Abstract
Purpose
(1) Determine the effect of 18Fluorodeoxyglucose Positron Emission Tomography (FDG-PET), magnetic resonance imaging (MRI), and electroencephalogram (EEG) on the decision for temporal lobe epilepsy (TLE) surgery. (2) Determine if FDG-PET, MRI, or EEG predict surgical outcome.
Procedures
All PET scans ordered (2000–2010) for epilepsy or seizure were tabulated. Medical records were investigated to determine eligibility and collect data. Statistical analysis included odds ratios, kappa statistics, univariate analysis, and logistic regression.
Results
186 patients had an FDG-PET, 124 patients had TLE, 50 were surgical candidates, and 27 had operations with > 6 months follow-up. Median length of follow-up was 24 months. MRI, FDG-PET, and EEG were significant predictors of surgical candidacy (p<0.001) with odds ratio of 42.8, 20.4, and 6.3 respectively. PET was the only significant predictor of post-operative outcome. (p<0.01)
Conclusion
MRI had a trend toward most influence on surgical candidacy, but only FDG-PET predicted the surgical outcome.
Keywords: temporal lobe epilepsy, medication refractory epilepsy, epilepsy surgery, fluorodeoxyglucose (FDG), positron emission tomography (PET), magnetic resonance imaging (MRI), Electroencephalogram (EEG)
Introduction
Surgical treatment of medication refractory temporal lobe epilepsy (TLE) has dramatically improved the quality of life for these patients, and 18Fluorodeoxyglucose (FDG) Positron Emission Tomography (PET) has been an integral part in the work-up of these surgical candidates. However, some challenge the usefulness of this test in the setting of advanced MR imaging, as well as its ability to provide prognostic information. [1] [2]
Epilepsy is a prevalent chronic disease with an incidence of approximately 61/100,000. [3] First-line therapy is treatment with antiepileptic drugs (AED). Drug therapy results in successful control in approximately 2/3 of patients. This leaves a substantial portion (up to 1/3) of patients who are either refractory to anti-epileptic drugs (AEDs) or have developed AED resistance. [4] Surgery has been validated as an effective treatment strategy for this patient group. Following surgery, seizure-free rates have been reported between 46% and 81% at a year with up to 72% remaining seizure free at 10 years. [5–7] The only randomized controlled trial investigating the efficacy of surgery found that 58% of patients in the surgical group were free from seizures at one year versus 8% of medically managed controls. [8]
The key to successful epilepsy surgery is defining the epileptogenic focus whether it is an apparent anatomic focus as in cortical dysplasia or a more functional one such as in non-lesional temporal epilepsy. [9] To this end several instruments are employed. The most widely used and clinically validated tools are magnetic resonate imaging (MRI), scalp and intracranial electroencephalogram (EEG), interictal FDGPET, and ictal-interictal single photon emission computed tomography (SPECT). How these tools are used in the most clinically efficacious manner is a matter of some debate. PET has a long-standing tradition in this capacity. Interictal hypometabolism (HM) was one of the first clinical findings during the development of PET, and its diagnostic efficacy has been validated in numerous studies.[10] [11] [12] In the past it has been noted to have predictive value independent of EEG and MRI.[13] The exact mechanism behind the HM is not entirely understood, but studies have found that the degree of HM is greater than expected for simple neuronal cell loss with partial volume averaging. [14–15]
The role of FDG-PET in epilepsy continues to be refined, and its use differs among ordering providers and among institutions. Several studies showed that temporal lobe HM had post-surgical prognostic significance. [11, 16] A study even found that FDG-PET had a greater concordance with successful temporal resection than MRI or MR spectroscopy. [17] FDG-PET uses a functional measure, and this has resulted in investigators proposing that specific sub-groups of TLE that do not exhibit characteristic anatomic abnormalities (e.g. non-lesional lateral temporal lobe epilepsy) are defined with FDG-PET.[18] FDG-PET findings other than the classic temporal lobe HM have yielded the observation that extra-temporal hypometabolism in TLE is correlated with worse post-operative outcomes. [19] Despite this body of research supporting the use of FDG-PET in TLE, the use of MRI along with greater clinical experience has allowed some to suggest that FDG-PET is not necessary in all instances. DellaBadia et al suggests that EEG and MRI alone may be sufficient for a screening test.[1] Gailard et al suggests that FDG-PET does not augment the predictive power of MRI when hippocampal atrophy is present.[2] The aim of this study is to determine what FDG-PET’s relative contribution is in the surgical decision-making for patients with medication refractory epilepsy, and to examine whether PET localization portends a positive surgical outcome.
