Abstract
Smoking during pregnancy exposes the fetus to nicotine, resulting in nicotine-stimulated neurotransmitter release. Recent evidence suggests that the hippocampus develops differently in males and females with delayed maturation in males. We show that chronic nicotine exposure during the first postnatal week has sex-specific long-term effects. Neonatal rat pups were chronically treated with nicotine (6 mg/kg/day) (CNN) from postnatal day 1 to 7 or milk only (Controls), and hippocampal slices were prepared from Control- and CNN-treated young adults. Field excitatory postsynaptic potentials (fEPSPs) or population spikes (PSs) were recorded from the CA1 hippocampus following CA1 s. radiatum stimulation. Input/Output curves constructed from fEPSP data indicated that CNN-males, but not females, had significantly increased excitatory responses compared to Controls (p<0.05, n=10 Con, n=11 CNN). Long-term potentiation (LTP) was not significantly changed by CNN. In the presence of bicuculline, which blocks inhibitory GABAA receptors, an epileptiform burst consisting of a series of PSs was evoked. The amplitude of the first PS was significantly larger in CNN-males and females compared to Controls (males: p<0.01, n=8 Con, n=8 CNN; females: p<0.05, n=9 Con, n=7 CNN). Only CNN-males also had significantly larger second PSs (p<0.05, n=8 con, n=8 CNN). Epileptiform activity evoked by zero Mg2+ incubation did not differ in amplitude or duration of bursts in CNN-males or females compared to Controls. These data indicate that neonatal nicotine exposure has long lasting effects and results in increased excitation within the CA1 hippocampus in adulthood, with males showing increased sensitivity to nicotine's effects.
1. Introduction
Nicotine binds to nicotinic acetylcholine receptors (nAChRs); pentameric, ligand-gated cation channels composed of α and β subunits combined in a homomeric or heteromeric fashion (Gotti et al., 2006; Kalamida et al., 2007; Sargent, 1993). In the hippocampus, nAChRs are expressed on GABAergic interneurons as well as on pyramidal cells (Jones and Yakel, 1997; Son and Winzer-Serhan, 2008; Sudweeks and Yakel, 2000). nAChRs can be located presynaptically, allowing them to regulate neurotransmitter release (Gray et al., 1996; Wonnacott, 1997), or postsynaptically on inhibitory interneurons within the hippocampus (Alkondon et al., 1998; Frazier et al., 1998; Jones and Yakel, 1997).
In the developing brain, nAChRs have been shown to regulate a variety of processes, including gene expression, neurite outgrowth, and developmentally regulated cell death (Dwyer et al., 2008). During hippocampal development, nicotine is able to stimulate GABA release (Le Magueresse et al., 2006; Maggi et al., 2001) and alter glutamatergic transmission by inducing spontaneous activity in previously silent glutamatergic synapses (Maggi et al., 2003) or decreasing activity at highly active synapses (Maggi et al., 2004). Developmental nicotine exposure results in alterations to hippocampal morphology (Huang et al., 2007; Roy and Sabherwal, 1998; Roy et al., 2002), and expression of neurotrophic factors (Son and Winzer-Serhan, 2009), a troubling outcome given that 15% of women continue to smoke during pregnancy (SAMHSA, 2010).
Hippocampal development is a complex process that relies heavily on appropriate GABAergic signaling mechanisms. During early development, GABA acts as an excitatory neurotransmitter via ionotrophic GABAA receptors (Ben-Ari et al., 1989; Ben-Ari, 2002; Cherubini et al., 1991), and excitatory GABAergic transmission is critical for the formation of giant depolarizing potentials (GDPs) in the postnatal rat hippocampus (Ben-Ari et al., 1989). Excitatory GABAergic transmission is important for a variety of developmental processes, including increasing the expression of growth factors such as brain-derived neurotrophic factor (BDNF) (Berninger et al., 1995; Represa and Ben-Ari, 2005) and enhancing synaptic strength (Kasyanov et al., 2004).
Recent evidence suggests that the hippocampus develops differently in males and females, and that the switch from depolarizing to hyperpolarizing GABAA receptor-mediated responses occurs earlier in females than in males (Galanopoulou, 2008a; Galanopoulou, 2008b; Nunez and McCarthy, 2007). Within the rat hippocampus, this switch appears to take place during the first postnatal week in females, but not until the end of the second postnatal week in males (Galanopoulou, 2008b). Neuronal α7 nAChRs are involved in the appropriate timing of the switch from excitatory to inhibitory GABAergic signaling, suggesting a critical role for endogenous nicotinic cholinergic activity in regulating the maturation of the GABA system (Liu et al., 2006; Liu et al., 2007). This interplay of nicotinic signaling with GABAergic transmission could have a significant impact on hippocampal synapse development which could also affect circuit formation.
In order to address the effects of nicotine exposure on hippocampal development, we developed a neonatal exposure model (Huang et al., 2006). The early postnatal period in rats is equivalent to the third trimester in humans (Dobbing and Sands, 1979), and a particularly critical time for hippocampal development (Winzer-Serhan, 2008). This period is characterized by strong expression of heteromeric and homomeric nAChRs (Adams et al., 2002; Son and Winzer-Serhan, 2006; Winzer-Serhan and Leslie, 2005), and high rates of axon sprouting, dendritic development and synaptogenesis (Danglot et al., 2006; de Graaf-Peters and Hadders-Algra, 2006; Dwyer et al., 2009). Additionally, during early postnatal development, nAChR subunits and receptors are transiently upregulated in the rat hippocampus, with particularly robust upregulation of α7, α5 and α2 subunits (Adams et al., 2002; Son and Winzer-Serhan, 2006; Winzer-Serhan and Leslie, 2005), possibly conferring an increased sensitivity to nicotine's effects during this time.
Because this is also a period in which GABA becomes hyperpolarizing in females, but remains depolarizing in males (Galanopoulou, 2008b), nAChR-mediated increased GABA release may have a sexually dimorphic effect during this treatment window. It is the aim of this study to use extracellular field potential recordings to determine if nicotine exposure during the first postnatal week in rats is capable of producing long-lasting functional changes affecting the overall balance of excitation and inhibition within the CA1 region of the hippocampus of adult male and female rats. We hypothesize that chronic neonatal nicotine treatment (CNN) will result in a change in the balance of excitation and inhibition in young adult rats, and that due to an extended period of excitatory GABA signaling, males will be more affected by the neonatal treatment than females.
