Abstract
Objective
The International Association of Diabetes and Pregnancy Study Groups (IADPSG) recently proposed new criteria for diagnosing gestational diabetes mellitus (GDM). We compared prevalence rates, risk factors, and the effect of ethnicity using the World Health Organization (WHO) and modified IADPSG criteria.
Methods
This was a population-based cohort study of 823 (74% of eligible) healthy pregnant women, of whom 59% were from ethnic minorities. Universal screening was performed at 28±2 weeks of gestation with the 75 g oral glucose tolerance test (OGTT). Venous plasma glucose (PG) was measured on site. GDM was diagnosed as per the definition of WHO criteria as fasting PG (FPG) ≥7.0 or 2-h PG ≥7.8 mmol/l; and as per the modified IADPSG criteria as FPG ≥5.1 or 2-h PG ≥8.5 mmol/l.
Results
OGTT was performed in 759 women. Crude GDM prevalence was 13.0% with WHO (Western Europeans 11%, ethnic minorities 15%, P=0.14) and 31.5% with modified IADPSG criteria (Western Europeans 24%, ethnic minorities 37%, P< 0.001). Using the WHO criteria, ethnic minority origin was an independent predictor (South Asians, odds ratio (OR) 2.24 (95% confidence interval (CI) 1.26–3.97); Middle Easterners, OR 2.13 (1.12–4.08)) after adjustments for age, parity, and prepregnant body mass index (BMI). This increased OR was unapparent after further adjustments for body height (proxy for early life socioeconomic status), education and family history of diabetes. Using the modified IADPSG criteria, prepregnant BMI (1.09 (1.05–1.13)) and ethnic minority origin (South Asians, 2.54 (1.56–4.13)) were independent predictors, while education, body height and family history had little impact.
Conclusion
GDM prevalence was overall 2.4-times higher with the modified IADPSG criteria compared with the WHO criteria. The new criteria identified many subjects with a relatively mild increase in FPG, strongly associated with South Asian origin and prepregnant overweight.
Introduction
Gestational diabetes mellitus (GDM), defined as any degree of glucose intolerance with onset or first recognition during pregnancy, was first described about half a century ago (1). The diagnostic criteria for GDM were initially developed to predict future diabetes in the mother, although its link with macrosomia was recognized. Today, a variety of screening procedures and diagnostic criteria are used (2). This lack of a standardized approach hampers the understanding, research and clinical care of GDM (3). Prevalence rates of GDM in population-based studies range from 1 to 22% (4). This diversity also reflects differences between the study populations in ethnic origin and age, and an increasing prevalence associated with the global epidemic of obesity and diabetes (4).
Recently, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) proposed new criteria for GDM (5) based on the findings from the Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) study (6). The HAPO study showed a continuous and graded relationship between maternal glycemia and adverse fetal outcomes. The cutoff values in the new criteria were set to reflect an odds ratio (OR) of at least 1.75 for an adverse fetal outcome, defined as above the 90th percentile for birth weight, cord C-peptide or percent body fat compared with subjects having glucose values equal to or below the mean value in the full cohort, although other ORs were discussed.
The proposed diagnostic cutoff values for glucose in the IADPSG criteria are slightly lower than those in the criteria that are currently most widely used in North America (3). Furthermore, one single glucose value above the cutoff value (fasting or during the oral glucose tolerance test (OGTT)) is sufficient to diagnose GDM, as opposed to two elevated glucose values. Universal instead of selective screening is recommended (5). In Europe, either the World Health Organization (WHO) criteria based on the cutoff values for diabetes and impaired glucose tolerance outside pregnancy (7) or the slightly modified European Association for the Study of Diabetes (EASD) criteria (8) are used most frequently when diagnosing GDM. Compared with these criteria, the IADPSG criteria's glucose cutoff values are lowered for the fasting and raised for the post-OGTT values.
