started to routinely identify these patients. To estimate the annual number of children aged < 5 years with influenza-associated hospitalizations in Bangladesh, we multiplied the incidence of influenza-associated SARI calculated from the four catchment areas by the population of children aged < 5 years in Bangladesh during 2009.22 Similarly, to estimate the number of persons aged ≥ 5 years with influenza-associated hospitalizations, investigators multiplied the incidence of influenza-associated SARI among those aged ≥ 5 years calculated from the four catchment areas by the populations of persons of that age group during 2009. We performed Wilcoxon rank sum, χ2 and Fisher’s exact tests where appropriate.
Human subjects
We requested written informed consent from patients at inpatient and outpatient departments in sentinel sites and from adult heads of household before enrolling participants in the surveillance system or in the household survey. The protocol was reviewed and approved by ICDDR,B and by the institutional review board of the Centers for Disease Control and Prevention in Atlanta, United States. During the pandemic, the team encouraged hospital staff to administer olsetamivir to all SARI and ILI patients who were aged less than 5 years or 65 years or over, who were pregnant, or who had any of the following: diabetes; chronic heart, lung or liver disease; asthma; neurologic or neuromuscular, haematologic or metabolic disorders; immune suppression; cancer or obesity, in accordance with Government of Bangladesh national treatment guidelines.
Results
Hospital-based influenza surveillance
From May 2008 to September 2010, surveillance physicians identified SARI in 358 patients aged less than 5 years and 495 aged 5 years or more; they also identified 2208 persons with ILI (Table 1). Most patients lived close to a sentinel hospital. The median age of 399 children less than 5 years old who were admitted to the paediatric ward with SARI was 4 months (range: 2–9). Only 101 (25%) of these children were female, compared with 413 (42%) of the 976 children less than 5 years old who had ILI (P < 0.001). Medical ward inpatients were significantly older than outpatients (median age: 37 and 20 years, respectively; P < 0.0001). The proportion of females aged 5 years or older admitted to hospital wards with SARI (168 [34%] of 495 with SARI) was significantly lower (P < 0.001) than the proportion that had ILI (643 [52%] of 1232 with ILI).
Table 1. Demographics of patients in hospital-based influenza surveillance during three influenza seasons,a Bangladesh, 2008–2010.
| Patient age and presentation | Enrolment period |
Age |
Females |
Patients positive for influenza viruses |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| May–Dec 2008 | Jan–Dec 2009 | Jan–Sep 2010 | Median | IQR | No. | % | No. | % | ||||
| ILI in children < 5 years old | 274 | 392 | 312 | 1 year | 0–2b | 413 | 42 | 110 | 11 | |||
| ILI in people ≥ 5 years old | 312 | 552 | 366 | 20 years | 11–30b | 643 | 52 | 256 | 21 | |||
| SARI in children < 5 years old | 82 | 100 | 176 | 3 months | 1 mo–6 yr | 95c | 27 | 16 | 4 | |||
| SARI in people ≥ 5 years old | 21 | 236 | 238 | 37 years | 21–55 | 168 | 34 | 110 | 22 | |||
ILI, influenza-like illness; IQR, interquartile range; SARI, severe acute respiratory infection.
a The influenza season typically lasts from May to October.
b Median age differed among patients with ILI in outpatient departments and in inpatient wards (P < 0.0001).
c Of adults who presented with severe acute respiratory infection, a higher proportion were women among those who visited outpatient departments than among those who were admitted to hospital (Fisher’s exact test, P < 0.001).
Clinical characteristics
ILI and SARI patients presented for care a median of 4 days after fever onset. Of 358 SARI patients aged less than 5 years, 314 (88%) had had fever. Of these 358 patients, 17 (5%) had known pre-existing lung disease, compared with 9 (1%) of 970 ILI patients of the same age (P < 0.001). Similarly, of 495 SARI patients aged 5 years or older, 89 (1%) had known pre-existing lung disease, such as chronic obstructive pulmonary disease or asthma, compared with 39 (3%) of 1216 ILI patients the same age (P < 0.001).