2. Results
2.1 CNN results in increased excitation in adult male but not female CA1 hippocampus
Following Schaeffer collateral pathway stimulation, fEPSPs were recorded from CA1 stratum (s.) radiatum in young adult male and female subjects. Representative field excitatory postsynaptic potential (fEPSP) recordings are shown to demonstrate the relationship between increasing voltage input and increased slope of fEPSPs (Fig. 1A). Responses from each sex were normalized to controls and analyzed using a two-way repeated measures ANOVA with Holm-Sidak post-hoc analysis. There were no differences in the slope of fEPSPs between CNN-or control-treated male or female rats in response to voltage inputs less than or equal to 15 V. However, in males but not females, CNN treatment resulted in significantly larger fEPSP slopes at higher voltage inputs (Fig. 1B). Additionally, there was an overall treatment effect in male but not female rats (Fig. 1B; p<0.05). Thus, neonatal nicotine exposure results in increased excitation in adult male but not female CA1 subfield.
Fig. 1.
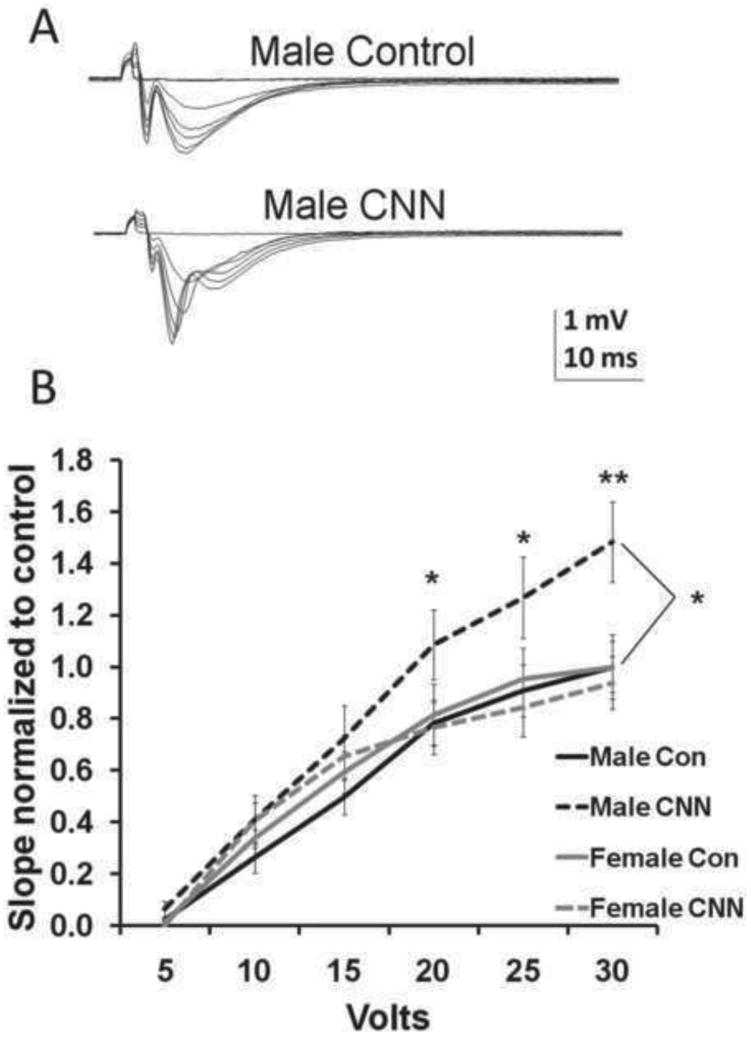
Input/Output (I/O) curves in slices from control- and CNN-treated males and female rats (A) Examples of fEPSPs elicited in CA1 s. radiatum at 5V increments from male control and male CNN-treated rats. (B) I/O curves, constructed from slopes of fEPSPs recorded from slices from P60-P87 male and female rats and normalized to the maximum slope of controls from that sex, show significantly greater responses to higher voltage inputs in CNN-treated male rats compared to male controls. (n=10, 11, 6, 8; control male, CNN male, control female, CNN female, respectively). Data analyzed by two-way repeated measures ANOVA, Holm-Sidak post hoc analysis. Significance between control and CNN-treated males *=p<0.05 **=p<0.01.
2.2 CNN does not alter long-term potentiation (LTP) in adult male or female CA1 hippocampus
In order to determine if CNN exposure affects excitatory synaptic plasticity in adult rats, we measured LTP from fEPSPs within CA1 hippocampus. LTP was induced using a theta-burst stimulation protocol (TBS) and recorded for at least 30 min. In the few cases where responses were recorded for 1 hr following TBS, there was no significant difference between the slope at 30 min and the slope at 1 hr. Representative traces are shown to illustrate the change in fEPSP following TBS (Fig. 2A), and a representative chart shows the change in slope following TBS in a male control rat (Fig. 2B). There was no difference in the increase in the slope fEPSPs following TBS in slices from control- versus CNN-treated male (Fig. 2C; p=0.91) or female (Fig. 2D, p=0.55) rats.
Fig. 2.
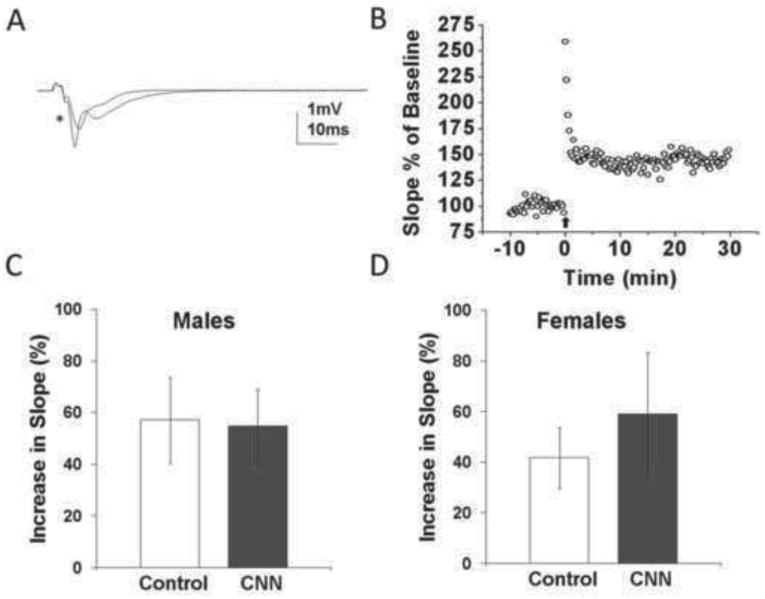
Long-term potentiation in hippocampal slices from young adult control- and CNN-treated males and female rats. (A) Sample fEPSP recorded from CA1 s. radiatum of male control rat before and 30 min after (*) theta-burst stimulation (TBS). (B) Sample time course of fEPSP slopes from a male control rat prior to and following TBS. (C) There was no difference in the percent increase in slope of fEPSPs 30 min following TBS in CNN- versus control-treated males. (n=4, 5; control and CNN, respectively). (D) There was also no difference in the percent increase in slope of fEPSPs 30 min following TBS in CNN- versus control- treated females. n=4.