Materials and Methods
Subject Selection
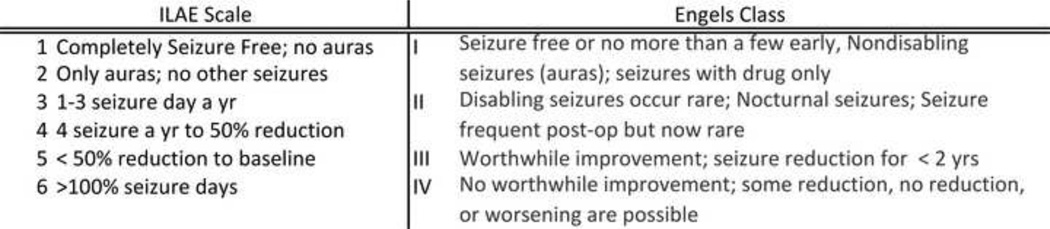
Local IRB approval was obtained to access the imaging and medical records for all adult patients (age 18 years or greater) that received brain FDG-PET for the purpose of evaluation of epilepsy (2000–2010). A query was carried out of a departmental database of brain FDG-PET and all studies with epilepsy, seizure, or temporal lobe epilepsy in the requisition were isolated. The medical and imaging records of these subjects were investigated for the following inclusion and exclusion criteria. Inclusion criteria: diagnosis or suspicion of TLE, consideration for surgical treatment of epilepsy, age ≥ 18 years, medical documentation of age, gender, seizure onset, seizure frequency, AED trials, EEG report, PET report, MRI report, and post-operative follow-up, and post-operative seizure frequency and character when applicable. Exclusion criteria: history of cerebral vascular accident (CVA), brain tumor, head trauma, tuberous sclerosis, prior cranial surgery, and hemispheric congenital malformations (e.g. porencephaly, lissencephaly, perisylvian polymicrogyria, hemimegalencephaly). After eligibility for the study was satisfied the medical records were further reviewed and an anonymized database was created with postoperative outcome graded according to the Engels and International League Against Epilepsy (ILAE) scales. [20] [21] Figure 1 is a summary of these scales.
Figure 1.
Summary of the international league against epilepsy (ILAE), and Engel’s classification for post-operative outcome after epilepsy surgery. [20] [21]
To be included in the surgical outcome data the patient had to have at least one post-operative assessment of their post-operative seizure course within the electronic medical record. Surgical candidacy was determined from the medical records by the findings of a multi-disciplinary epilepsy conference that included neurologists, neurosurgeons, and radiologists. The FDG-PET was deemed positive if unilateral temporal lobe hypometabolism was present. Other areas of hypometabolism were identified as extratemporal hypometabolism. The MRI was deemed positive if mesiotemporal sclerosis, hippocampal atrophy, unilateral temporal atrophy, or temporal gliosis were indentified. The EEG was deemed localizing if reports indicated that seizures originated from one temporal lobe.
Imaging protocols
Patients fasted for 6 hours prior to injections. Diabetic patients withheld diabetic medications for 6 hours and blood glucose measurements were required to be < 200 mg/dL at the time of tracer injection. Patients were injected intravenously with 0.14 mCi/kg (minimum of 10mCi) of 18F-FDG. Patients then relaxed quietly for 45 minutes in a dimly lit room, avoiding unnecessary activity. Patients were imaged at 60 minutes with one of three scanners: GE Advance, GE Discover LS, or GE Discovery VCT. Scans were performed in 3D and 2D modes and were reconstructed with and without attenuation correction. The primary MR sequences used in the epilepsy protocol were the 3D T1 spoiled gradient (SPGR) and proton density (PD)/T2 (dual echo) sequence, with diffusion tensor imaging (DTI) being a latter addition. The MR scanners were GE Signa 1.5 Tesla.
