Abstract
Mechanical forces play an increasingly recognized role in modulating cell function. This report demonstrates mechanosensing by T cells, using polyacrylamide gels presenting ligands to CD3 and CD28. Naive CD4 T cells exhibited stronger activation, as measured by attachment and secretion of IL-2, with increasing substrate elastic modulus over the range of 10–200 kPa. By presenting these ligands on different surfaces, this report further demonstrates that mechanosensing is more strongly associated with CD3 rather than CD28 signaling. Finally, phospho-specific staining for Zap70 and Src family kinase proteins suggests that sensing of substrate rigidity occurs at least in part by processes downstream of T-cell receptor activation. The ability of T cells to quantitatively respond to substrate rigidly provides an intriguing new model for mechanobiology.
Cells have the remarkable ability to respond to the mechanical rigidity of the extracellular environment. This has been explored predominantly in anchorage-dependent cells and the specific context of integrin- and cadherin-based adhesion. As a complementary system, we demonstrate here mechanosensing by T lymphocytes, key modulators of adaptive immunity. T cells are activated through engagement of the T-cell receptor (TCR) by peptide-bearing major histocompatibility complex proteins on antigen presenting cells within a small (∼70 μm2) cell-cell contact area termed the immune synapse (1). This interface hosts additional receptor-ligand interactions; engagement of CD28 on the T cell surface provides a costimulatory signal that augments TCR function and is required for activation of naive T cells. The immune synapse is also characterized by a dynamic cytoskeleton (2) that transports clusters of signaling molecules, suggesting a role of physical forces in T cell activation. Indeed, recent studies show that the TCR is sensitive to forces (3, 4), but the full impact and mechanism of mechanosensing in T cells remains unexplored.
In this report, the antigen presenting cell is replaced with polyacrylamide gels presenting two activating antibodies (see the Supporting Material), one against CD3 (epsilon subunit, which upon binding activates the TCR complex) and the other to CD28. Concurrent engagement of these two receptors by appropriate antibodies immobilized on rigid beads or planar surfaces is sufficient to induce T cell activation. Notably, activation is not induced by soluble anti-CD3 and anti-CD28. In this report, gel rigidity was controlled by varying the amount of bis-acrylamide cross-linker (5) yielding a core set of materials of bulk Young's moduli (E) between 10 and 200 kPa. Biotinylated anti-CD3 and anti-CD28 antibodies were tethered to the polyacrylamide using an acrylamide-modified streptavidin, yielding a thin (micrometers thick), layer of antibodies (Fig. S1 in the Supporting Material). The concentration of acrylamide-streptavidin was adjusted to produce a single, standard surface density of tethered proteins that will be used for this study across all substrates. Please see the Supporting Material for additional analysis of this approach.
Mouse naive CD4+ T cells were seeded onto polyacrylamide gels presenting a 1:1 mix of anti-CD3 and anti-CD28, and secretion of IL-2 over a 6-h period was compared across gels as a functional measure of activation using a fluorescence-based, surface capture method (6). IL-2 secretion was lowest on the softest (E = 10 kPa) gels, and increased with substrate rigidity (Fig. 1 A). A small, not statistically significant decrease was observed on the 200 kPa vs. 100 kPa gel, possibly reflecting lower T cell accessibility to the antibodies due to smaller gel pore size. IL-2 secretion was not detectable on surfaces containing either anti-CD3 or anti-CD28 alone (data not shown), reflecting the need for both signals in T cell activation. Cell attachment also responds to substrate rigidity, with a lower density of cells observed on the softest surface compared to the three stiffer preparations (Fig. 1 A). Decreasing the gel rigidity below 10 kPa presented no further change in either cell response (Fig. S2); the rest of this study focuses on the core range of 10–200 kPa. These results suggest that cell response can be divided into two ranges on the basis of Young's modulus; for rigidities of 25 kPa or higher, cells exhibit strong attachment and a positive (but saturating) correlation of IL-2 secretion with stiffness, whereas below this range, cells reduce both attachment and IL-2 secretion. An alternative interpretation of this data is that at the lower rigidities IL-2 production is modulated by cell attachment density and not directly by elastic modulus. This was addressed by seeding cells at higher densities, as detailed in the Supporting Material. On the 10 kPa gels, an increase in cell attachment density to match that of the three stiffer gels resulted in a minor, not statistically significant increase in IL-2 secretion. Much larger increases in cell seeding density resulted in a minor statistically significant increase IL-2 secretion, but this was associated with a much larger increase in attachment density. Cooperativity between cells thus has an effect on cell activation that is small compared to mechanosensing.