Patients with positive samples
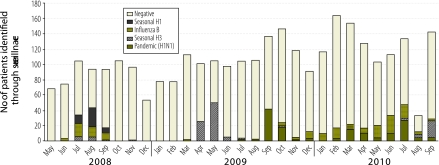
Respiratory samples from 7 (9%) of 82 SARI patients aged less than 5 years, 1 (5%) of 21 SARI patients aged 5 years or older, and 93 (16%) of 586 ILI patients of all ages tested positive for influenza viruses during 2008. This was in contrast to respiratory samples from 5 (5%) of 100 SARI patients aged less than 5 years, 55 (23%) of 236 SARI patients aged 5 years or older, and 115 (12%) of 944 ILI patients tested positive for influenza during 2009; and in contrast to 4 (2%) of 181 SARI patients aged less than 5 years, 54 (23%) of 238 SARI patients aged 5 years or older, and 158 (23%) of 678 ILI patients during 2010. Among 631 patients whose samples tested positive for influenza viruses, 325 (64%) tested positive for influenza A (i.e. 74 [12%] for seasonal influenza type A H1, 164 [26%] for type A H3, and 174 [28%] for type A pH1); 162 (32%) tested positive for influenza B, and 5 (1%) tested positive for both influenza A and B (Fig. 1). During 2008, 78% of patients infected with seasonal influenza viruses presented to the hospitals from May to October and peak activity occurred in July.
Fig. 1.
Patients with severe acute respiratory infection and influenza-like illness who were confirmed to have influenza, Bangladesh, 2008–2010
Health care utilization survey
From July 2008 to January 2009, field workers interviewed participants from 3840 households and obtained information on health care utilization for 17 495 household members. The median age of household members was 22 years and 53% were female. Participants identified 2435 (13.9%) household members who had had fever and cough or a sore throat (ILI) the week before the interview (Table 2). Participants reported that 484 children aged less than 5 years had sought care for ILI from some type of provider during the 2 months before the interview, and that 16 (3%) of these children had sought care at the sentinel hospitals. Similarly, 1950 people aged 5 years or older sought care for ILI from some type of provider during the 2 months before the interview, and 84 (4%) of them had done so at the sentinel hospitals. Respondents also reported that 165 (0.9%) of the 17 495 household members had been admitted to hospital for an illness that had started with fever and cough or a sore throat (i.e. SARI) during the year before the interview. Of these 165 SARI patients, 61 (37%) had been admitted to a sentinel hospital.
Table 2. Health-care-seeking patterns among household members with influenza-like illness (ILI), by sex, age and shortness of breath/difficulty breathing, Bangladesh, 2008.
| Population | Household members |
Source of care among those who sought care for ILI in past 2 months |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) total enrolled | No. (%) with ILI in past 7 daysa | No. (%) who sought care for ILI in past 2 weeks | No. (%) licensed physicians | No. (%) traditional healers, homeopath, unlicensed provider | No. (%) pharmacies | No. (%) sentinel hospital | No. (%) other hospital | ||
| Sex | |||||||||
| Female | 9465 (53) | 1238 (13) | 1757 (19) | 360 (21) | 690 (39) | 506 (29) | 54 (3) | 175 (10) | |
| Male | 8480 (47) | 1197 (14) | 1837 (22) | 372 (20) | 696 (38) | 560 (30) | 58 (3) | 145 (8) | |
| Age (years) | |||||||||
| 0–4 | 2066 (12) | 486 (23) | 748 (36) | 200 (27) | 314 (42) | 151 (20) | 18 (2) | 85 (11) | |
| 5–19 | 6078 (34) | 646 (11) | 1131 (19) | 191 (17) | 469 (42) | 379 (41) | 27 (2) | 69 (6) | |
| 20–49 | 7164 (40) | 842 (12) | 1162 (16) | 235 (20) | 396 (34) | 366 (32) | 43 (4) | 128 (11) | |
| 50–64 | 1686 (9) | 294 (17) | 352 (21) | 62 (18) | 129 (37) | 110 (31) | 16 (5) | 26 (7) | |
| 65+ | 951 (5) | 168 (18) | 201 (21) | 44 (22) | 78 (39) | 60 (30) | 8 (4) | 12 (6) | |
| Shortness of breath or difficulty breathing | 651 (3) | 392 (2) | 651 (100) | 186 (29) | 252 (39) | 118 (18) | 38 (6) | 92 (14) | |
a Pearson’s χ2 test yielded a P < 0.001 when the proportions of patients who developed ILI in hospitals with and without electricity were compared.
Hospitalizations and clinic visits for influenza
From 2008 to 2010, seven SARI patients aged less than 5 years whose samples tested positive for influenza viruses were admitted from the paediatric outpatient department (i.e. 3 in 2008, 3 in 2009 and 1 in 2010). Similarly, 17 SARI cases whose samples tested positive for influenza viruses were admitted from the adult outpatient department (i.e. 8 in 2009 and 9 in 2010). Based on these findings, the estimated incidence of SARI associated with influenza among children aged less than 5 years was 6.7 cases per 1000 person–years (py) (95% CI: 0–18.3) during the 2008 season, 4.4 per 1000 py during the 2009 season (95% CI: 0–13.4) and again 6.5 per 1000 py (95% CI: 0–8.3) during the 2010 season. The incidence of SARI among people aged 5 years or over was 1.1 cases per 10 000 py (95% CI: 0.4–2.0) during 2009 and 1.3 per 10 000 py (95% CI: 0.5–2.2) during 2010.