In many parts of the world, ethnic minority groups, which are often socially disadvantaged (9), are disproportionally more affected by type 2 diabetes (10) and GDM (11). The present population-based STORK Groruddalen Study was conducted in the district of Oslo, Norway, covering 82 000 inhabitants, of whom 40% have an ethnic minority background (12). This study was aimed to determine the prevalence of GDM and its risk factors with the WHO (7) and the IADPSG criteria, slightly modified due to lack of 1-h glucose values (5) overall, in the largest ethnic groups. Furthermore, we wanted to assess the association between ethnic origin and these criteria after adjusting for covariates and discuss the implications of the proposed criteria for public health preventive strategies.
Design and methods
Design, study population and data collection
This population-based cohort study was conducted at three public Child Health Clinics in Groruddalen, Oslo, Norway (12). Antenatal care for pregnant women in Norway is carried out in primary care, either at the public Child Health Clinic alone or in combination with the general practitioner (GP), or by the GP alone (13). Groruddalen covers affluent as well as more deprived residential areas and has a population with a diverse socioeconomic status. The majority (75–85%) of pregnant women residing in this area attend the Child Clinics for antenatal care.
From May 6, 2008 to May 15, 2010, 823 (74% of eligible) women were included. All information material and questionnaires were translated to Arabic, English, Sorani, Somali, Tamil, Turkish, Urdu and Vietnamese, and quality controlled by bilingual health professionals. Women were eligible if they i) lived in the districts, ii) planned to give birth at one of the two study hospitals, iii) were <20 weeks pregnant, iv) could communicate in Norwegian or any of the above specified languages and v) were able to give a written consent to participate. Women with pregestational diabetes or other diseases necessitating intensive hospital follow-up during pregnancy were excluded. The Regional Ethics Committee and the Norwegian Data Inspectorate approved the study protocol.
We have previously published a paper in which the study population is described in detail (12). At baseline, participants were representative for women attending the Child Health Clinics with respect to ethnicity and age. A slight selection toward lower parity (South Asians) and age (Africans) was found. The study methods have been presented elsewhere (12). In short, data from questionnaires, anthropometric measurements and venous fasting blood samples drawn after an overnight fast, were collected by specially trained midwives at <20 and at 28±2 weeks of gestation. All staff members were certified after extensive education and on-site supervision and were assisted by professional translators when needed. Collected data included demographic and socioeconomic factors (education, employment and body height), family history of diabetes, medical and obstetric history and information related to the pregnancy. Body height was measured to the nearest 0.1 cm (fixed stadiometer checked against a standard meter) and body weight was measured to the nearest 0.1 kg using a Tanita-BC 418 MA scale (Tanita Corp., Tokyo, Japan). Self-reported prepregnant body weight correlated strongly with weight at inclusion (r=0.97, P<0.001, mean difference: 2.0 kg) and was used to calculate prepregnant body mass index (BMI).
Ethnicity may be defined as the social group a person belongs to, which implies shared culture, history, geographical origins, language, lifestyle factors, and physical, genetic and other factors (14). In this study, ethnic origin was defined by the participant's country of birth or the participant's mother's country of birth if the participant's mother was born outside of Europe or North America. Women with ethnic origin from Eastern Europe, Asia, Africa and South America and Central America are referred to as ethnic minority women. The reference group participants, Western Europeans, were born in Norway (93.6%), Sweden or Denmark (3.2%), or other Western European countries (3.2%, includes North America, n=3).
GDM definitions and laboratory methods
A 75 g OGTT was performed at 28 weeks of gestation after an overnight fast. GDM was diagnosed with the WHO criteria (fasting plasma glucose (FPG) ≥7.0 or 2-h PG ≥7.8 mmol/l (7)) and the slightly modified IADPSG criteria (FPG ≥5.1 or 2-h PG ≥8.5 mmol/l (5)), as 1-h glucose values were not available. For the diagnosis and handling of the GDM cases during the study, the WHO criteria were used. In accordance with the national guidelines (15), women with FPG ≥7.0 mmol/l or 2-h PG ≥9.0 mmol/l were referred to secondary care and those with 2-h PG 7.8–9.0 mmol/l were referred to their GP, after lifestyle advice had been given.
