Abstract
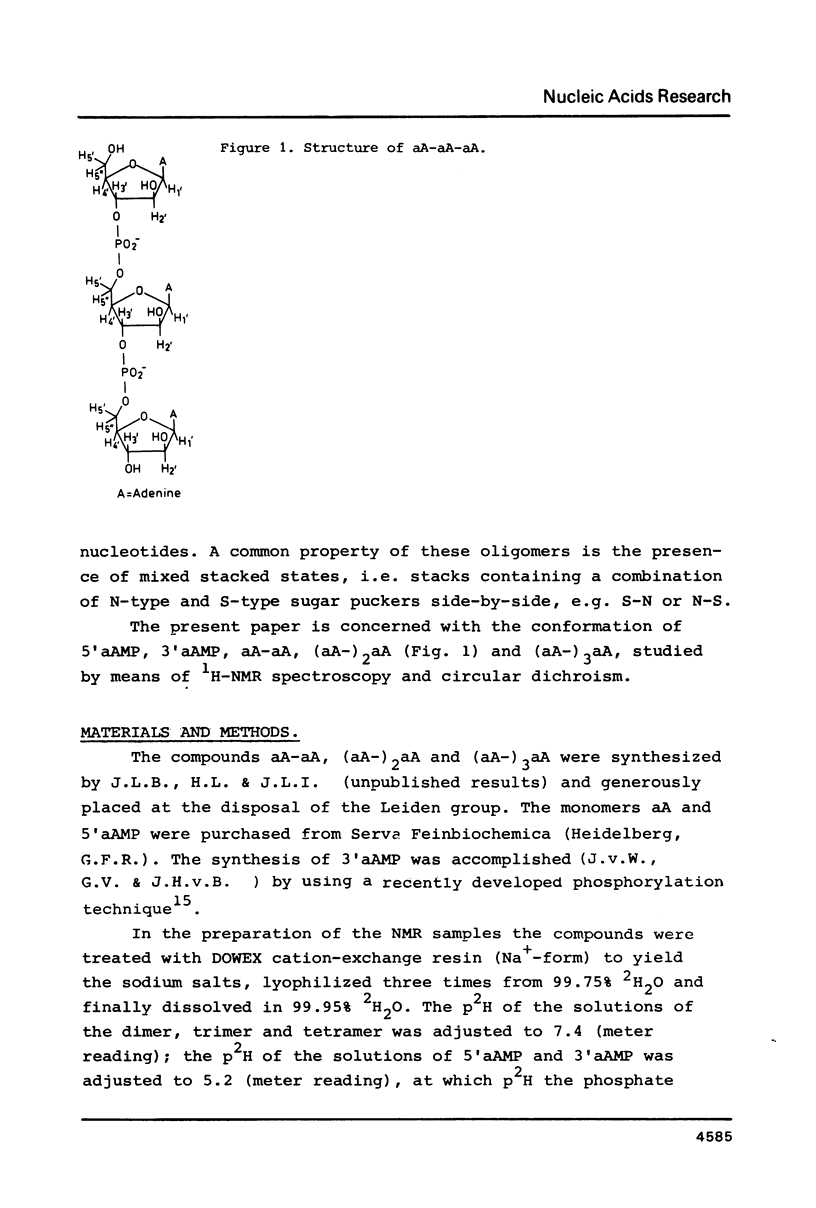
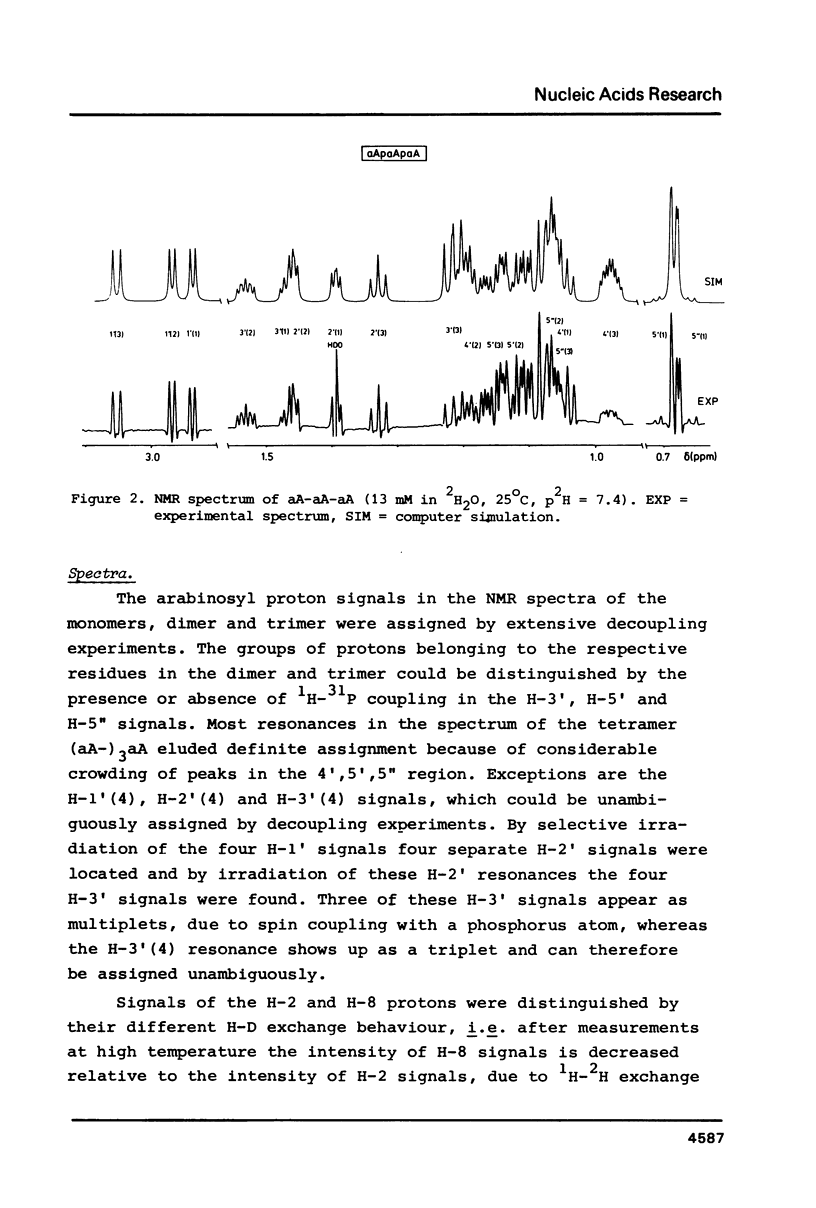
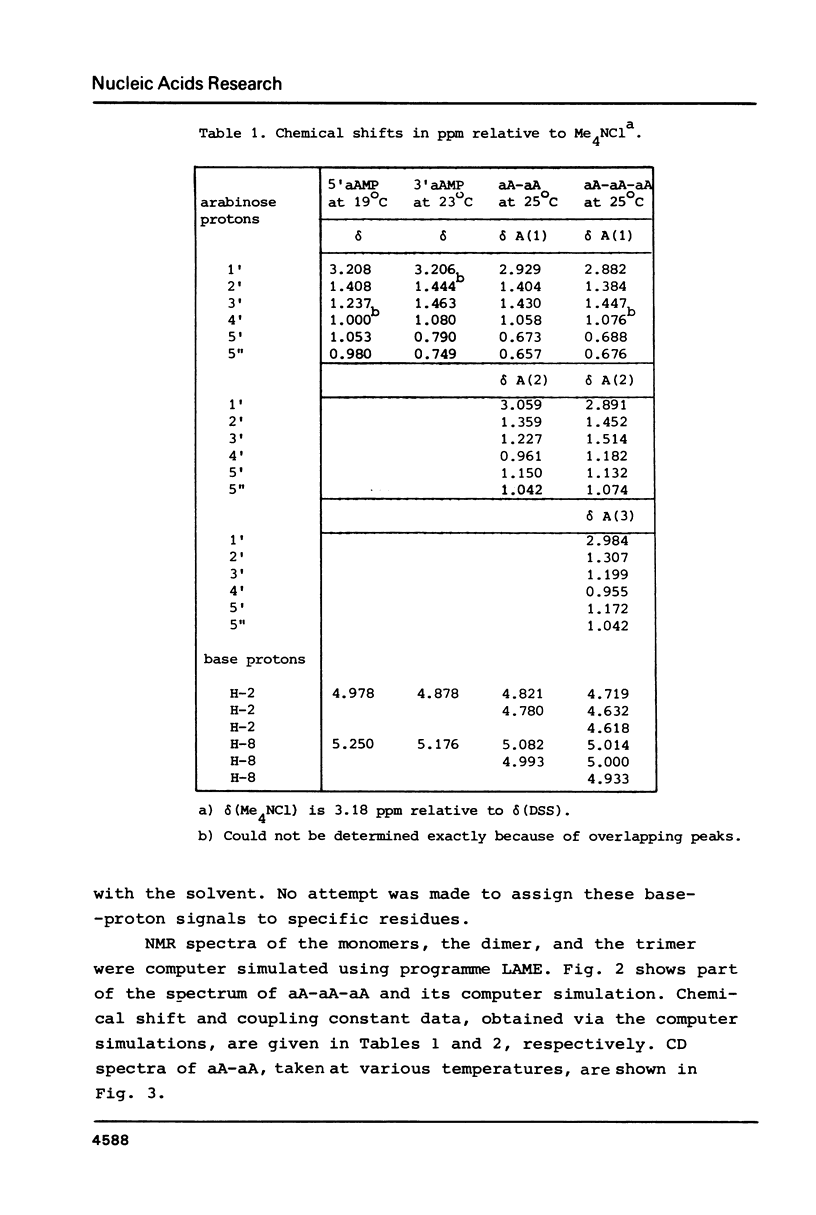
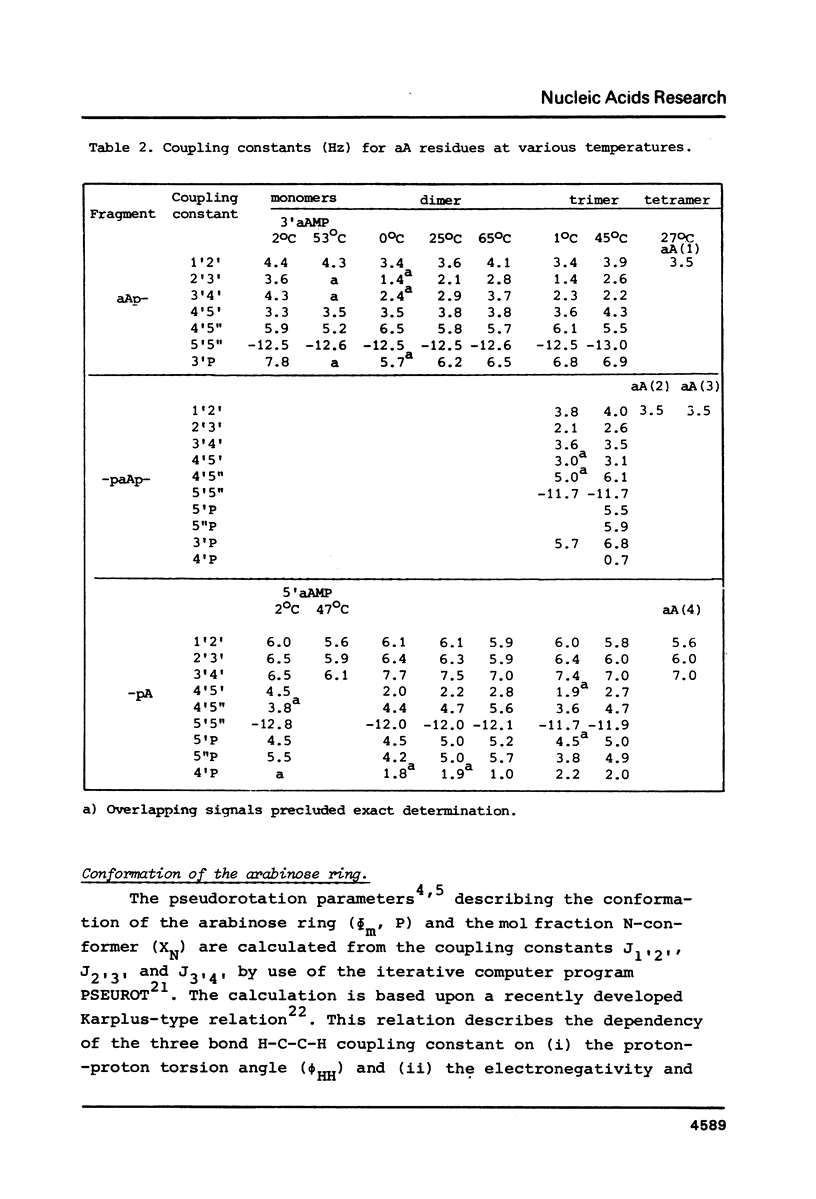
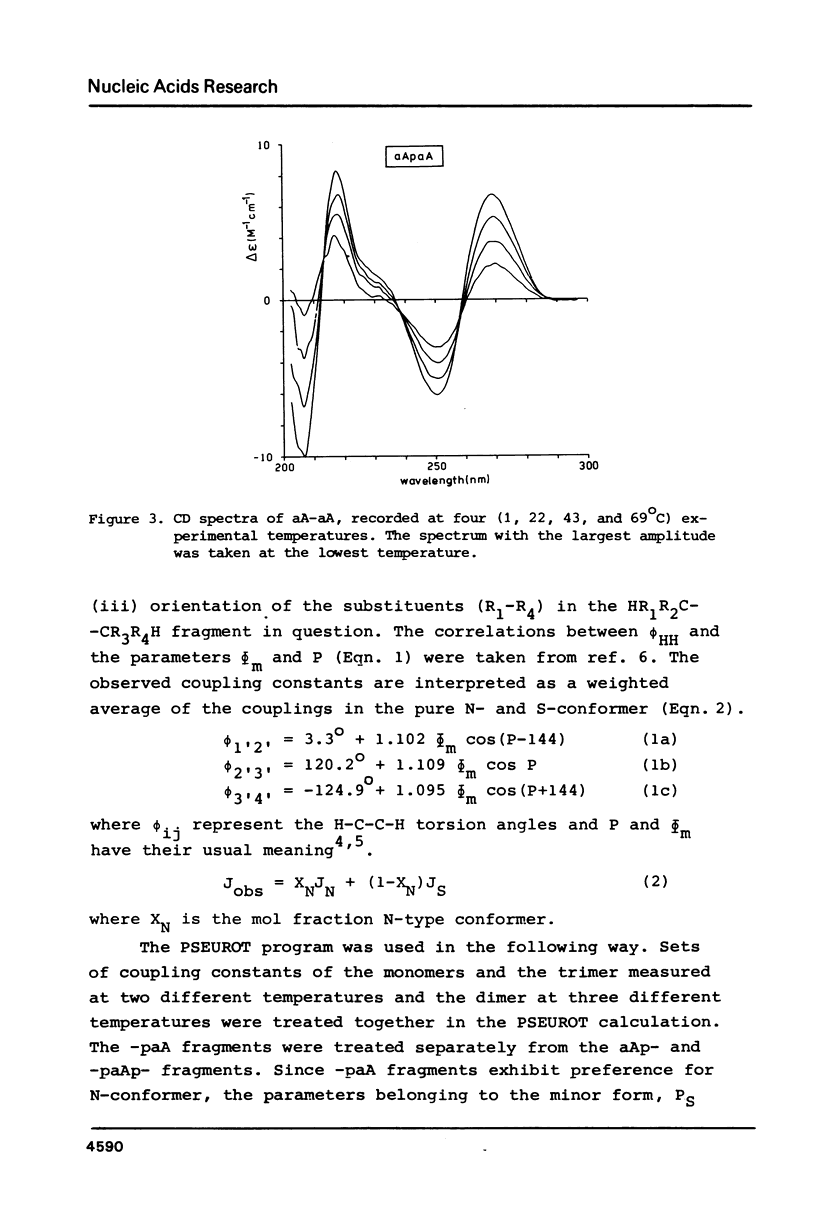