2.3 CNN leads to increased responses after GABAA receptor blockade in adult male and female CA1 hippocampus
To further examine the long-term effects of CNN on excitation within the hippocampus, we examined population spikes (PSs) recorded from CA1 s. pyramidale following application of the GABAA receptor (GABAAR) antagonist bicuculline (BIC), which has been shown to be a potent antagonist of inhibitory activity in the hippocampus (Curtis et al., 1971; Kemp et al., 1986). Baseline activity was not significantly different between control- and CNN-treated groups (1.96 mV and 2.22 mV, respectively, p=0.11; and females 1.97 mV and 1.99 mV, respectively, p=0.75). After BIC application to the slices for 30 min, stimulation of the Schaeffer collaterals resulted in epileptiform bursts in CA1 s. pyramidale of young adult control- and CNN-treated males and females (Fig. 3). Following the 30 min of continuous BIC administration, PSs from CNN-treated male rats were significantly larger than those from control-treated males. (Fig. 3B, control= 2.78 mV, CNN= 3.90 mV, p<0.01). Additionally, a newly formed second PS following BIC administration was also significantly larger in CNN-treated males compared to controls (Fig. 3C, control= 1.88 mV, CNN= 2.62 mV, p<0.05). The change in the duration of the epileptiform bursts, measured as the time from reaching 35% maximum amplitude to the time to return to the same amplitude, was unchanged between treatment groups (Fig. 3D, control= 328.58 mV, CNN= 286.04 mV, p=0.62). As in males, CNN treatment in females resulted in a larger amplitude of the first PS following BIC application compared to controls (Fig. 3E, control= 3.03 mV, CNN= 3.75 mV p<0.05). However, in contrast to males, in females there was no difference in the amplitude of the second PS (Fig. 3F, control= 1.9 mV, CNN= 2.04 mV, p=0.58). There was no change in the percent increase in epileptiform burst duration in CNN compared to control females (Fig. 3G, control= 283.74 mV, CNN= 216.55 mV, p=0.20). Thus, excitatory responses, most likely through fast glutamatergic receptors, are increased in young adults treated neonatally with nicotine, but the effect is more pronounced in CNN-treated males than females.
Fig. 3.
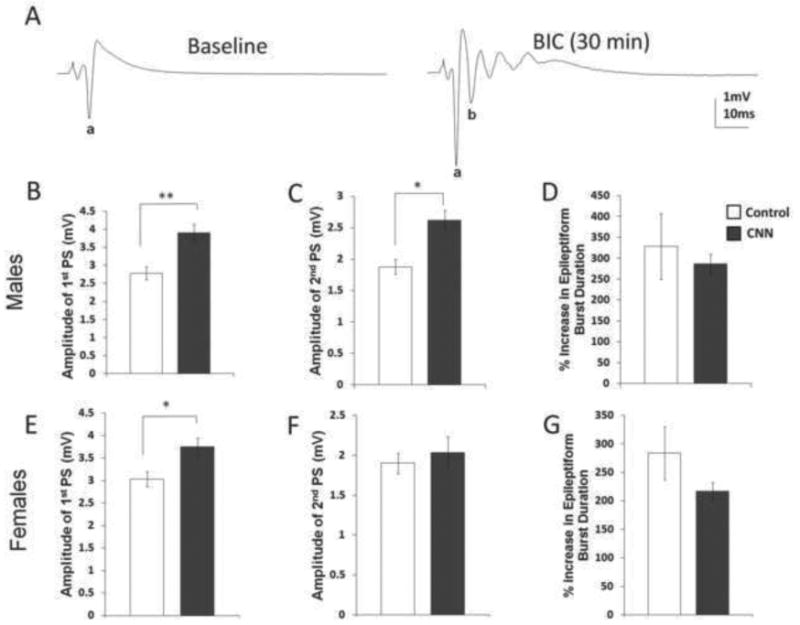
Effects of bicuculline (BIC) (3 μM) bath application on PSs from hippocampal slices from young adult control- and CNN-treated male and female rats. (A) BIC produces an epileptiform burst in population spikes (PSs) recorded from CA1 s. pyramidale. In males, following 30 min bath application of BIC, the amplitude of the first PS (B), and amplitude of the second PS (C) were significantly greater in CNN- compared to control- treated rats. There was no change in the percent increase in epileptiform burst duration (D) (n=8). In females, there was an increase in amplitude of the first PS (E), but no change in the amplitude of the second PS (F) or percent increase in epileptiform burst duration (G) in control- versus CNN-treated rats (n=9, 7, control and CNN, respectively). control= white bars, CNN= grey bars; a= first PS, b= second PS. *=p<0.05, **=p<0.01.
2.4 CNN does not alter NMDA receptor activation in CA1 hippocampus
Epileptiform activity in the presence of BIC has been shown to have both AMPA receptor (AMPAR) and NMDA receptor (NMDAR) components (Dingledine et al., 1986). In order to more closely examine the role of NMDAR-mediated input, we recorded PSs in the absence of Mg2+ (0-Mg2+) which leads to removal of the voltage dependent block of NMDA receptor activation and results in an epileptiform burst in response to stimulation (Coan and Collingridge, 1985; Tancredi et al., 1990). There were no differences in the amplitude of the PSs in baseline somatic responses in slices from control and CNN male (2.03 mV and 2 mV, respectively; p=0.70) and female rats (2.05 mV and 1.99 mV, respectively, p=0.56). Following 30 min of continuous exposure to 0-Mg2+, epileptiform bursts were recorded in all treatment groups (Fig. 4). There was a strong but non-significant trend towards increased amplitude of the first PS in CNN-treated males compared to controls (Fig. 4B, control= 3.05 mV, CNN= 3.80 mV, p= 0.056), and no significant difference in the amplitude of the second PS (Fig. 4C, control= 1.35 mV, CNN= 1.53 mV, p=0.54). Additionally, there was no change in the duration of the epileptiform burst in CNN compared to control male rats (Fig. 4D, control=222.68 mV, CNN= 209.47 mV, p=0.87). In female rats, there was no difference in the amplitude of the first (Fig. 4E, control= 4.04 mV, CNN= 4.76 mV, p= 0.075) or second (Fig. 4F, control= 2.3 mV, CNN= 1.98 mV, p=0.44) PS or in the percent increase of epileptiform burst duration (Fig. 4G, control= 238.79 mV, CNN= 318.98 mV, p=0.17) following 0-Mg2+ application.