Statistical Analysis
Statistical analysis was carried out with JMP 9.0.0 (copyright SAS Institute Cary, North Carolina). The surgical and non-surgical groups were assessed for differences in clinic factors and diagnostic modalities. For this analysis Fisher-exact tests were used for comparisons of categorical data, and logistic regression for comparisons of categorical and continuous variables. The relative predictive strength for surgical candidacy was assessed for PET, MRI, and EEG first with 2x2 contingency tables generating odds ratios, sensitivities, and specificities. These ratios were calculated with 95% confidence intervals generated with the adjusted Wald method. [22] These statistics were also used on combinations of the three diagnostic modalities for prediction of surgical candidacy. PET, MRI, and EEG were then used in a logistic multivariate model. PET, MRI, and EEG were then each left out of the model to find the change in R2. The R2 values reported in this manuscript are McFadden’s R2. This measure ranges from 0 to 1. The closer to 1 the R2 is the better the predictive strength of the logistic model. [23] Another logistic model was generated with independent variables including all the diagnostic modalities and all of the clinical factors. Kappa agreement statistics between the modalities were calculated, and the results were analyzed with the following convention for level of agreement (0–0.20 slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial, 0.81–1 near perfect). [24]
To detect predictors of post-operative outcome univariate statistics of potential predictors were assessed in the same manner as the surgical candidacy analysis. A logistic regression model was again used with complete seizure freedom (ILAE class I) as one category and any seizure activity post-surgery (ILAE class 2–5) as the other category. The multivariate model was run with only significant factors on univariate analysis and then again with all potential predictors. Length of follow-up was used as a covariate in all multivariate models to account for the varying length of follow-up between subjects (a potential source of bias). The alpha level was set at 0.05 for all statistical tests.
Results
Patient selection
A total of 184 adult subjects underwent FDG-PET with epilepsy or seizure stated in the requisition. Of these 60 were excluded because of tumor (19), no medical record (15), prior CVA (2), prior trauma (5), non-TL epilepsy (8), large hemorrhage (2), no documentation of seizures (6), no MRI or EEG (4). That left 124 patients that meet the inclusion and exclusion criteria of this study. Five patients had no documented surgical decision. Thirty-six (36) underwent epilepsy surgery and an additional 14 patients were considered surgical candidates, but had not yet undergone surgery. Two of the 36 patients that had surgery did not have an assessment of post-operative seizure frequency in the electronic medical record at the time of data collection. Table 1 summarizes the distribution of demographic and clinical factors of the study population.
Table 1.
Patient Characteristics
| Sex | male: female | 48:76 |
| Age (yrs) | median; IQR | 38.5; 48-28 |
| Age at onset of Seizures (yrs) | median; IQR | 12; 4–22 |
| Frequency of Seizures (per month) | median; IQR | 2; 1–6 |
| Duration of Epilepsy (yrs) | median; IQR | 22; 13–33 |
| Anti-Epileptic Drug Trials | median; IQR | 3; 2–5 |
| Unilateral Temporal Lobe Hypometabolism | yes:no | 61:64 |
| Extratemporal Hypometabolism | yes:no | 19:25 |
| Mesiotemporal Sclerosis on MRI | yes:no | 57:67 |
| EEG localize to one temporal lobe | yes:no | 73:51 |
| Surgical Candidate | yes:no:unknown | 50:69:5 |
| Length of Follow-Up* (months) | median; IQR | 24; 6.5–42 |
| ILAE* (at last follow-up) | median; IQR | 1; 1–2 |
IQR, inter-quartile range (25–75%); yrs, years; ILAE, International League Against Epilepsy;
n=34 (only patients that received surgery and had follow-up)
Surgical Candidacy Analysis
Patients under consideration for epileptic surgery are presented at a multi-disciplinary conference attended by neurologists, neuroradiologists, and neurosurgeons. The consensus of this group was utilized to determine surgical candidacy. Several patients (14) had disease likely amenable to surgery, but have not yet undergone temporal resection. Reasons for not proceeding with surgery were that surgical risk outweighs benefit (2), the patient declined surgery (6), patient seizure control improved with another medication trial (1), patient is currently planned for surgery (3), and unknown reasons (2). A decision about the surgical candidacy had not been made for five patients. This left n=119 of the 124 for subsequent analysis. 50 of these patients were deemed surgical candidates and 69 were not.