Glucose was measured on site (within 5 min after vein puncture) in venous EDTA blood according to a standardized protocol, using a patient-near method (HemoCue 201+, Angelholm, Sweden) calibrated for plasma. This method was preferred to: i) avoid preanalytical glucose reductions due to glycolysis during shipment to the laboratory; and ii) receive immediate results to allow optimal patient information and necessary actions when GDM was diagnosed. The total system variation at 2.0 and 7.0 mmol/l was 1 s.d.=0.21 and 0.25 mmol/l respectively. The same batch number of cuvettes and controls (run weekly, all within 1 s.d.=0.5 mmol/l from mean) was used at the three study sites. The three instruments were externally validated one to two times per year against recently drawn patient blood as controls with mean 6.7 mmol/l (maximum 1 s.d.=0.11 mmol/l) and three controls with mean 3.0, 7.0 and 11.0 mmol/l (maximum 1 s.d.=0.20 mmol/l for all).
The on-site measured glucose values were checked in a large subgroup of samples against the glucose values measured by standard clinical chemistry laboratory (Vitros 5.1 FS, Ortho Clinical Diagnostics, Rochester, MN, USA, slide adapted colorimetric method at the Department of Multidisciplinary Laboratory Medicine and Medical Biochemistry, Akershus University Hospital). For this analysis, venous blood was drawn in gel tubes, allowed to clot for 30 min, and thereafter stored at +4 °C until daily shipment to the laboratory. The mean difference in glucose values between the on-site measurements and the measurements performed at the hospital laboratory for the 2-h PG value was 0.30 mmol/l; the measurements highly correlated with R2=0.913, and a linear regression equation: 2-h PG (HemoCue)=0.989+0.883×2-h PG (Hospital). HbA1c was measured with HPLC (Tosoh G8, Tosoh Corporation, normal reference range 4–6%).
When valid HemoCue FPG or 2-h PG values were missing, glucose values from the hospital laboratory (FPG, n=14; 2-h PG, n=9) or values extracted from the medical records (FPG, n=11; 2-h PG, n=14) were used. One woman started insulin treatment at 20 weeks of gestation and OGTT was not performed at 28 weeks.
Statistical analyses
The main outcome variable was GDM. The study was designed to be large enough to show differences in prevalence rates of GDM with the WHO criteria between the major ethnic groups, based on the following assumed prevalences: Western Europeans, 5%; South Asians, 20%; and other ethnic minority groups, 10%. We aimed to enroll at least 800 women, which would give ∼100 GDM cases (12).
Differences in characteristics between groups were tested with t-tests and one-way ANOVA for normally distributed continuous variables and χ2 tests for categorical variables, and with the Mann–Whitney test for non-normally distributed continuous data. Pearson's or Spearman correlation coefficients were calculated as appropriate.
Univariate and multiple logistic regression analyses were performed to identify the effect of ethnic origin on GDM with the WHO and modified IADPSG criteria separately, after adjusting for covariates. In model A, age, prepregnant BMI and parity were entered as adjustment covariates, whereas in model B, education and body height (used as a proxy for early life socioeconomic status) were further added. In the final model C, all covariates, including first-degree relatives with diabetes, were entered. Interactions were tested on the relative scale by adding into each model a cross product term between two-by-two factors at a time. Statistical significance level was set to P<0.05. ORs with 95% CI were estimated. SPSS version 18 (IBM SPSS Statistics, NY, USA) was used.
Results
Characteristics of women
Of the 823 included women, 18 (2%) had an abortion or delivery before the visit at 28 weeks of gestation and 33 (4%) did not attend the clinic. Of the 772 (93.8%) women who attended the clinic at 28 weeks of gestation, 13 were not able to complete the OGTT, and thus the OGTT data were available only for 759 (92.2%) women.