Fig. 4.
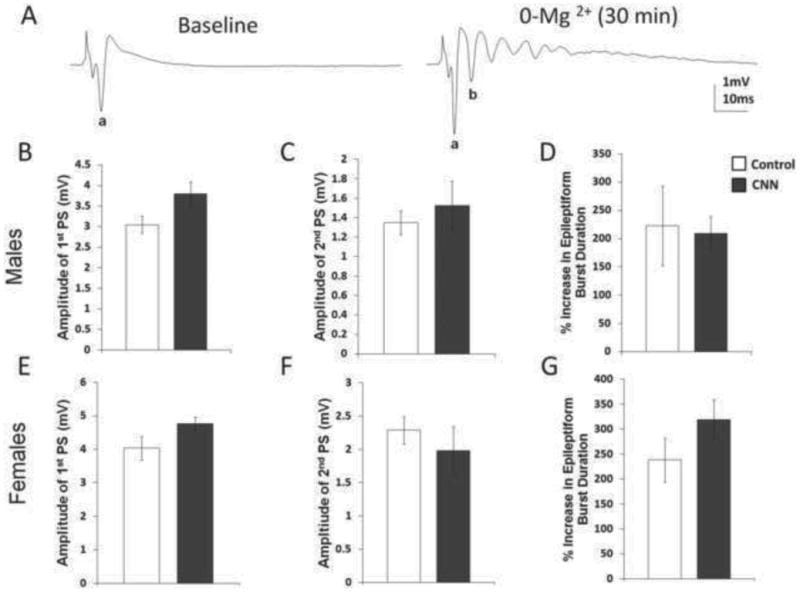
Effects of 0-Mg2+ bath application on PSs from hippocampal slices from young adult control- and CNN-treated male and female rats. (A) 0-Mg2+ produces an epileptiform burst in PSs recorded from CA1 s. pyramidale. In males, there was no change in the amplitude of the first PS (B), the amplitude of the second PS (C), or the percent increase in epileptiform burst duration (D). n=8. In females, there was also no change in the amplitude of the first PS (E), the amplitude of the second PS (F), or the percent increase in epileptiform burst duration (G) in control- versus CNN-treated females. control= white bars, CNN= grey bars; a= first PS, b= second PS.
3. Discussion
This study provides evidence for permanently altered electrophysiological responses in the adult hippocampus following chronic neonatal (P1-7) nicotine exposure during a time period equivalent to the third trimester of human development. Our results are in line with other functional studies which have shown that developmental nicotine exposure can induce long-lasting changes in functional responses (Britton et al., 2007; Jiang and Role, 2008; Pilarski et al., 2011; Vaglenova et al., 2008). Furthermore, our data indicate that males and females are differentially susceptible to the effects of early postnatal nicotine exposure with regards to hippocampal functions during adulthood, which might explain some of the long-term sex-specific behavioral effects seen with developmental nicotine exposure due to maternal smoking in humans (Weissman et al., 1999).
3.1 Increased excitatory responses in CNN-treated adults
Using field potential recordings from hippocampal slices, we were able to examine gross changes in hippocampal electrophysiological responses in adult male and female rats exposed to nicotine during the first postnatal week of development. Following CNN treatment, adult male rats displayed significantly larger slopes of fEPSPs in the CA1 hippocampus in response to increasing presynaptic stimulation. In contrast, this increase in fEPSP slope was not observed in slices from female rats.
Multiple mechanisms may explain the changes in the slopes of fEPSP and hippocampal function that we observed in CNN-treated male subjects. These include an overall increase in excitatory transmission involving excitatory glutamatergic receptors, an overall decrease in inhibitory transmission involving GABAergic receptors, changes in the connectivity of excitatory projections from the Schaeffer collaterals to CA1, or changes in the connectivity of regulatory inhibitory interneurons. Alone or in combination, these changes could contribute to the altered responses in adult hippocampus seen in male rats treated neonatally with nicotine.
In order to more closely examine changes to excitatory responses, GABAARs were blocked with the specific GABAAR antagonist BIC (Curtis et al., 1971; Kemp et al., 1986), which resulted in epileptiform responses recorded in the CA1 pyramidal cell layer in all treatment groups. CNN-treated males had significantly larger amplitudes of the first and second PSs indicating a larger excitatory response. In contrast, CNN-treated female rats exhibited larger amplitudes of only the first PS, indicating a somewhat smaller increased excitation compared to CNN-treated males. Since our recording solution contained Mg2+, which serves as a voltage-dependent block for NMDARs (Coan and Collingridge, 1985; Collingridge et al., 1988; Mayer et al., 1984), these PSs represent excitatory activity mostly via AMPA/kainate glutamatergic receptors (Collingridge et al., 1983), suggesting that long-term changes in hippocampal excitatory responses may be mediated via alterations in AMPAR activity. Additional work will be required to verify changes in these pathways.
There is evidence for a small NMDAR component which may contribute to the epileptiform activity induced by GABAAR blockage (Dingledine et al., 1986). In order to ascertain if NMDAR-mediated signaling contributed to the increased excitation observed in CNN-treated rats, slices were continuously washed with a 0-Mg2+ solution. This removed the Mg2+ block from NMDARs, leading to voltage-independent activation by endogenous glutamate, and resulting in epileptiform activity in response to stimulation despite the presence of endogenous GABA and inhibition via GABAARs (Coan and Collingridge, 1985; Tancredi et al., 1990). Under these recording conditions, Schaeffer collateral stimulation caused epileptiform wave activity which was of similar duration in all treatment groups, suggesting similar inhibitory input in all groups. The first PS was slightly larger in CNN-treated males compared to controls but failed to reach significance. In females, there was no difference in first or second PS amplitude following 0-Mg2+ application. This suggests that there are little or no long-term changes in the NMDAR component of fEPSPs as a consequence of neonatal nicotine exposure in either male of female hippocampus; a conclusion supported by the lack of effect of CNN treatment on LTP, which is highly dependent on NMDAR function (Bliss and Collingridge, 1993).