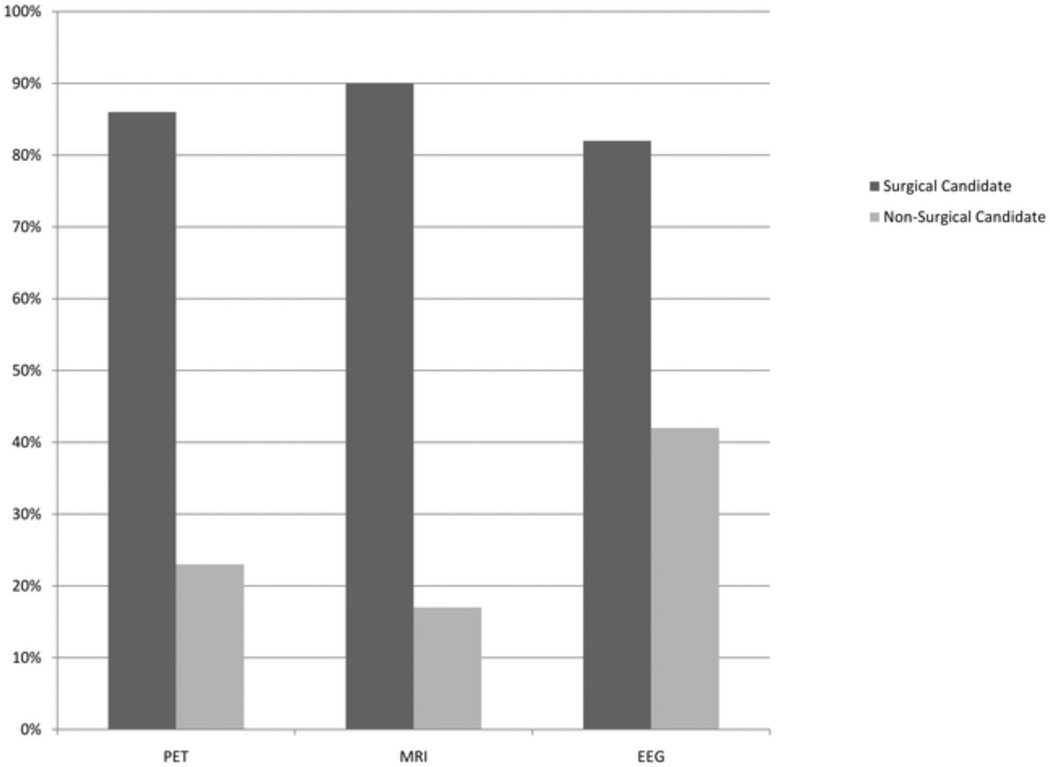
Univariate analysis demonstrated several factors that were different between these groups, including age of onset of epilepsy, duration of epilepsy, number of AED drug trials, and percentage of patients that had localizing findings on FDG-PET, MRI, and EEG. Sex, age at PET study, frequency of seizure (preoperative), and non-temporal lobe hypometabolism (extra-temporal lobe hypometabolism) were not statistically different between these groups. Table 2 summarizes this data. Figure 2 illustrates the differences in the percentage of subjects that had a positive FDG-PET (86%, 23%), MRI (90%, 17%), or EEG (82%, 42%) if they were deemed a surgical candidate versus not surgical candidate respectively. The odds ratios, sensitivity, and specificity for surgical candidacy were calculated for FDG-PET, MRI, and EEG. MRI had the highest odds ratio (42.8), sensitivity (0.9), and specificity (0.83) followed by FDGPET (20.4, 0.86, 0.77), and then EEG (6.3, 0.82, 0.58), but the 95% confidence intervals overlapped for all three modalities for all three measures. The odds ratio, sensitivity, and specificity were also calculated for cases where all three modalities were positive and for cases when MRI and PET, MRI and EEG, and PET and EEG were respectively positive. When all three modalities were positive the odds ratio was the highest at 50.3 and next was when MRI and EEG were positive at 41.8. Table 3 presents this data along with the data from the other combinations of diagnostic modalities.
Table 2.
Surgical Candidacy Univariate Analysis
| Surgical Candidate |
Non-Surgical Candidates | p-value | ||
|---|---|---|---|---|
| Age (yr) | median; IQR | 41.5 (29.5–48) | 37 (27–48) | 0.34 |
| Sex | % female | 60% | 62% | 0.85 |
| Age of Onset (yr) | median; IQR | 6.5 (2–18) | 16 (7–25) | <0.01 |
| Duration of Epilepsy (yr) | median; IQR | 29 (16–39.25) | 19 (11–29) | <0.001 |
| Frequency (per month) | median; IQR | 4 (2–11) | 2 (1–3) | 0.23 |
| Anti-Epileptic Drug | ||||
| Trials | median; IQR | 4 (3–6) | 2 (1–3) | <0.01 |
| Extra-temporal HM | % with extra temporal HM | 18% | 13% | 0.61 |
| <0.000 | ||||
| FDG-PET | % unilateral TL HM | 86% | 23% | 1 |
| <0.000 | ||||
| MRI | % TL abn | 90% | 17% | 1 |
| <0.000 | ||||
| EEG | % localize unilateral TL | 82% | 42% | 1 |
IQR, inter-quartile range (25–75%); TL abn, temporal lobe abnormality; HM, hypometabolism; TL, temporal lobe; yr, years
Figure 2.