Figure 1.
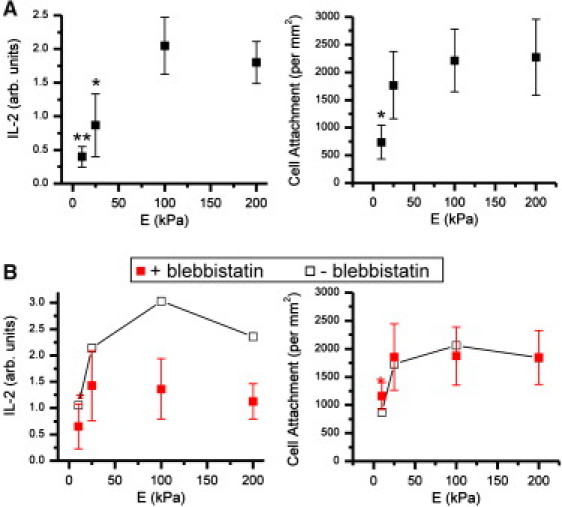
Rigidity-dependent activation of CD4+ T cells. (A) 6-h IL-2 secretion and cell attachment correlate with Young's modulus, E. ∗P < 0.05, ∗∗P < 0.005 compared to 200 kPa surface. Data are mean ± SD, n = 7. (B) Inhibition of myosin-based contractility abrogates mechanosensing on surfaces of 25 kPa or greater. ∗P < 0.05 compared to 200 kPa surface, n = 3. Error bars for nonblebbistatin controls are omitted for clarity.
Treatment of cells with blebbistatin (100 μM, Fig. 1 B) abrogated the sensitivity of IL-2 secretion across the three stiffest gels, indicating a role of cytoskeletal contractility in cell response. This builds upon an earlier study showing TCR signaling in response to B cells and anti-CD3 presenting bilayers is reduced by blebbistatin treatment (7). Notably, both IL-2 secretion and cell attachment on the 10 kPa gel remained lower than on the three stiffer gels, supporting the idea that cell interaction with the 10 kPa gel is fundamentally different than on the stiffer counterparts.
We next sought to determine whether mechanosensing is mediated by CD3 or CD28. For these experiments, one of the activating antibodies was tethered to the planar polyacrylamide gels while the other was immobilized onto rigid, 4-μm diameter polystyrene beads (Fig. 2 A). Varying the rigidity of gels presenting anti-CD3 modulated IL-2 secretion resembling that on surfaces presenting both anti-CD3 and anti-CD28 (Fig. 2 B). In contrast, cells on gels presenting anti-CD28 showed only a small and statistically insignificant (analysis of variance (ANOVA), α = 0.05, n = 3) decreasing trend in IL-2 secretion with increasing Young's modulus. A longer incubation time (16 h.) was required to obtain measurable IL-2 secretion from these cells. This delayed response may be related to the smaller surface presented by individual beads compared to a gel, or that CD3 and CD28 were engaged on different faces of the T cell, a configuration termed trans-costimulation that earlier studies show induces lower levels of activation than the cis- counterpart (8). We also note that a well-established method for activating T cells is to provide anti-CD3 on a solid support and anti-CD28 in solution. Surprisingly, soluble CD28 at concentrations of 2–20 μg/ml was ineffective in stimulating IL-2 secretion by cells on gels presenting anti-CD3. Together, these results suggest that T cell mechanosensing is associated with CD3 rather than CD28.
Figure 2.