Our results suggest that the increase in excitatory responses in CNN-treated animals might be due to an increase in AMPAR function or numbers without significant changes in NMDARs or inhibitory activity. Future experiments are needed to test these assumptions. In support of our findings and conclusions, a recent study found that prenatal nicotine exposure results in increased excitatory responses in brainstem neurons (Fregosi and Pilarski, 2008), and the authors were able to show that the increase was linked to an upregulation of AMPA but not NMDA receptor function (Fregosi and Pilarski, 2008; Pilarski and Fregosi, 2009). However, another study showed that prenatal nicotine treatment results in decreased AMPAR-mediated mEPSCs in CA1 pyramidal cells (Vaglenova et al., 2008). Because the developmental exposure times were different in that study, it is difficult to compare directly their results to the data presented here.
Although our results did not provide evidence for long-term alterations of NMDAR-mediated excitatory transmission by CNN, there is some evidence that developmental nicotine exposure can cause long lasting changes that involve the slow excitatory transmission regulated by NMDA receptors (Aramakis and Metherate, 1998; Aramakis et al., 2000; Metherate, 2004). The lack of an effect on NMDAR function in our study might reflect the immature state of glutamatergic synaptic connectivity in the neonatal hippocampus. Excitatory glutamatergic synapses gradually develop during the first 2 weeks of life and do not reach maturity until after P14 (Fiala et al., 1998; Hsia et al., 1998; Tyzio et al., 1999). During the first postnatal week, the majority of glutamatergic Schaeffer collateral-CA1 synapses are believed to be functionally silent (Durand and Konnerth, 1996; Isaac et al., 1995). At these silent synapses, nicotine enhances synaptic efficacy and converts ‘presynaptically silent’ synapses into conductive ones (Maggi et al., 2003). However, during the second postnatal week, active high probability synapses are more prominent, and nicotine induces a persistent reduction of synaptic efficacy in active synapses (Maggi et al., 2004). This difference is seen in results from Aramakis and Metherate (1998) which reported alterations in NMDAR function after nicotine exposure during the second but not first postnatal week. Thus, depending on the developmental exposure time and the maturational state of a brain region, nicotine could differentially affect NMDARs.
Because field potentials are a measure of overall activity within the hippocampus, it is possible that the observed changes in excitatory responses could be, at least in part, a reflection of altered functional connectivity within the hippocampus. There is strong evidence from other studies that nAChRs are important for regulating connectivity patterns during development. For example, altered synapse formation has been observed at thalamocortical and cortico-amygdala synapses following developmental nicotine exposure (Aramakis et al., 2000; Heath and Picciotto, 2009; Jiang and Role, 2008). In addition, loss of heteromeric β2-containing nAChRs in β2 knock-out mice alters wiring and refinement of visual circuits with subsequent consequences for visual acuity (Bansal et al., 2000; Rossi et al., 2001). In these β2 knock-out mice, retinocollicular synapses appear to remain in an immature state for a longer period than in wild type mice (Shah and Crair, 2008). It is possible that the repeated activation of nAChRs by nicotine during the neonatal period distorts the normal trajectory of synaptogenesis in the postnatal hippocampus, thereby contributing to permanently altered response properties found in our CNN-treated adult rats. At this point, it remains unclear how much of the increased excitatory responses is caused by altered receptor functions versus alterations in the development of the hippocampal circuits.
3.2 Sex differences in the increased excitatory responses in CNN-treated adults
The results of this study strongly indicate that nicotine exposure during the first postnatal week affects males to a greater extent than females. This is in agreement with other studies that suggest that nicotine can have sex-specific effects. It has been shown that in adult rats, nicotine-induced nAChR upregulation occurs more readily in males than in females (Koylu et al., 1997). There have also been reported sex differences in nicotinic currents in the prefrontal cortex during development (Alves et al., 2010), and sex-specific responses to prenatal nicotine exposure (Vaglenova et al., 2004; Vaglenova et al., 2008).
One possible explanation for our sex-specific effects could be the different rate of maturation of the GABAergic system in males and females. GABAergic signaling within the hippocampus becomes hyperpolarizing in females during the first postnatal week, but remains depolarizing in males into the second postnatal week (Galanopoulou, 2008a; Galanopoulou, 2008b). Because endogenous nicotinic signaling has also been shown to contribute to the switch from depolarizing to hyperpolarizing GABAergic signaling (Liu et al., 2006), it is possible the exogenous nicotine may be able to speed up this switch, which may in turn have a larger effect in males due to a normally longer period of excitatory GABAergic signaling.
nAChR activity is capable of stimulating presynaptic GABA release in neonatal hippocampus, (Maggi et al., 2001), suggesting that by merely increasing GABA tone, nicotine could differential affect developmental processes in males and females. Increasing the release of GABA, and therefore causing either depolarizing or hyperpolarizing events in male and female hippocampus, respectively, is likely to have drastically different consequences. For example, excitatory GABA increases BDNF expression, whereas inhibitory GABA decreases BDNF expression (Berninger et al., 1995; Represa and Ben-Ari, 2005). Increased BDNF expression in males but not females could differentially affect glutamatergic synapse development (Mohajerani et al., 2007). Nicotine's effects on BDNF levels could also alter survival of neurons during development (Huang et al., 2007). It is also possible that other differences between males and females, such as different levels of sex hormones, are responsible for the sex-specific long-term effects of neonatal nicotine exposure. It has previously been shown that progesterone can inhibit nAChR activity (Valera et al., 1992), and progesterone and 17βEstradiol, but not testosterone, are able to block nicotine's antinociceptive effects in female mice (Damaj, 2001). Further studies will need to be conducted to determine the cause of the sex-specific effects described here.
3.3 Conclusions
Overall, our results suggest that nicotine treatment during a brief developmental time period is able to have long-lasting effects on hippocampal activity in both male and female offspring. However, males and females are not affected in precisely the same way, and it appears that in at least some aspects, males may be more susceptible to nicotine's effects during this developmental period. It remains to be identified exactly what makes males more sensitive to nicotine's effects during this time period and whether or not there are other sex-specific outcomes following developmental nicotine exposure.