The Percentage of PETs, MRIs, and EEGs that are positive for localizing the epileptogenic focus in patients that were Surgical Candidates and Non-Surgical Candidates
Table 3.
Surgical Candidacy Relative Predictive Strength
| Odds Ratio* | Sensitivity* | Specificity* | p-value# | delta R2 | |
|---|---|---|---|---|---|
| FDG-PET (TL HM) | 20.4 [7.7–54.0] | 0.77 [0.85-0.65] | 0.77 [0.85-0.65] | <0.001 | 0.08 |
| MRI (TL abn) | 42.8 [14.0–130.3] | 0.90 [0.96–0.78] | 0.83 [0.90-0.72] | <0.001 | 0.23 |
| EEG | 6.3 [2.6–14.9] | 0.82 [0.90-0.69] | 0.58 [0.69-0.46] | <0.001 | 0.07 |
| MRI, EEG, & PET positive | 50.3 [11.0-228.8] | 0.60 [0.72-0.46] | 0.97 [1.00-0.89] | ||
| MRI & PET positive | 33.3 [11.5–95.9] | 0.76 [0.86-0.62] | 0.91 [0.96-0.82] | ||
| EEG & PET positive | 22.8 [8.4–61.7] | 0.72 [0.83-0.58] | 0.90 [0.95-0.80] | ||
| MRI & EEG positive | 41.8 [12.8-136.5] | 0.72 [0.83-0.58] | 0.94 [0.98-0.86] | ||
95% Confidence interval in […];
delta R2, change in R2 if variable left out of regression model; yr, year; TL HM, temporal lobe hypometabolism; abn, abnormality; #logistic regression model with PET, MRI, and EEG; Total R2=0.60;
On multivariate analysis with MRI (p<0.01), EEG (p<0.01), FDG-PET (<0.01), AED trials (p=0.80), duration of epilepsy (p=0.39), and age of onset of epilepsy (p=0.24), only MRI, FDG-PET, and EEG were significant. Multivariate analysis was carried out with only MRI, FDG-PET, and EEG. MRI (p<0.01), PET (p<0.01), and EEG (P<0.01) all remained significant predictors of surgical candidacy. (The total R2 for the model was 0.60. The logistic regression was recalculated leaving out each specific factor to see how that would affect the R2. Leaving out MRI caused the R2 to drop 0.23 (delta R2). PET had the next highest delta R2 of 0.08, followed by EEG with a delta R2 of 0.07. A logistic model with dependent variables including all of the clinical factors and all three of the diagnostic modalities yielded an R2 of 0.64. The only factors that remained significant were FDG-PET, MRI, and EEG (all p<0.001). Kappa statistics were used to see the level of agreement between PET, MRI, and EEG. PET & MRI had a moderate agreement (kappa 0.515) while PET & EEG (kappa 0.292), and MRI & EEG (kappa 0.205) had only fair agreement.
Predictors of Surgical Outcomes
Thirty-four (34) patients received TLE surgery and had post-surgical seizure assessment. Twenty-eight (28) of these had an Engel’s Class I outcome, 4 had an Engel’s Class II outcome, and 2 had an Engel’s class III outcome. The patient’s ILAE outcomes were 22 patients with a class 1 outcome, 6 patients with a class 2 outcome, 4 patients with a class 3 outcome, 1 patient with a class 4 outcome, and 1 patient with a class 5 outcome. Potential predictive factors (independent variables) were assessed with a univariate logistic regression using post-operative seizure freedom (ILAE class 1) as the dependent variable. Factors assessed included length of follow-up, duration of epilepsy, age at onset of epilepsy, number of AEDs trials, localization of seizure focus with PET, temporal lobe abnormality on MRI, localization of seizure focus with EEG, seizure frequency, AED trials, and extra-temporal hypometabolism. On univariate analysis the only factors significant in predicting post-operative seizure were unilateral temporal lobe hypometabolism on FDG-PET (p=0.04) and length of follow-up (p=0.03). MRI (p=0.54), EEG (p=0.42), seizure frequency (p=0.75), AED trials (p=0.82), duration of epilepsy (p=0.89), age of onset of epilepsy (p=0.34), and extra-temporal hypometabolism (p=0.64) were not significant. Multivariate analysis with logistic regression model using only FDG-PET (p=0.02) and length of follow-up (p=0.02) (to correct for bias from post-operative duration) as independent variables found both factors to still be significant with an R2 of 0.26. With only length of follow-up used a covariate, MRI (0.99) was still not a predictor of surgical outcome, and neither was EEG (p=0.98). On multivariate analysis with all the factors from univariate analysis found that FDG-PET (p=0.02), and length of follow-up (p=0.03) remained significant and the model had an R2 of 0.40. Of the 34 patients 8 patients had either only PET or MRI positive while the remaining 26 had MRI and PET both positive. The five patients that had mesiotemporal sclerosis on MRI and did not have unilateral TL HM on PET had 1 patient with a ILAE class 1 outcome, 3 patients had a class 2 outcome, and 1 patient had a class 5 outcome, while the three patients that were MRI negative and FDG-PET positive all had ILAE class 1 outcomes. Of patients with mesiotemporal sclerosis (n=31), a greater proportion had seizure freedom (69%) if they had temporal hypometabolism of FDG-PET, than patients without this finding (20%) (p=0.06).