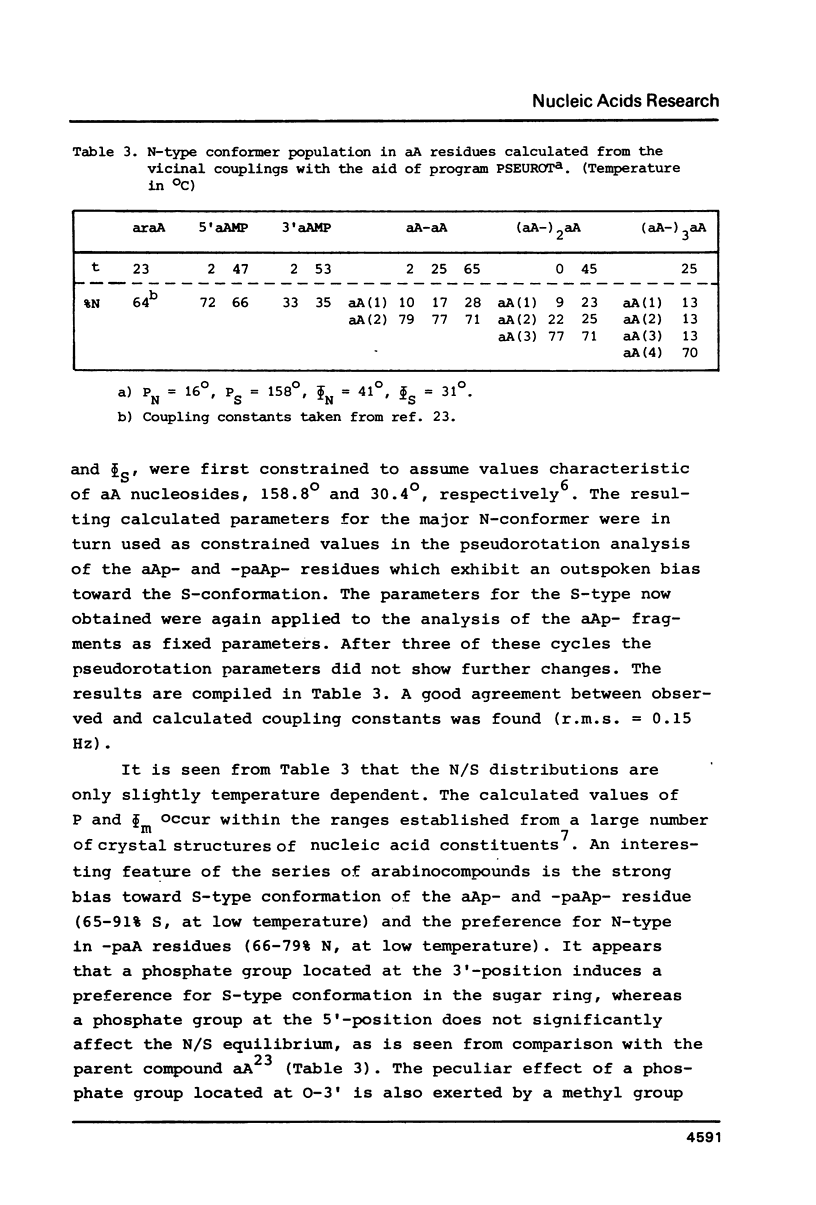
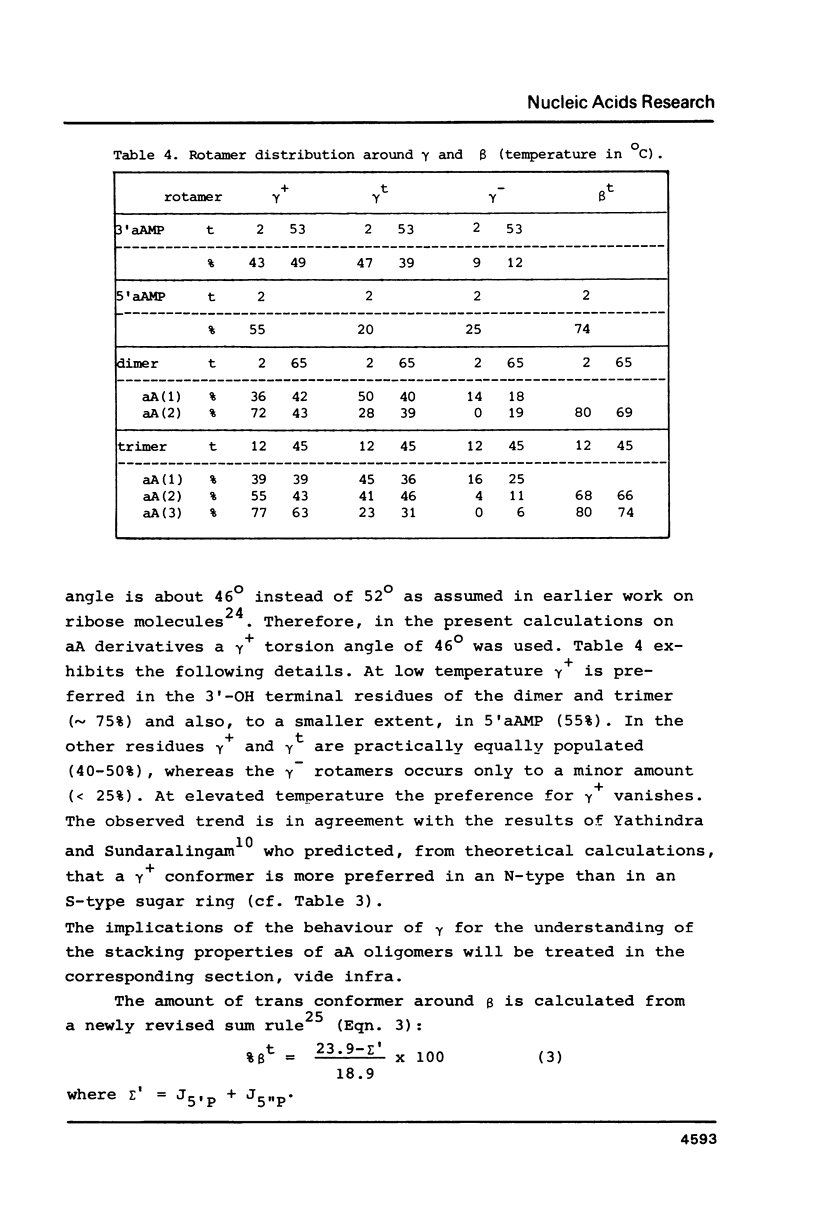
A 500 and 300 MHz proton NMR study of the series of oligoarabinonucleotides 5'aAMP, 3'aAMP, aA-aA, (aA-)2aA and (aA-)3aA is presented. In addition, circular dichroism is used to study the stacking behaviour of aA-aA. The complete 1H-NMR spectral assignment of the compounds (except the tetramer) is given. Proton-proton and proton-phosphorus coupling constants, obtained by computer simulation of the high-field region of the spectra, yield information on the conformation of the arabinose rings (N- or S-type) and on the intramolecular stacking properties of the dimer and the trimer. The monomers 5'aAMP and 3'aAMP exhibit a preference for N- and S-type sugar conformation, respectively. It is shown that the dimer aA-aA at low temperature prefers a mixed stacked state of the type aA(S)-aA(N). In the trimer the aA(2)-aA(3) fragment exhibits a conformation similar to that found in the dimer, whereas the aA(1) residue prefers to adopt S-type sugar and has some tendency to stack upon residue aA(2).