4. Experimental Procedures
4.1 Nicotine treatment
Virgin-mated pregnant Sprague-Dawley rats (Harlan, Houston, TX) arrived between gestational day 14 and 16, and were housed in accordance with the rules stipulated by the Texas A&M University Animal Care Committee. The day the pups were born was termed postnatal day (P) 0 (P0). On P1, litters were culled to 8-10 pups. Starting on P1, pups were treated using a CNN treatment model as previously described (Huang et al., 2006). Briefly, pups were given milk formula at a volume 1/36 their total body weight three times a day using the oral gastric intubation method. One half of the pups from each litter were given 2 mg/kg/dose nicotine (Sigma Chemical, St. Louis, MO) mixed in milk formula (Enfamil with iron; Mead Johnson & Company, Evansville, IN), the control pups were given milk formula only. The treatment period lasted from P1-P7. At P21, rats were weaned and housed two-to-a-cage and grown under standard care conditions into adulthood.
4.2 Tissue Preparation
Between P60 and P87, rats were decapitated under isoflurane anesthesia, and their brains were removed. The hippocampus was blunt dissected from each hemisphere, and briefly placed in ice-cold cutting solution containing (in mM) 0.3 Kynurenic acid, 120 NaCl, 11 D-Glucose, 26 NaHCO3, 6 MgCl2, 3 KCl, 0.5 CaCl2, and 5 HEPES. 500 μm transverse slices were cut from the middle third of each hippocampus using a McIlwain tissue chopper (Mickle Laboratory Engineering Co. LTD.), and slices were transferred to artificial cerebrospinal fluid (ACSF) containing (in mM) 124 NaCl, 3 KCl, 1.5 MgSO47H20, 1.2 NaH2PO4, 2.4 CaCl2, 26 NaHCO3, and 10 D-Glucose bubbled with 95%O2 /5%CO2. Slices were warmed to 34°C for one hour, and then cooled to 25°C, where they were held for the remainder of the day.
4.3 Extracellular Field Potential Recordings
4.3.1 Input/Output Curves
Slices were transferred to a recording chamber where they were continuously washed with oxygenated ACSF warmed to 27-28°C. Extracellular field potentials were elicited every 15 s via a bipolar stimulating electrode placed in the Schaeffer collateral pathway of the CA1 hippocampus. fEPSPs were recorded using a glass micropipette filled with 1 M NaCl placed in the CA1 s. radiatum. The Schaeffer collateral pathway was stimulated at voltage steps of 5 V from 5-30 V, and the resulting fEPSPs were recorded. Four fEPSPs were recorded from each voltage input, and the average of these traces was used for data analysis. The slopes of the resulting fEPSPs were measured and normalized to controls in order to construct an Input/Output (I/O) curve (see below).
4.3.2 Long-Term Potentiation
An I/O curve was constructed using varying voltage inputs while recording fEPSPs from the CA1 s. radiatum. Baseline fEPSPs were recorded for 10 min at 15 s intervals using the voltage input which elicited approximately 50-70% maximum response. The Schaeffer collateral pathway was then stimulated using a TBS protocol consisting of 3 bursts of 4-6 100 Hz pulses at 1 s intervals at a voltage 5 V higher than the baseline recording voltage. fEPSPs were then recorded for 30 min or 1 hr following TBS.
4.3.3 Responses to Bicuculline and 0-Mg2+
For field PSs, a glass recording electrode was placed in the CA1 s. pyramidale, and slices were stimulated as described above. Baseline PSs were recorded every 15 s for 2½ min. The average of these 10 PSs was used for data analysis. ACSF containing bicuculline (BIC, 3 μM; Sigma Chemical, St. Louis, MO), or ACSF made without Mg2+ (0-Mg2+) was then bath applied to the slice. Extracellular responses were recorded every 15 s for 30 min while the oxygenated BIC or 0-Mg2+ solution flowed continuously over the slice. The final three PSs at the end of the 30 min time period were averaged for data analysis.
4.4 Data Analysis
Clampex 9.0 was used for data acquisition and PClamp 9 was used for data analysis. For I/O curves, the slope of the averaged fEPSPs to each voltage input was measured. Slopes were normalized to the average maximum slope of sex-specific control-treated rats; males were normalized to male control maximums and females were normalized to female control maximums. The normalized values of fEPSP slopes in control and CNN rats at each voltage input were compared using a two-way repeated measures ANOVA with Holm-Sidak post-hoc analysis. Outlying responses more than two standard deviations away from the mean were removed (one female control). For BIC and 0-Mg2+ PSs, the amplitude of PS of the averaged baseline recording was measured as the average of the distance from the peak of the initial positive potential to the initial negative afterpotential, and the distance from the initial negative afterpotential to the peak of the second positive potential. Following the 30 min drug application, the amplitude of the first PS was again measured. The amplitude of a newly-formed second PS was measured as the distance from the start of the second afterpotential to the negative peak of the afterpotential. Additionally, the epileptiform burst duration, as defined as the time to reach 35% maximum height to the time to return to 35% maximum height (Anderson and Collingridge, 2001), was measured from baseline responses as well as from responses following BIC or 0-Mg2+ application. The amplitude of the first and second peak, as well as the increase in epileptiform burst duration following BIC or 0-Mg2+ application were compared separately using a Student's t-test. Slices in which BIC application did not result in increased amplitude of the first PS were excluded from data analysis. For LTP experiments, the percent increase in the slopes of fEPSPs 30 min after TBS was compared in control and CNN rats using a Student's t-test. Slices in which a decrease in slope was recorded following TBS were excluded from data analysis.
For all experiments, “n” refers to the number of animals recorded from. If recordings were made in more than one slice from an animal, the results from each slice were averaged and the animal average for that measure was used for data analysis.
Highlights.
> Chronic neonatal nicotine treatment leads to long-term changes in hippocampal field potentials. > Both male and female rats exhibit changes in field potential responses. >Long-term effects are less pronounced in adult females than in males. >Young adult males show increased excitation following neonatal nicotine.