Multiplying our average SARI incidence (5.9 per 1000 py) by the population of children aged less than 5 years in Bangladesh during 2010 (i.e. 19 331 302),22 we estimated that annually 113 000 children in this age group seek hospital care for influenza. Multiplying the average SARI incidence (i.e. 1.2 cases per 10 000 py) by the population of persons aged 5 years or older in Bangladesh during 2010 (i.e. 132 920 875),22 we also estimated that 16 000 people in this age group annually seek hospital care for influenza.
The incidence of medically attended, laboratory-confirmed seasonal influenza among outpatients with ILI was 10 cases per 100 py (95% CI: 8–14) during the 2008 season, 6.6 per 100 py (95% CI: 5–9) during the 2009 season and 17 per 100 py (95% CI: 13–22) during the 2010 season (Table 3). Similarly, the incidence of ambulatory ILI among patients infected with the pandemic (H1N1) virus was 4.5 cases per 100 py (95% CI: 3–6) during 2009.
Table 3. Incidence of influenza-associated influenza-like illness (ILI) per 100 person–years based on health care utilization survey data from hospital catchment areas and surveillance data for three influenza seasons, Bangladesh, 2008–2010.
| Parameter | Kishorgonj | Comilla | Barisal | Bogra | Total seasonal influenza |
|---|---|---|---|---|---|
| Number of patients with ILI with samples that tested positive for influenza viruses during 2008 (numerator) | 15 | 22 | 38 | 18 | 93 |
| Census population in catchment area in 2008 (denominator) | 407 276 | 680 113 | 513 894 | 770 318 | 2 371 601 |
| Sampling fraction (i.e. surveillance 2 days per month) (denominator) | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| Patients with ILI who sought care at sentinel site during past 2 months (divided by 8 to extrapolate to 7 days)/Number with ILI during 7 days (denominator) | 4/687 | 2/528 | 7/624 | 1/596 | 14/2435 |
| Incidence of influenza-associated ILI (per 100 person–years) during 2008 |
10 |
13 |
10 |
21 |
10 |
| Number of patients with ILI whose samples tested positive for influenza viruses during 2009 (numerator) | 23 | 41 | 33 | 18 | 115 |
| Census population in catchment area during 2009 (denominator) | 413 386 | 690 315 | 521 602 | 781 873 | 2 407 175 |
| Sampling fraction (i.e. surveillance 2 days per month) (denominator) | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| Number of patients with ILI who sought care at sentinel site during past 2 months (divided by 8 to extrapolate to 7 days)/Number with ILI during 7 days (denominator) | 4/687 | 2/528 | 7/624 | 1/596 | 14/2435 |
| Incidence of influenza-associated ILI (per 100 person–years) during 2009 |
15 |
24 |
9 |
21 |
13 |
| Number of patients with ILI whose samples tested positive for influenza during 2010 (numerator) | 26 | 55 | 46 | 31 | 158 |
| Census population in catchment area in 2009 (denominator) | 419 586 | 700 669 | 529 426 | 793 601 | 2 443 283 |
| Sampling fraction (i.e. surveillance 2 days per month) (denominator) | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| Number of patients with ILI who sought care at sentinel site during past 2 months (divided by 8 to extrapolate to 7 days)/Number with ILI during 7 days (denominator) | 4/687 | 2/528 | 7/624 | 1/596 | 14/2435 |
| Incidence of influenza-associated ILI (per 100 person–years) in 2010 | 16 | 32 | 35 | 35 | 17 |
Discussion
In our study, the rate of clinic visits for influenza during non-pandemic years was similar to that observed in other sites in Bangladesh and southern Asia.25 In an urban community-based surveillance site in Bangladesh, the incidence of influenza among children aged less than 5 years with acute respiratory infection was 10 per 100 py.15 The incidence of ILI (10 per 100 py in 2008) is also similar to that observed in rural India in children aged less than 3 years with acute respiratory infection and influenza (14 per 100 py).26 Our ILI estimates, however, are an order of magnitude higher than in Thailand (14 per 1000 py).27 In Thailand they sampled ILI patients with measured fever (≥ 38 °C), whereas in Bangladesh we sampled ILI patients with subjective fever, an approach likely to capture a greater number of influenza patients. In addition, in Thailand they used health care utilization data for cases with pneumonia to determine the effective catchment population of their sites and this may have underestimated the percentage of people seeking care for ILI outside their surveillance facilities.