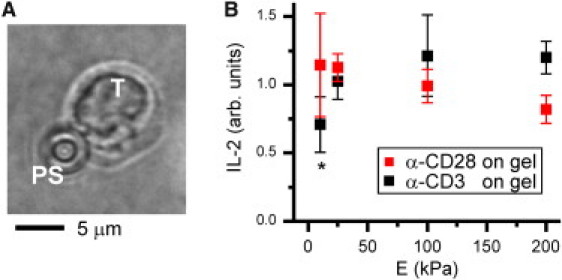
CD3-mediated mechanosensing. (A) T cell (T) interacting with an antibody-coated polystyrene (PS) bead and underlying gel. (B) 16-h secretion of IL-2. ∗P < 0.05 compared to 200 kPa gel. Data are mean ± SD, n = 3.
We next focused on proteins involved in T cell activation as potential mechanisms of mechanosensing. Phospho-specific antibodies were used to detect Zap70 (Tyr-493) and an activation loop that is conserved across many Src family kinase proteins (SFK) (9, 10); available antibodies cannot distinguish between phosphorylated Lck (Tyr-394) and Fyn (Tyr-420), the two major SFK proteins involved in T cell signaling. By 2 min following seeding, both antibodies detected clusters of proteins in the cell-substrate interface on the three stiffest surfaces (Fig. 3). In contrast, cells on the 10 kPa gels were devoid of pZap70 and pSFK clusters within the interior of the cell-substrate interface, exhibiting only minor accumulations along the cell edge (Fig. 3 A). Whole-cell measurement of pZap70 and pSFK followed a similar pattern, being lower on the 10 kPa gel than the 200 kPa preparation (Fig. 3 B). The 2 min time point captures the early burst of Zap70 and SFK activity, but similar patterns were also observed for sustained signaling at 30 min (Fig. S3, A and C). Notably, cells on the three stiffest surfaces were more spread than on the 10 kPa preparation (Fig. S3 A). Application of blebbistatin did not affect the distribution or cellular levels of pZap70 and pSFK (Fig. S3, B and C; α = 0.05, two-way ANOVA). Together, these results suggest that loss of cell attachment and activation on the 10 kPa gel is associated with loss of early TCR signaling, whereas mechanosensing on the stiffest gels is mediated by mechanisms downstream of Lck/Fyn and Zap70. We note that for human cells interacting with B cells or lipid bilayers, blebbistatin reduces pZap70 at both the whole cell level and in microclusters at the cell-bilayer interface (7). This may reflect differences in species or ligand presentation, but the use of total internal reflection microscopy to probe the thin (200 nm) cell-bilayer interface (not possible at cell-gel contacts) may also explain these results. Finally, we followed phosphorylation of Pyk2 (Tyr-580), a protein related to focal adhesion kinase, which has additional roles in TCR signaling (11). Similar to SFK and Zap70, clusters of pPyk2 were found in the cell-substrate interface on the three stiffest gels, but were restricted to the interface edge on the 10 kPa preparations (Fig. S3, A and B). Unlike SFK and Zap70, whole-cell levels of pPyk2 were independent of rigidity (Fig. S3 C). However, blebbistatin induced a minor but statistically significant decrease in pPyk2 (P < 0.01, two-way ANOVA) across all substrates, suggesting that Pyk2 responds to cell contractility and may contribute to T cell mechanosensing.
Figure 3.
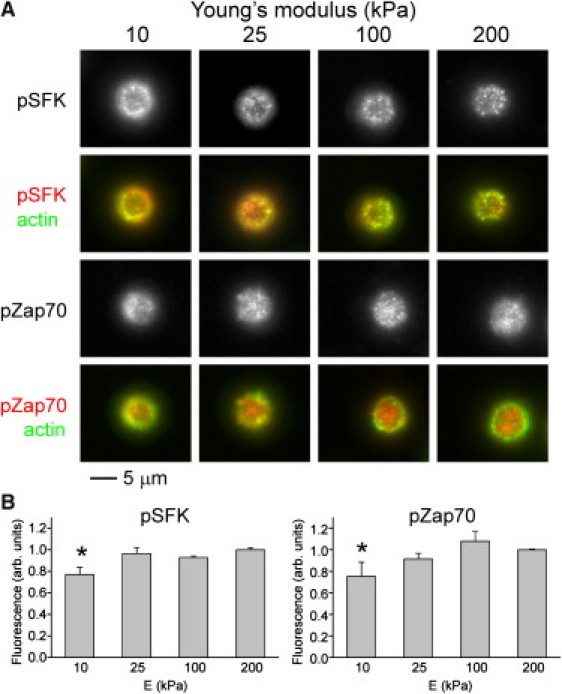
Rigidity-dependent early signaling. (A) Phospho-specific immunostaining 2 min after seeding. (B) Comparison of whole-cell phosphorylation of early signaling proteins. Data are mean ± SD, n = 3. ∗P < 0.05 compared to 200 kPa surface.