Acknowledgments
This research was supported by NIH grants R01DA016487 (U.W.S.) and T32-MH065728 (J.C.D).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams CE, Broide RS, Chen Y, Winzer-Serhan UH, Henderson TA, Leslie FM, Freedman R. Development of the alpha7 nicotinic cholinergic receptor in rat hippocampal formation. Brain Res Dev Brain Res. 2002;139:175–87. doi: 10.1016/s0165-3806(02)00547-3. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX. alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 1998;810:257–63. doi: 10.1016/s0006-8993(98)00880-4. [DOI] [PubMed] [Google Scholar]
- Alves NC, Bailey CD, Nashmi R, Lambe EK. Developmental sex differences in nicotinic currents of prefrontal layer VI neurons in mice and rats. PLoS One. 2010;5:e9261. doi: 10.1371/journal.pone.0009261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WW, Collingridge GL. The LTP Program: a data acquisition program for online analysis of long-term potentiation and other synaptic events. J Neurosci Methods. 2001;108:71–83. doi: 10.1016/s0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- Aramakis VB, Metherate R. Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex. J Neurosci. 1998;18:8485–95. doi: 10.1523/JNEUROSCI.18-20-08485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramakis VB, Hsieh CY, Leslie FM, Metherate R. A critical period for nicotine-induced disruption of synaptic development in rat auditory cortex. J Neurosci. 2000;20:6106–16. doi: 10.1523/JNEUROSCI.20-16-06106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20:7672–81. doi: 10.1523/JNEUROSCI.20-20-07672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–25. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–39. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Berninger B, Marty S, Zafra F, da Penha Berzaghi M, Thoenen H, Lindholm D. GABAergic stimulation switches from enhancing to repressing BDNF expression in rat hippocampal neurons during maturation in vitro. Development. 1995;121:2327–35. doi: 10.1242/dev.121.8.2327. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Britton AF, Vann RE, Robinson SE. Perinatal nicotine exposure eliminates peak in nicotinic acetylcholine receptor response in adolescent rats. J Pharmacol Exp Ther. 2007;320:871–6. doi: 10.1124/jpet.106.112730. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–9. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Coan EJ, Collingridge GL. Magnesium ions block an N-methyl-D-aspartate receptor-mediated component of synaptic transmission in rat hippocampus. Neurosci Lett. 1985;53:21–6. doi: 10.1016/0304-3940(85)90091-6. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Herron CE, Lester RA. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Duggan AW, Felix D, Johnston GA, McLennan H. Antagonism between bicuculline and GABA in the cat brain. Brain Res. 1971;33:57–73. doi: 10.1016/0006-8993(71)90305-2. [DOI] [PubMed] [Google Scholar]
- Damaj MI. Influence of gender and sex hormones on nicotine acute pharmacological effects in mice. J Pharmacol Exp Ther. 2001;296:132–40. [PubMed] [Google Scholar]
- Danglot L, Triller A, Marty S. The development of hippocampal interneurons in rodents. Hippocampus. 2006;16:1032–60. doi: 10.1002/hipo.20225. [DOI] [PubMed] [Google Scholar]
- de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev. 2006;82:257–66. doi: 10.1016/j.earlhumdev.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Hynes MA, King GL. Involvement of N-methyl-D-aspartate receptors in epileptiform bursting in the rat hippocampal slice. J Physiol. 1986;380:175–89. doi: 10.1113/jphysiol.1986.sp016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Durand GM, Konnerth A. Long-term potentiation as a mechanism of functional synapse induction in the developing hippocampus. J Physiol Paris. 1996;90:313–5. doi: 10.1016/s0928-4257(97)87905-3. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C Embryo Today. 2008;84:30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122:125–39. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–11. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998;18:1187–95. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregosi RF, Pilarski JQ. Prenatal nicotine exposure and development of nicotinic and fast amino acid-mediated neurotransmission in the control of breathing. Respir Physiol Neurobiol. 2008;164:80–6. doi: 10.1016/j.resp.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS. Sexually dimorphic expression of KCC2 and GABA function. Epilepsy Res. 2008a;80:99–113. doi: 10.1016/j.eplepsyres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS. Dissociated gender-specific effects of recurrent seizures on GABA signaling in CA1 pyramidal neurons: role of GABA(A) receptors. J Neurosci. 2008b;28:1557–67. doi: 10.1523/JNEUROSCI.5180-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–91. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–6. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Heath CJ, Picciotto MR. Nicotine-induced plasticity during development: modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56 1:254–62. doi: 10.1016/j.neuropharm.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia AY, Malenka RC, Nicoll RA. Development of excitatory circuitry in the hippocampus. J Neurophysiol. 1998;79:2013–24. doi: 10.1152/jn.1998.79.4.2013. [DOI] [PubMed] [Google Scholar]
- Huang LZ, Hsiao SH, Trzeciakowski J, Frye GD, Winzer-Serhan UH. Chronic nicotine induces growth retardation in neonatal rat pups. Life Sci. 2006;78:1483–93. doi: 10.1016/j.lfs.2005.07.047. [DOI] [PubMed] [Google Scholar]
- Huang LZ, Abbott LC, Winzer-Serhan UH. Effects of chronic neonatal nicotine exposure on nicotinic acetylcholine receptor binding, cell death and morphology in hippocampus and cerebellum. Neuroscience. 2007;146:1854–68. doi: 10.1016/j.neuroscience.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–34. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Jiang L, Role LW. Facilitation of cortico-amygdala synapses by nicotine: activity-dependent modulation of glutamatergic transmission. J Neurophysiol. 2008;99:1988–99. doi: 10.1152/jn.00933.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Yakel JL. Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J Physiol. 1997;504(Pt 3):603–10. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamida D, Poulas K, Avramopoulou V, Fostieri E, Lagoumintzis G, Lazaridis K, Sideri A, Zouridakis M, Tzartos SJ. Muscle and neuronal nicotinic acetylcholine receptors. Structure, function and pathogenicity. FEBS J. 2007;274:3799–845. doi: 10.1111/j.1742-4658.2007.05935.x. [DOI] [PubMed] [Google Scholar]