This manuscript has several important limitations. We assumed that we could accurately determine the average proportion of samples that tested positive for influenza viruses by randomly sampling patients two days a month, since positivity changed gradually over the 6-month period of influenza endemicity in Bangladesh. In addition, health care utilization surveys were conducted sequentially during 6 months in each catchment area before the pandemic and it is possible that health care seeking patterns documented in the survey differed from those observed during the pandemic. Nevertheless, health care utilization patterns are limited by the capacity of the health-care system to treat patients in Bangladesh, where 11 patients are hospitalized for every 10 hospital beds.28 Incidence was estimated from four sentinel sites that may not have been representative of all of Bangladesh, particularly its urban areas, which are densely populated and more prosperous than rural areas and which represent approximately 25% of the country’s population. We were unable to reliably differentiate ILI patients with mild disease from those with severe respiratory illness in the community who were never hospitalized. This may have led us to underestimate SARI incidence among catchment area residents. Nevertheless, our estimated incidence of influenza was similar to the incidence detected through active community-based surveillance in urban Dhaka (i.e. 10.2 per 100 py).15
Conclusion
Influenza illness in Bangladesh is often associated with health care seeking in the outpatient setting. In addition, children aged less than 5 years seem disproportionately affected by influenza-associated hospitalization, with rates that were higher than those seen among Bangladeshi adults or young children in the United States.29 Our incidence figures for outpatient visits and hospitalizations for influenza suggest that influenza control strategies would be beneficial in countries like Bangladesh.
Acknowledgements
ICDDR,B acknowledges with gratitude the commitment of the Centers for Disease Control and Prevention to its research. We would also acknowledge the contributions of Dorothy Southern, Jo Grzelinska, Peter Kim Streatfield, Salah Uddin Khan, Sk. Masum Billah, Nancy Cox, Carolyn Bridges, Ann Moen, Karen Siener, Sonja Olsen, Kimberly Lindblade, Paul Gargiullo and the surveillance staff. The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the institutions with which the authors are affiliated.
Funding:
This study was funded by the United States Centers for Disease Control and Prevention; grant number U01/CI000628-02.
Competing interests:
None declared.
References
- 1.Pandemic influenza preparedness and response: a WHO guidance document Geneva: Global Influenza Programme, World Health Organization; 2009. Available from: http://whqlibdoc.who.int/publications/2009/9789241547680_eng.pdf [accessed 22 August 2011]. [PubMed]
- 2.Azziz-Baumgartner E, Smith N, González-Álvarez R, Daves S, Layton M, Linares N, et al. National pandemic influenza preparedness planning. Influenza Other Respi Viruses. 2009;3:189–96. doi: 10.1111/j.1750-2659.2009.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox W, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 4.Molinari NAM. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–96. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 5.Mereckiene J, Cotter S, Nicoll A, Lévy-Bruhl D, Ferro A, Tridente G, et al. National Seasonal Influenza Vaccination Survey in Europe. Euro Surveill. 2008;13:1–7. doi: 10.2807/ese.13.43.19017-en. [DOI] [PubMed] [Google Scholar]
- 6.Heikkinen T, Ziegler T, Peltola V, Lehtinen P, Toikka P, Lintu M, et al. Incidence of influenza in Finnish children. Pediatr Infect Dis J. 2003;22:204–6. doi: 10.1097/00006454-200302000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Thompson WW, Comanor L, Shay DK. Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. J Infect Dis. 2006;194:S82–91. doi: 10.1086/507558. [DOI] [PubMed] [Google Scholar]
- 8.Simmerman JM, Uyeki TM. The burden of influenza in East and South-East Asia: a review of the English language literature. Influenza Other Respi Viruses. 2008;2:81–92. doi: 10.1111/j.1750-2659.2008.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leo YS, Lye DC, Barkham T, Krishnan P, Seow E, Chow A. Influenza in the tropics. Lance Infect Dist. 2009;9:457–8. doi: 10.1016/S1473-3099(09)70182-3. [DOI] [PubMed] [Google Scholar]