Finally, we note that TCR and CD28 signaling is very distinct in mechanism than the integrin and cadherin pathways. Specifically, although CD3 and CD28 signaling influences cytoskeleton dynamics, direct mechanical connections between these structures have not been identified. Mechanosensing through these pathways is thus a new model in mechanobiology that sets a wider role of physical forces in biology.
Acknowledgments
We thank E. U. Azeloglu (Mount Sinial School of Medicine, New York, NY) for assistance with mechanical testing of polyacrylamide gels.
This study was supported by National Institutes of Health grants PN2 EY016586 and R01AI088377.
Editor: Michael Edidin.
Footnotes
Materials and Methods, three figures, and references (12, 13) are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(11)05408-7.
Supporting Material
References and Footnotes
- 1.Grakoui A., Bromley S.K., et al. Dustin M.L. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 2.Dustin M.L. Cell adhesion molecules and actin cytoskeleton at immune synapses and kinapses. Curr. Opin. Cell Biol. 2007;19:529–533. doi: 10.1016/j.ceb.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim S.T., Takeuchi K., et al. Reinherz E.L. The alphabeta T cell receptor is an anisotropic mechanosensor. J. Biol. Chem. 2009;284:31028–31037. doi: 10.1074/jbc.M109.052712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y.C., Chen B.M., et al. Roffler S.R. Cutting Edge: mechanical forces acting on T cells immobilized via the TCR complex can trigger TCR signaling. J. Immunol. 2010;184:5959–5963. doi: 10.4049/jimmunol.0900775. [DOI] [PubMed] [Google Scholar]
- 5.Pelham R.J., Jr., Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen K., Thomas V.K., et al. Kam L.C. Micropatterning of costimulatory ligands enhances CD4+ T cell function. Proc. Natl. Acad. Sci. USA. 2008;105:7791–7796. doi: 10.1073/pnas.0710295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ilani T., Vasiliver-Shamis G., et al. Dustin M.L. T cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nat. Immunol. 2009;10:531–539. doi: 10.1038/ni.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Lockhart M., Miller J. Engagement of CD28 outside of the immunological synapse results in up-regulation of IL-2 mRNA stability but not IL-2 transcription. J. Immunol. 2006;176:4778–4784. doi: 10.4049/jimmunol.176.8.4778. [DOI] [PubMed] [Google Scholar]
- 9.Chan A.C., Dalton M., et al. Kurosaki T. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995;14:2499–2508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veillette A., Fournel M. The CD4 associated tyrosine protein kinase p56lck is positively regulated through its site of autophosphorylation. Oncogene. 1990;5:1455–1462. [PubMed] [Google Scholar]
- 11.Ostergaard H.L., Lysechko T.L. Focal adhesion kinase-related protein tyrosine kinase Pyk2 in T-cell activation and function. Immunol. Res. 2005;31:267–282. doi: 10.1385/IR:31:3:267. [DOI] [PubMed] [Google Scholar]
- 12.Costa K.D., Yin F.C. Analysis of indentation: implications for measuring mechanical properties with atomic force microscopy. J. Biomech. Eng. 1999;121:462–471. doi: 10.1115/1.2835074. [DOI] [PubMed] [Google Scholar]
- 13.Sims T.N., Soos T.J., et al. Dustin M.L. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