Discussion
The aim of this study was to investigate the effect FDG-PET has on surgical decision making relative to EEG and MRI, and if the differences in these variables translates to differences in surgical outcomes. This study found that MRI had a trend toward the strongest influence on surgical candidacy with the highest sensitivity, specificity, and odds ratio. PET and EEG had a smaller influence, but confidence intervals that overlapped with MRI and each other. For patients that had surgery and at least 6 months of follow-up, only PET (p=0.005) and length of follow-up (p=0.045) were significant predictors of surgical outcome on univariate and multivariate analysis. MRI and EEG were not.
This study shows that over the decade from 2000–2010 MR imaging is likely the most influential imaging modality in determining surgical candidacy for TLE at least at our institution. It is not clear if this finding is because of the familiarity and comfort of neurologist, neurosurgeons, and radiologists with MRI or because of actual better clinical efficacy. To create a side by side comparison and a more homogenous patient population only subjects that had MRI, FDG-PET, and EEG were allowed in this study. Still within this group MRI predominated over scalp EEG and FDG-PET. A similar study performed in 2002 with n=69 found the odds ratio for PET was (8.57), MRI (4.01), and EEG (4.01) in predicting surgical candidacy. [1] That study found that when EEG and PET were positive it resulted in the highest odds ratio (for any two of the three tests) (10.00) while our study found that was true for MRI and EEG (odds ratio 41.8). These differences are potentially either due to institutional bias or changing practice patterns over the last decade. A prospective study randomizing patients to MRI, EEG, and PET only in the cases that remain indeterminate versus mandatory MRI, EEG, and PET for all patients is needed to fully investigate PET cost-effectiveness and affect on surgical outcomes.
Contrary to the surgical decision analysis, the analysis of post-operative outcome yielded only FDG-PET as a significant predictor of post-operative outcome. MRI and EEG were not significant predictors of post-operative outcomes. Only four patients had divergent findings between FDG-PET and MRI. The patients that were MRI negative and PET positive fared much better than the patients that were PET negative and MRI positive. The five patients that were MRI positive, PET negative fared better than those, than the three patients that were MRI negative, PET positive. Of MRI positive patients the patients that were also PET positive did better than the PET negative with a trend toward statistical significance (p=0.06). This finding is in contrast to a study that found that FDG-PET offered no increased prognostic significance when MRI showed mesiotemporal sclerosis. [2] But this finding is similar to prior studies that found that PET hypometabolism correlates well with post-operative outcomes. [11, 16–17] One study even found that temporal hypometabolism had a higher correlation with post-operative outcome than post-surgical pathology. [26] The duration of epilepsy has also been correlated with the degree of hypometabolism, but this study did not confirm this finding. [27] [28] Other PET imaging variables including extratemporal hypometabolism and bilateral temporal hypometabolism, have been correlated negatively with surgical outcomes, but that was not the case in this study. [29] [30]
This study is limited primarily by its retrospective nature. A full elucidation of this topic needs a prospective intention to treat design. Nonetheless this is the largest study (n=124) of its kind. The multidisciplinary conference as the means for deciding the surgical candidacy of patients introduces bias. In this setting the images are all re-interpreted with the insight provided by experts in the other diagnostic modalities as well as clinicians. This collaboration can alter “calls” that may have been marginal, giving more nuance to the yes/no variable used to gather clinical information for this study. Only patients that had all three diagnostic modalities were included in this study. This likely biases the study toward more complex cases with greater diagnostic uncertainty. The order in which the MRI, FDG-PET, and EEG were obtained also affects their interpretation. MRIs are typically obtained prior to FDG-PETs and this likely influences the FDG-PET reader’s interpretations. The results of the post-operative outcome analysis are tempered by the small sample size (n=34), and because of the limited variability in MRI and PET findings (most patients were positive on both of these tests). The only method to completely account for the interpretation and patient selection biases is a prospective randomized trial. In such a trial it is important to consider clinical factors and routine test results. Ideally based on these factors the extensiveness of the pre-operative work-up could be determined. Important factors that should be investigated include: age of onset of epilepsy, duration of epilepsy, # of antiepileptic drug trials, history of febrile seizures, semiology, suspicion of a lateral temporal of extratemporal focus, and equivocal scalp EEG findings.