Fifty-nine percent were from an ethnic minority group, with the largest groups being composed of South Asians (25%) and Middle Easterners (15%). Baseline characteristics did not differ between those with and without OGTT data. Western European women were older, taller, had lower parity and higher education compared with the ethnic minority women (Table 1). Mean prepregnant BMI did not differ between Western European and ethnic minority women, although a considerable heterogeneity between the ethnic minority groups was observed for several variables, such as prepregnant BMI, parity and education, as shown in Table 1.
Table 1.
Baseline characteristics of the total cohort stratified into Western European and ethnic minority women, and further into ethnic origin. Data are mean (s.d.), unless otherwise stated.
| Total | Western Europea | Ethnic minority | Eastern Europeb | South Asiac | East Asiad | Middle Easte | Somalia | Sub-Saharan Africa/South Americaf | |
|---|---|---|---|---|---|---|---|---|---|
| n (%) | 759 (100.0) | 313 (41.2) | 446 (58.8) | 42 (5.5) | 188 (24.8) | 39 (5.1) | 112 (14.8) | 35 (4.6) | 30 (4.0) |
| Years of maternal age | 29.9 (4.8) | 30.9 (4.5) | 29.1 (4.9) | 28.7 (4.1) | 28.7 (4.5) | 31.0 (4.6) | 29.4 (5.4) | 28.5 (5.8) | 29.4 (5.8) |
| Parity, n (%) | |||||||||
| Nulliparous | 347 (45.7) | 162 (51.7) | 185 (41.5) | 27 (64.3) | 78 (41.5) | 16 (41.0) | 38 (33.9) | 13 (37.1) | 13 (43.3) |
| Uniparous | 261 (34.4) | 116 (37.1) | 145 (32.5) | 12 (28.6) | 62 (33.0) | 16 (41.0) | 39 (34.8) | 5 (14.3) | 11 (36.7) |
| Multiparous (≥ 2) | 151 (19.9) | 35 (11.2) | 116 (26.0) | 3 (7.1) | 48 (25.5) | 7 (18.0) | 35 (31.3) | 17 (48.6) | 6 (20.0) |
| Educational levelg, n (%) | |||||||||
| <10 y schooling | 123 (16.3) | 10 (3.2) | 113 (25.3) | 5 (12.2) | 33 (17.6) | 8 (20.5) | 42 (37.5) | 21 (60.0) | 4 (13.3) |
| Secondary level, 10–12 y | 297 (39.5) | 95 (30.6) | 202 (45.7) | 14 (34.1) | 94 (50.3) | 16 (41.0) | 50 (45.5) | 11 (31.4) | 17 (56.7) |
| University/college | 333 (44.2) | 206 (66.2) | 127 (28.7) | 22 (53.7) | 60 (32.1) | 15 (38.5) | 18 (16.4) | 3 (8.6) | 9 (30.0) |
| Employedg, n % | 525 (70.0) | 272 (87.5) | 253 (57.6) | 31 (75.6) | 115 (62.2) | 31 (79.5) | 52 (46.8) | 9 (26.5) | 15 (51.7) |
| First-degree relatives with diabetesg, n % | 194 (25.6) | 40 (13.0) | 154 (35.6) | 8 (19.5) | 88 (47.3) | 6 (15.8) | 42 (38.5) | 6 (20.0) | 4 (13.4) |
| Gestational week at inclusion | 15 (3.4) | 14 (2.3) | 16 (3.9) | 15 (3.1) | 16 (3.9) | 16 (3.9) | 15 (3.3) | 18 (5.5) | 16 (3.8) |
| Body height (cm) | 163.7 (6.7) | 167.4 (5.6) | 161.1 (6.1) | 166.2 (5.8) | 160.0 (5.6) | 157.2 (6.1) | 161.2 (5.5) | 164.1 (5.8) | 161.6 (6.3) |
| BMI prepregnantg (kg/m2) | 24.6 (4.8) | 24.6 (4.8) | 24.6 (4.9) | 23.8 (4.4) | 23.7 (4.1) | 22.3 (3.4) | 25.9 (5.1) | 26.8 (6.5) | 26.3 (5.8) |
Norway, 93.6%; Sweden and Denmark, 3.2% and other Western Europe (includes North America n=3), 3.2%.
