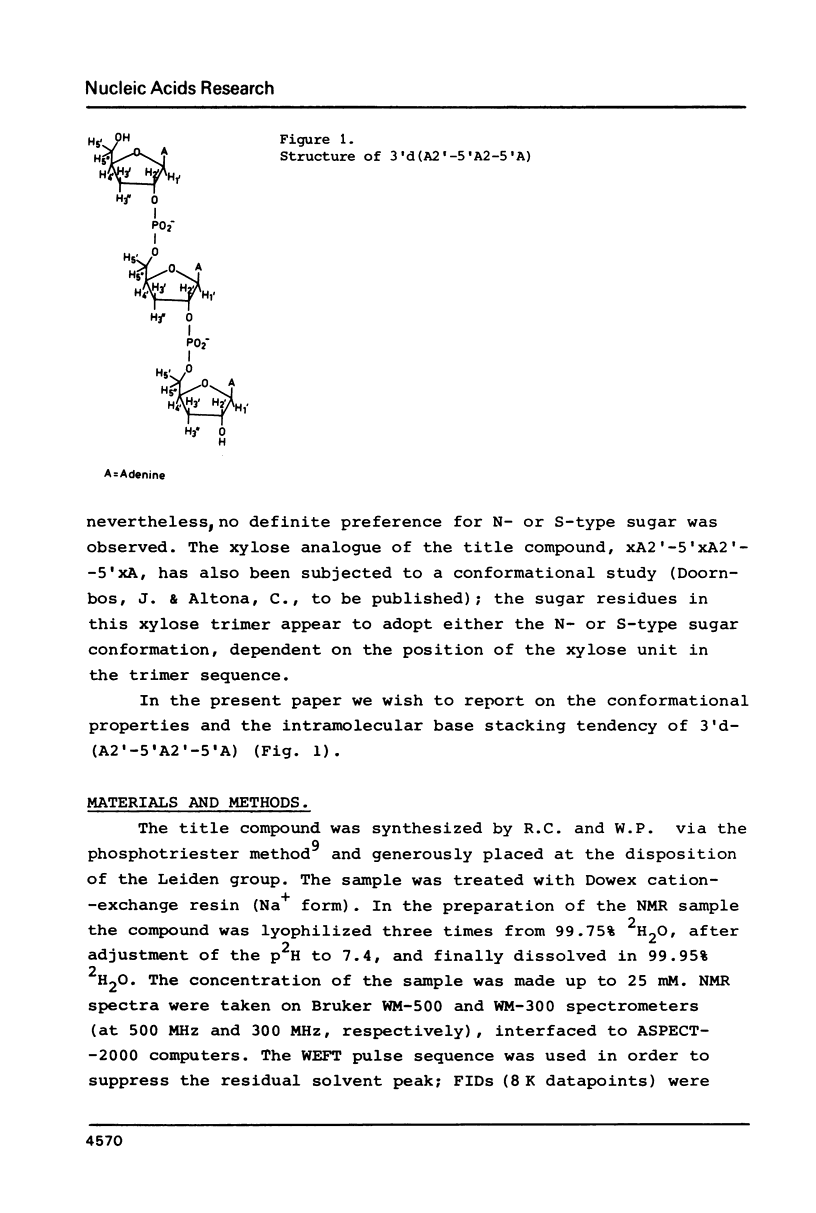
Abstract
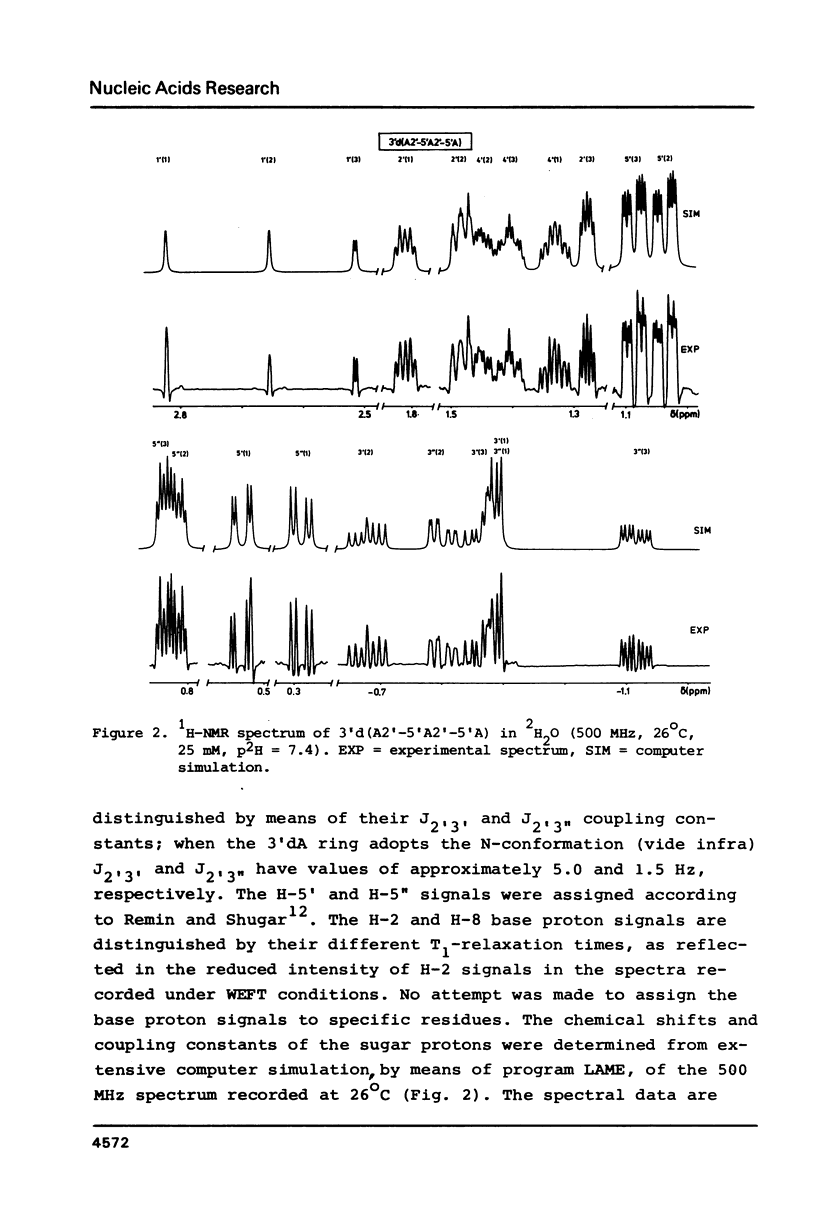
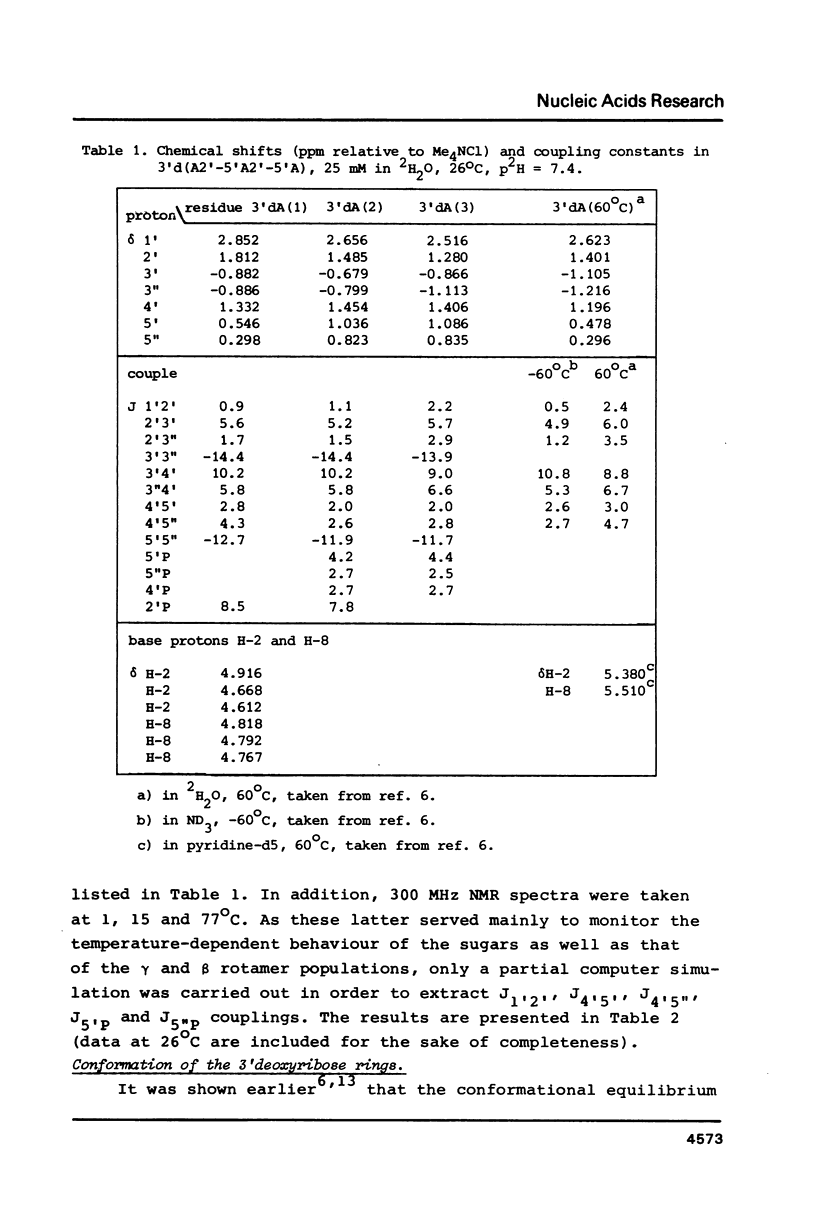
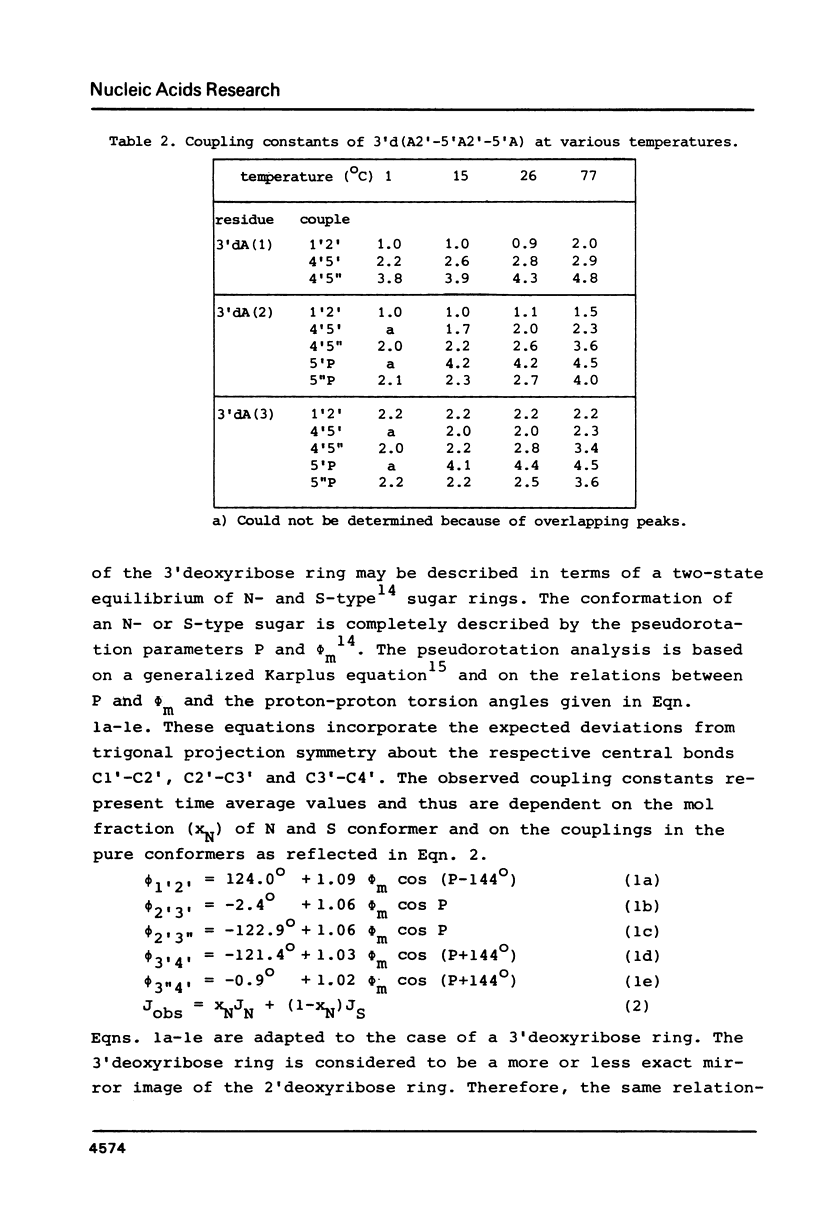
A 500 MHz and 300 MHz NMR study of the trinucleoside diphosphate 3'd(A2'-5'A2'-5'A) is presented. In addition, circular dichroism is used to study base stacking in the title compound. The complete 1H-NMR spectral assignment of the sugar ring proton signals is given. Information about the sugar ring (N- or S-type conformation) and about the backbone geometry along C4'-C5' and C5'-O5' bonds is obtained from the NMR coupling constants. It is shown that the trimer mainly occurs in the N-N-N stacked state at low temperatures; the presence of a minor amount of N-N-S conformational sequence is indicated.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. A new description using the concept of pseudorotation. J Am Chem Soc. 1972 Nov 15;94(23):8205–8212. doi: 10.1021/ja00778a043. [DOI] [PubMed] [Google Scholar]