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. A new description using the concept of pseudorotation. J Am Chem Soc. 1972 Nov 15;94(23):8205–8212. doi: 10.1021/ja00778a043. [DOI] [PubMed] [Google Scholar]
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. Improved method for the interpretation of proton magnetic resonance coupling constants. J Am Chem Soc. 1973 Apr 4;95(7):2333–2344. doi: 10.1021/ja00788a038. [DOI] [PubMed] [Google Scholar]
- Brahms J., Maurizot J. C., Pilet J. Interactions contributing to the stability of a polynucleotide helical chain role of the 2'-hydroxyl and of the phosphate groups. Biochim Biophys Acta. 1969 Jul 22;186(1):110–123. doi: 10.1016/0005-2787(69)90493-6. [DOI] [PubMed] [Google Scholar]
- Doornbos J., den Hartog J. A., van Boom J. H., Altona C. Conformational analysis of the nucleotides A2'-5'A, A2'-5'A2'-5'A and A2'-5'U from nuclear magnetic resonance and circular dichroism studies. Eur J Biochem. 1981 May 15;116(2):403–412. doi: 10.1111/j.1432-1033.1981.tb05349.x. [DOI] [PubMed] [Google Scholar]
- Ekiel I., Remin M., Darzynkiewicz E., Shugar D. Correlations of conformational parameters and equilibrium conformational states in a variety of beta-D-arabinonucleosides and their analogues. Biochim Biophys Acta. 1979 Apr 26;562(2):177–191. doi: 10.1016/0005-2787(79)90164-3. [DOI] [PubMed] [Google Scholar]
- Hartel A. J., Lankhorst P. P., Altona C. Thermodynamics of stacking and of self-association of the dinucleoside monophosphate m2(6)A-U from proton NMR chemical shifts: differential concentration temperature profile method. Eur J Biochem. 1982 Dec 15;129(2):343–357. doi: 10.1111/j.1432-1033.1982.tb07057.x. [DOI] [PubMed] [Google Scholar]
- Maurizot J. C., Wechter W. J., Brahms J., Sadron C. Comparison of conformational characteristics of arabinose and ribose containing dinucleoside phosphates. Nature. 1968 Jul 27;219(5152):377–379. doi: 10.1038/219377a0. [DOI] [PubMed] [Google Scholar]
- Olsthoorn C. S., Bostelaar L. J., Van Boom J. H., Altona C. Conformational characteristics of the trinucleoside diphosphate dApdApdA and its constituents from nuclear magnetic resonance and circular dichroism studies. Extrapolation to the stacked conformers. Eur J Biochem. 1980 Nov;112(1):95–110. doi: 10.1111/j.1432-1033.1980.tb04991.x. [DOI] [PubMed] [Google Scholar]
- Olsthoorn C. S., Doornbos J., de Leeuw H. P., Altona C. Influence of the 2'-hydroxyl group and of 6-N-methylation on the conformation of adenine dinucleoside monophosphates in solution. A nuclear magnetic resonance and circular dichroism study. Eur J Biochem. 1982 Jul;125(2):367–382. doi: 10.1111/j.1432-1033.1982.tb06693.x. [DOI] [PubMed] [Google Scholar]
- Olsthoorn C. S., Haasnoot C. A., Altona C. Circular dichroism studies of 6-N-methylated adenylyladenosine and adenylyluridine and their parent compounds. Thermodynamics of stacking. Eur J Biochem. 1980 May;106(1):85–95. doi: 10.1111/j.1432-1033.1980.tb05999.x. [DOI] [PubMed] [Google Scholar]
- Remin M., Darzynkiewicz E., Ekiel I., Shugar D. Conformation in aqueous medium of the neutral, protonated and anionic forms of 9-beta-D-arabinofuranosyladenine. Biochim Biophys Acta. 1976 Jul 16;435(4):405–416. doi: 10.1016/0005-2787(76)90205-7. [DOI] [PubMed] [Google Scholar]
- Remin M., Shugar D. Conformation of the exocyclic 5'-CH 2 OH in nucleosides and nucleotides in aqueous solution from specific assignments of the H 5' and H 5'' signals in the NMR spectra. Biochem Biophys Res Commun. 1972 Aug 7;48(3):636–642. doi: 10.1016/0006-291x(72)90395-6. [DOI] [PubMed] [Google Scholar]
- Yathindra N., Sundaralingam M. Conformational analysis of arabinonucleosides and nucleotides. A comparison with the ribonucleosides and nucleotides. Biochim Biophys Acta. 1979 Sep 27;564(2):301–310. doi: 10.1016/0005-2787(79)90227-2. [DOI] [PubMed] [Google Scholar]
- van der Marel G. A., van Boeckel C. A., Wille G., van Boom J. H. A general method for the synthesis of 5'-monophosphates of DNA fragments via phosphotriester intermediates. Nucleic Acids Res. 1982 Apr 10;10(7):2337–2351. doi: 10.1093/nar/10.7.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]