This study shows that MRI is likely the most influential pre-operative diagnostic modality in patients with medical refractory temporal lobe epilepsy at our institution, which differs from a study in a different institution in 2002 where FDG-PET was the most influential. This study also demonstrates that FDG-PET still has prognostic and diagnostic information that is independent of clinical presentation, MRI, and EEG.
Highlights.
Reviewed the records of patients receiving FDG-PET for temporal lobe epilepsy.
Modeled surgical candidacy and surgical outcomes based of PET, MRI, and EEG results.
124 subjects had TLE, median follow-up (24 months), 34 subjects had TLE surgery.
MRI results affected surgical candidacy most (OR 42.8), PET (OR 20.4), EEG (OR 6.3)
FDG-PET was only significant predictor of post-operative outcome (p=0.04).
Acknowledgements
Dr. Bermans Iskandar1 MD, Dr. Alejandro Rio del Munzo2 PhD, Christine Jaskowiak2, Mark McNall2, and Brook Peters2
University of Wisconsin School of Medicine and Public Health Department of Neurologic Surgery1 and Department of Radiology2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure: None of the authors involved in this study have any conflicts of interests to disclose.
Ethics: This study was approved by the institutional review board (IRB) of the University of Wisconsin, and was performed in accordance of the ethical standards laid down in the 1964 Declaration of Helsinki.
References
- 1.DellaBadia J, Jr, et al. Assessment and cost comparison of sleep-deprived EEG, MRI and PET in the prediction of surgical treatment for epilepsy. Seizure. 2002;11(5):303–309. doi: 10.1053/seiz.2001.0648. [DOI] [PubMed] [Google Scholar]
- 2.Gaillard WD, et al. FDG-PET and volumetric MRI in the evaluation of patients with partial epilepsy. Neurology. 1995;45(1):123–126. doi: 10.1212/wnl.45.1.123. [DOI] [PubMed] [Google Scholar]
- 3.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34(3):453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- 4.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 5.Yoon HH, et al. Long-term seizure outcome in patients initially seizure-free after resective epilepsy surgery. Neurology. 2003;61(4):445–450. doi: 10.1212/01.wnl.0000081226.51886.5b. [DOI] [PubMed] [Google Scholar]
- 6.Foldvary N, et al. Seizure outcome after temporal lobectomy for temporal lobe epilepsy: a Kaplan-Meier survival analysis. Neurology. 2000;54(3):630–634. doi: 10.1212/wnl.54.3.630. [DOI] [PubMed] [Google Scholar]
- 7.Jeha LE, et al. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology. 2006;66(12):1938–1940. doi: 10.1212/01.wnl.0000219810.71010.9b. [DOI] [PubMed] [Google Scholar]
- 8.Wiebe S, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 9.Rosenow F, Luders H. Presurgical evaluation of epilepsy. Brain. 2001;124(Pt 9):1683–1700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- 10.Manno EM, et al. Predictors of outcome after anterior temporal lobectomy: positron emission tomography. Neurology. 1994;44(12):2331–2336. doi: 10.1212/wnl.44.12.2321. [DOI] [PubMed] [Google Scholar]
- 11.Theodore WH, et al. Temporal lobectomy for uncontrolled seizures: the role of positron emission tomography. Ann Neurol. 1992;32(6):789–794. doi: 10.1002/ana.410320613. [DOI] [PubMed] [Google Scholar]
- 12.Casse R, et al. Positron emission tomography and epilepsy. Mol Imaging Biol. 2002;4(5):338–351. doi: 10.1016/s1536-1632(02)00071-9. [DOI] [PubMed] [Google Scholar]