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. Improved method for the interpretation of proton magnetic resonance coupling constants. J Am Chem Soc. 1973 Apr 4;95(7):2333–2344. doi: 10.1021/ja00788a038. [DOI] [PubMed] [Google Scholar]
- Doetsch P., Wu J. M., Sawada Y., Suhadolnik R. J. Synthesis and characterization of (2'-5')ppp3'dA(p3'dA)n, an analogue of (2'-5')pppA(pA)n. Nature. 1981 May 28;291(5813):355–358. doi: 10.1038/291355a0. [DOI] [PubMed] [Google Scholar]
- Doornbos J., den Hartog J. A., van Boom J. H., Altona C. Conformational analysis of the nucleotides A2'-5'A, A2'-5'A2'-5'A and A2'-5'U from nuclear magnetic resonance and circular dichroism studies. Eur J Biochem. 1981 May 15;116(2):403–412. doi: 10.1111/j.1432-1033.1981.tb05349.x. [DOI] [PubMed] [Google Scholar]
- Eppstein D. A., March Y. V., Schryver B. B., Larsen M. A., Barnett J. W., Verheyden J. P., Prisbe E. J. Analogs of (A2'p)nA. Correlation of structure of analogs of ppp(A2'p)2A and (A2'p)2A with stability and biological activity. J Biol Chem. 1982 Nov 25;257(22):13390–13397. [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Hovanessian A. G. Nature of inhibitor of cell-free protein synthesis formed in response to interferon and double-stranded RNA. Nature. 