Poland, 16.7%; Russia, 14.3%; Kosovo, 14.3% and other Eastern Europe, 54.8%.
Pakistan, 62.2%; Sri Lanka, 30.9% and other South Asia, 6.9%.
Vietnam, 43.6%; Philippines, 28.2%; Thailand, 10.3% and other Eastern Asia, 17.9%.
Iraq, 30.4%; Turkey, 22.3%; Morocco, 19.6%; Afghanistan, 10.7% and other Middle East, 16.8%.
Sub-Saharan Africa, 53.3%; South/Central America, 26.7% and Nigeria, 33.3%.
Incomplete data on the variables because of missing values for 6–19 women.
Prevalence of GDM
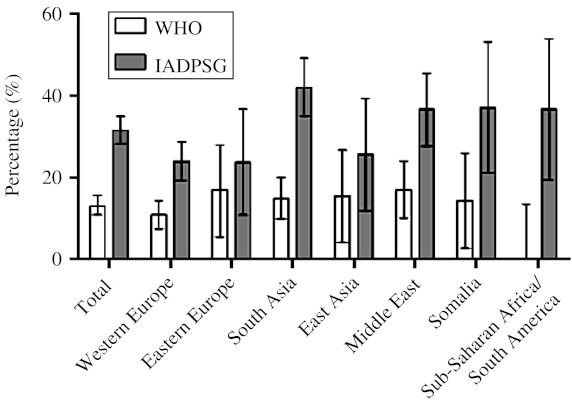
The number of women diagnosed as having GDM with the WHO criteria and the modified IADPSG criteria was 99 and 239 respectively, giving crude prevalence rates of 13.0 and 31.5% respectively. The prevalence rates in Western European (10.9%) and ethnic minority women (14.6%) with the WHO criteria were not significantly different (P=0.14), but with the modified IADPSG criteria, the difference was highly significant (24 and 36.8% respectively, P<0.001; Fig. 1, Table 2). The GDM prevalence increased 2.8 times in South Asian and 2.2 times in Western European and Middle Eastern women (2.4 overall), after applying the modified IADPSG criteria, compared with the WHO criteria.
Figure 1.
Crude GDM prevalence, with 95% CI, based on the WHO and the modified IADPSG criteria for the total cohort and the main ethnic minority groups.
Table 2.
Characteristics for the gestational diabetes mellitus (GDM) groups identified by the WHO and the modified International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria and stratified into groups based on different diagnostic cutoff values for GDM. Data are mean (s.d.) unless otherwise stated.