- 10.Homaira N. Incidence of influenza-associated mortality in Bangladesh: 2009 Presented at the MISMS South Asia Meeting and Workshop, Kathmandu, Nepal, 9–13 August 2011. [Google Scholar]
- 11.International Centre for Diarrhoeal Disease Research. Bangladesh. Pandemic (H1N1) 2009 in Bangladesh. Health Sci Bul (English) 2009;7. Available from: https://centre.icddrb.org/pub/publication.jsp?classificationID=56&pubID=10462 [accessed 22 August 2011]. [Google Scholar]
- 12.Nasreen S, Azziz-Baumgartner E, Gurley ES, Winch PJ, Unicomb L, Sharker MA, et al. Prevalent high risk respiratory hygiene practices in urban and rural Bangladesh. Trop Med Int Health. 2010;15:762–71. doi: 10.1111/j.1365-3156.2010.02531.x. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003;362:1733–45. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray CJ, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918 pandemic: a quantitative analysis. Lancet. 2006;368:2211–8. doi: 10.1016/S0140-6736(06)69895-4. [DOI] [PubMed] [Google Scholar]
- 15.Brooks WA, Goswami D, Rahman M, Nahar K, Fry A, Balish A, et al. Influenza is a major contributor to childhood pneumonia in a tropical developing country. Pediatr Infect Dis J. 2010;29:216–21. doi: 10.1097/INF.0b013e3181bc23fd. [DOI] [PubMed] [Google Scholar]
- 16.Zaman RU, Alamgir AS, Rahman M, Azziz-Baumgartner E, Gurley ES, Sharker MA, et al. Influenza in outpatient ILI case-patients in national hospital-based surveillance, Bangladesh, 2007-2008. PLoS ONE. 2009;4:e8452. doi: 10.1371/journal.pone.0008452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.PAHO-CDC generic protocol for influenza surveillance Washington: Pan American Health Organization; 2006. Available from: http://www.paho.org/English/AD/DPC/CD/flu-snl-gpis.pdf [accessed 22 August 2011].
- 18.Sungu M, Sanders R. Influenza virus activity in Papua New Guinea. P N G Med J. 1991;34:199–203. [PubMed] [Google Scholar]
- 19.Recommendations and laboratory procedures for detection of avian influenza A(H5N1) virus in specimens from suspected human cases Geneva: World Health Organization; 2007. Available from: http://www.who.int/csr/disease/avian_influenza/guidelines/RecAIlabtestsAug07.pdf [accessed 22 August 2011].
- 20.CDC protocol of realtime RTPCR for influenza A (H1N1) Atlanta: WHO Collaborating Centre for influenza, Centers for Disease Control and Prevention; 2009. Available from: http://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf [accessed 22 August 2011].
- 21.Atmar RL, Baxter BD, Dominguez EA, Taber LH. Comparison of reverse transcription-PCR with tissue culture and other rapid diagnostic assays for detection of type A influenza virus. J Clin Microbiol. 1996;34:2604–6. doi: 10.1128/jcm.34.10.2604-2606.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Population census–2001, community series. Dhaka: Bangladesh Bureau of Statistics; 2001.
- 23.Chapter 3: Population and population changes. In: Health and demographic surveillance system [Internet]. Dhaka: International Centre for Diarrhoeal Disease Research, Bangladesh; 2008. Available from: http://centre.icddrb.org/images/Chakaria-HDSS-Report-SR105-Chapter-3.pdf [accessed 22 August 2011].
- 24.Efron B, Tibshirani RJ. An introduction to the bootstrap New York: Chapman & Hall; 1993. [Google Scholar]
- 25.Ng TPPK, Niti M, Goh LG. Influenza in Singapore: assessing the burden of illness in the community. Ann Acad Med Singapore. 2002;31:182–8. [PubMed] [Google Scholar]
- 26.Broor S, Parveen S, Bharaj P, Prasad VS, Srinivasulu KN, Sumanth KM, et al. A prospective three-year cohort study of the epidemiology and virology of acute respiratory infections of children in rural India. PLoS ONE. 2007;2:e491. doi: 10.1371/journal.pone.0000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simmerman JM, Lertiendumrong J, Dowell SF, Uyeki T, Olsen SJ, Chittaganpitch M, et al. The cost of influenza in Thailand. Vaccine. 2006;24:4417–26. doi: 10.1016/j.vaccine.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 28.Gurley ES, Yaman RU, Sultana R, Bell M, Fry AM, Srinivasan A, et al. Rates of hospital-acquired respiratory illness in Bangladeshi tertiary care hospitals: results from a low-cost pilot surveillance strategy. Clin Infect Dis. 2010;50:1084–90. doi: 10.1086/651265. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention Update: influenza activity — United States, 2009–10 season. . MMWR Morb Mortal Wkly Rep. 2010;59:901–8. [PubMed] [Google Scholar]