- Kasyanov AM, Safiulina VF, Voronin LL, Cherubini E. GABA-mediated giant depolarizing potentials as coincidence detectors for enhancing synaptic efficacy in the developing hippocampus. Proc Natl Acad Sci U S A. 2004;101:3967–72. doi: 10.1073/pnas.0305974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JA, Marshall GR, Woodruff GN. Quantitative evaluation of the potencies of GABA-receptor agonists and antagonists using the rat hippocampal slice preparation. Br J Pharmacol. 1986;87:677–84. doi: 10.1111/j.1476-5381.1986.tb14585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koylu E, Demirgoren S, London ED, Pogun S. Sex difference in up-regulation of nicotinic acetylcholine receptors in rat brain. Life Sci. 1997;61:PL 185–90. doi: 10.1016/s0024-3205(97)00665-6. [DOI] [PubMed] [Google Scholar]
- Le Magueresse C, Safiulina V, Changeux JP, Cherubini E. Nicotinic modulation of network and synaptic transmission in the immature hippocampus investigated with genetically modified mice. J Physiol. 2006;576:533–46. doi: 10.1113/jphysiol.2006.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–3. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang J, Berg DK. Role of endogenous nicotinic signaling in guiding neuronal development. Biochem Pharmacol. 2007;74:1112–9. doi: 10.1016/j.bcp.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Sher E, Cherubini E. Regulation of GABA release by nicotinic acetylcholine receptors in the neonatal rat hippocampus. J Physiol. 2001;536:89–100. doi: 10.1111/j.1469-7793.2001.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Le Magueresse C, Changeux JP, Cherubini E. Nicotine activates immature “silent” connections in the developing hippocampus. Proc Natl Acad Sci U S A. 2003;100:2059–64. doi: 10.1073/pnas.0437947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Sola E, Minneci F, Le Magueresse C, Changeux JP, Cherubini E. Persistent decrease in synaptic efficacy induced by nicotine at Schaffer collateral-CA1 synapses in the immature rat hippocampus. J Physiol. 2004;559:863–74. doi: 10.1113/jphysiol.2004.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–3. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Metherate R. Nicotinic acetylcholine receptors in sensory cortex. Learn Mem. 2004;11:50–9. doi: 10.1101/lm.69904. [DOI] [PubMed] [Google Scholar]
- Mohajerani MH, Sivakumaran S, Zacchi P, Aguilera P, Cherubini E. Correlated network activity enhances synaptic efficacy via BDNF and the ERK pathway at immature CA3 CA1 connections in the hippocampus. Proc Natl Acad Sci U S A. 2007;104:13176–81. doi: 10.1073/pnas.0704533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Evidence for an extended duration of GABA-mediated excitation in the developing male versus female hippocampus. Dev Neurobiol. 2007;67:1879–90. doi: 10.1002/dneu.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilarski JQ, Fregosi RF. Prenatal nicotine exposure alters medullary nicotinic and AMPA-mediated control of respiratory frequency in vitro. Respir Physiol Neurobiol. 2009;169:1–10. doi: 10.1016/j.resp.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilarski JQ, Wakefield HE, Fuglevand AJ, Levine RB, Fregosi RF. Developmental nicotine exposure alters neurotransmission and excitability in hypoglossal motoneurons. J Neurophysiol. 2011;105:423–33. doi: 10.1152/jn.00876.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28:278–83. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Rossi FM, Pizzorusso T, Porciatti V, Marubio LM, Maffei L, Changeux JP. Requirement of the nicotinic acetylcholine receptor beta 2 subunit for the anatomical and functional development of the visual system. Proc Natl Acad Sci U S A. 2001;98:6453–8. doi: 10.1073/pnas.101120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy TS, Sabherwal U. Effects of gestational nicotine exposure on hippocampal morphology. Neurotoxicol Teratol. 1998;20:465–73. doi: 10.1016/s0892-0362(97)00137-2. [DOI] [PubMed] [Google Scholar]
- Roy TS, Seidler FJ, Slotkin TA. Prenatal nicotine exposure evokes alterations of cell structure in hippocampus and somatosensory cortex. J Pharmacol Exp Ther. 2002;300:124–33. doi: 10.1124/jpet.300.1.124. [DOI] [PubMed] [Google Scholar]
- Samhsa. Office of Applied Studies, HHS Publication No SMA 10-4856Findings, ed.^eds. Rockville, MD: 2010. Results from the 2009 National Survey on Drug Use and Health: Volume I Summary of National Findings Vol., N.S.H.-A. [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–43. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Shah RD, Crair MC. Retinocollicular synapse maturation and plasticity are regulated by correlated retinal waves. J Neurosci. 2008;28:292–303. doi: 10.1523/JNEUROSCI.4276-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JH, Winzer-Serhan UH. Postnatal expression of alpha2 nicotinic acetylcholine receptor subunit mRNA in developing cortex and hippocampus. J Chem Neuroanat. 2006;32:179–90. doi: 10.1016/j.jchemneu.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JH, Winzer-Serhan UH. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs in rat hippocampal GABAergic interneurons. J Comp Neurol. 2008;511:286–99. doi: 10.1002/cne.21828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JH, Winzer-Serhan UH. Chronic neonatal nicotine exposure increases mRNA expression of neurotrophic factors in the postnatal rat hippocampus. Brain Res. 2009;1278:1–14. doi: 10.1016/j.brainres.2009.04.046. [DOI] [PubMed] [Google Scholar]
- Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol. 2000;527(Pt 3):515–28. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancredi V, Hwa GG, Zona C, Brancati A, Avoli M. Low magnesium epileptogenesis in the rat hippocampal slice: electrophysiological and pharmacological features. Brain Res. 1990;511:280–90. doi: 10.1016/0006-8993(90)90173-9. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Represa A, Jorquera I, Ben-Ari Y, Gozlan H, Aniksztejn L. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J Neurosci. 1999;19:10372–82. doi: 10.1523/JNEUROSCI.19-23-10372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaglenova J, Birru S, Pandiella NM, Breese CR. An assessment of the long-term developmental and behavioral teratogenicity of prenatal nicotine exposure. Behav Brain Res. 2004;150:159–70. doi: 10.1016/j.bbr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Vaglenova J, Parameshwaran K, Suppiramaniam V, Breese CR, Pandiella N, Birru S. Long-lasting teratogenic effects of nicotine on cognition: gender specificity and role of AMPA receptor function. Neurobiol Learn Mem. 2008;90:527–36. doi: 10.1016/j.nlm.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Valera S, Ballivet M, Bertrand D. Progesterone modulates a neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1992;89:9949–53. doi: 10.1073/pnas.89.20.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiatry. 1999;38:892–9. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Leslie FM. Expression of alpha5 nicotinic acetylcholine receptor subunit mRNA during hippocampal and cortical development. J Comp Neurol. 2005;481:19–30. doi: 10.1002/cne.20357. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–8. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]