- 13.Theodore WH, et al. Hippocampal volume and glucose metabolism in temporal lobe epileptic foci. Epilepsia. 2001;42(1):130–132. doi: 10.1046/j.1528-1157.2001.080874.x. [DOI] [PubMed] [Google Scholar]
- 14.Foldvary N, et al. Correlation of hippocampal neuronal density and FDG-PET in mesial temporal lobe epilepsy. Epilepsia. 1999;40(1):26–29. doi: 10.1111/j.1528-1157.1999.tb01984.x. [DOI] [PubMed] [Google Scholar]
- 15.Lamusuo S, et al. [18F]FDG-PET reveals temporal hypometabolism in patients with temporal lobe epilepsy even when quantitative MRI and histopathological analysis show only mild hippocampal damage. Arch Neurol. 2001;58(6):933–939. doi: 10.1001/archneur.58.6.933. [DOI] [PubMed] [Google Scholar]
- 16.Theodore WH, et al. Positron emission tomographic measurement of cerebral blood flow and temporal lobectomy. Ann Neurol. 1994;36(2):241–244. doi: 10.1002/ana.410360220. [DOI] [PubMed] [Google Scholar]
- 17.Meyer PT, et al. Inter-modality comparisons of seizure focus lateralization in complex partial seizures. Eur J Nucl Med. 2001;28(10):1529–1540. doi: 10.1007/s002590100602. [DOI] [PubMed] [Google Scholar]
- 18.Carne RP, et al. MRI-negative PET-positive temporal lobe epilepsy: a distinct surgically remediable syndrome. Brain. 2004;127(Pt 10):2276–2285. doi: 10.1093/brain/awh257. [DOI] [PubMed] [Google Scholar]
- 19.Wong CH, et al. The topography and significance of extratemporal hypometabolism in refractory mesial temporal lobe epilepsy examined by FDG-PET. Epilepsia. 2010;51(8):1365–1373. doi: 10.1111/j.1528-1167.2010.02552.x. [DOI] [PubMed] [Google Scholar]
- 20.Engel J, Jr, VNP, Rasmussen TB, Ojemann LM. Surgical treatment of the epliepsies. New York: 1993. Outcome with respect to epileptic seizures; pp. 609–621. [Google Scholar]
- 21.Wieser HG, et al. ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42(2):282–286. [PubMed] [Google Scholar]
- 22.Agresti A, Coull BA. Approximate Is Better than "Exact" for Interval Estimation of Binomial Proportions. The American Statistician. 1998;52(2):119–126. [Google Scholar]
- 23.McFadden D. Conditional logit analysis of qualitative choice behaviour. In: Zaremka P, editor. Frontiers in Econometrics. New York, NY: Academic Press; 1974. [Google Scholar]
- 24.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 25.Agresti A. Modelling ordered categorical data: recent advances and future challenges. Stat Med. 1999;18(17–18):2191–2207. doi: 10.1002/(sici)1097-0258(19990915/30)18:17/18<2191::aid-sim249>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 26.Radtke RA, et al. Temporal lobe hypometabolism on PET: predictor of seizure control after temporal lobectomy. Neurology. 1993;43(6):1088–1092. doi: 10.1212/wnl.43.6.1088. [DOI] [PubMed] [Google Scholar]
- 27.Akman CI, et al. Epilepsy duration impacts on brain glucose metabolism in temporal lobe epilepsy: results of voxel-based mapping. Epilepsy Behav. 2010;17(3):373–380. doi: 10.1016/j.yebeh.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theodore WH, et al. Epilepsy duration, febrile seizures, and cerebral glucose metabolism. Epilepsia. 2004;45(3):276–279. doi: 10.1111/j.0013-9580.2004.51803.x. [DOI] [PubMed] [Google Scholar]
- 29.Choi JY, et al. Extratemporal hypometabolism on FDG PET in temporal lobe epilepsy as a predictor of seizure outcome after temporal lobectomy. Eur J Nucl Med Mol Imaging. 2003;30(4):581–587. doi: 10.1007/s00259-002-1079-8. [DOI] [PubMed] [Google Scholar]
- 30.Blum DE, et al. Bilateral temporal hypometabolism in epilepsy. Epilepsia. 1998;39(6):651–659. doi: 10.1111/j.1528-1157.1998.tb01434.x. [DOI] [PubMed] [Google Scholar]