1977 Aug 11;268(5620):540–542. doi: 10.1038/268540a0. [DOI] [PubMed] [Google Scholar]
- Mellema J. R., Haasnoot C. A., Van Boom J. H., Altona C. Complete assignment and conformational analysis of a deoxyribotetranucleotide. d(TAAT). A 360 and 500 Mhz NMR study. Biochim Biophys Acta. 1981 Sep 28;655(2):256–264. doi: 10.1016/0005-2787(81)90016-2. [DOI] [PubMed] [Google Scholar]
- Olsthoorn C. S., Bostelaar L. J., Van Boom J. H., Altona C. Conformational characteristics of the trinucleoside diphosphate dApdApdA and its constituents from nuclear magnetic resonance and circular dichroism studies. Extrapolation to the stacked conformers. Eur J Biochem. 1980 Nov;112(1):95–110. doi: 10.1111/j.1432-1033.1980.tb04991.x. [DOI] [PubMed] [Google Scholar]
- Olsthoorn C. S., Haasnoot C. A., Altona C. Circular dichroism studies of 6-N-methylated adenylyladenosine and adenylyluridine and their parent compounds. Thermodynamics of stacking. Eur J Biochem. 1980 May;106(1):85–95. doi: 10.1111/j.1432-1033.1980.tb05999.x. [DOI] [PubMed] [Google Scholar]
- Remin M., Shugar D. Conformation of the exocyclic 5'-CH 2 OH in nucleosides and nucleotides in aqueous solution from specific assignments of the H 5' and H 5'' signals in the NMR spectra. Biochem Biophys Res Commun. 1972 Aug 7;48(3):636–642. doi: 10.1016/0006-291x(72)90395-6. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Chernajovsky Y., Shulman L., Federman P., Berissi H., Revel M. An interferon-induced phosphodiesterase degrading (2'-5') oligoisoadenylate and the C-C-A terminus of tRNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4788–4792. doi: 10.1073/pnas.76.10.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhof E., Plach H., Cuno I., Lüdemann H. D. Proton magnetic resonance studies of 2'-,3'-, and 5'-deoxyadenosine conformations in solution. Nucleic Acids Res. 1977 Apr;4(4):939–953. doi: 10.1093/nar/4.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]