| GDM | GDM | |||||||
|---|---|---|---|---|---|---|---|---|
| Total WHOa | Total IADPSGb | No GDM by any criteriac | WHO and IADPSGd | WHO onlye | IADPSG onlyf | P1 | P2 | |
| n (%) | 99 (13.0) | 239 (31.5) | 492 (64.8) | 71 (9.4) | 28 (3.7) | 168 (22.1) | ||
| Years of maternal age | 31.8 (4.8) | 30.2 (5.1) | 29.6 (4.7) | 32.2 (4.8) | 30.5 (4.6) | 29.4 (5.0) | <0.001 | 0.208 |
| Nulliparous, n (%) | 49 (59.5) | 104 (43.5) | 225 (45.7) | 31 (43.7) | 18 (64.3) | 73 (43.5) | 0.113 | 0.041 |
| Educational level <10 yearsg, n (%) | 24 (24.5) | 51 (21.4) | 68 (14.0) | 20 (28.6) | 4 (14.3) | 31 (18.5) | 0.161 | 0.594 |
| Ethnic minorities, n % | 65 (65.7) | 164 (68.6) | 267 (54.3) | 50 (70.4) | 15 (53.6) | 114 (67.9) | 0.257 | 0.140 |
| First-degree relatives with diabetesg, n (%) | 39 (41.1) | 75 (32.2) | 109 (22.7) | 29 (42.6) | 10 (37.0) | 46 (27.0) | 0.081 | 0.332 |
| Body height (cm) | 161.0 (6.6) | 163.1 (6.6) | 164.1 (6.7) | 160.8 (6.6) | 161.5 (6.6) | 164.0 (6.5) | 0.001 | 0.073 |
| BMI prepregnantg (kg/m2) | 25.7 (5.0) | 26.0 (5.6) | 23.9 (4.3) | 26.1 (5.3) | 24.7 (4.1) | 26.0 (5.8) | 0.514 | 0.171 |
| FPG <20 weeks gestationg (mmol/l) | 4.6 (0.5) | 4.7 (0.4) | 4.3 (0.4) | 4.7 (0.5) | 4.4 (0.3) | 4.6 (0.4) | 0.004 | <0.001 |
| FPG 28 weeks gestation (mmol/l) | 5.2 (0.8) | 5.5 (0.5) | 4.5 (0.3) | 5.4 (0.8) | 4.6 (0.3) | 5.5 (0.4) | <0.001 | <0.001 |
| 2-h PG 28 weeks gestationg (mmol/l) | 8.8 (0.9) | 7.1 (1.6) | 5.7 (1.0) | 9.1 (0.9) | 8.0 (0.2) | 6.3 (1.0) | <0.001 | <0.001 |
| HbA1c <20 weeks gestationg (%) | 5.2 (0.3) | 5.3 (0.3) | 5.1 (0.3) | 5.3 (0.3) | 5.1 (0.3) | 5.3 (0.3) | 0.023 | 0.023 |
| HbA1c 28 weeks gestationg (%) | 5.3 (0.4) | 5.3 (0.3) | 5.1 (0.3) | 5.4 (0.4) | 5.1 (0.3) | 5.3 (0.3) | <0.001 | 0.007 |
| Gestational week at OGTT | 28 (1.5) | 28 (1.2) | 28 (1.3) | 28 (1.5) | 28 (1.3) | 28 (1.1) | 0.029 | 0.079 |
P values for the differences between the GDM WHO and modified IADPSG, the WHO only and the modified IADPSG only (P1), and between the WHO only and the modified IADPSG only (P2).
Total GDM by the WHO criteria (FPG ≥7.0 mmol/l and/or 2-h PG ≥7.8 mmol/l).
Total GDM by the modified IADPSG criteria (FPG ≥5.1 mmol/l and/or 2-h PG ≥8.5 mmol/l).
The group without GDM.
GDM by both the WHO and the modified IADPSG criteria (FPG ≥5.1 mmol/l and 2-h PG ≥7.8).
GDM by the WHO criteria only (FPG <5.1 mmol/l and 2-h PG 7.8–8.4 mmol/l).
GDM by the modified IADPSG criteria only (FPG 5.1–6.9 mmol/l and 2-h PG <7.8 mmol/l).
Incomplete data on the variables because of missing values for 1–19 women.
With the modified IADPSG criteria, 24.2% were diagnosed exclusively by FPG ≥5.1 mmol/l, 3.3% exclusively by 2-h PG ≥8.5 mmol/l, and 4.0% by both FPG and 2-h PG above the cutoff values. FPG ≥5.1 mmol/l was found in 21.4% of Western European, 37.2% of South Asian and 35.7% of Middle Eastern women.
Comparison of the GDM groups identified with both the WHO and the modified IADPSG criteria (9.4%), with the WHO criteria only (3.7%), and with the modified IADPSG criteria only (22.1%; Table 2) revealed significant differences between the groups for age, body height, glucose and HbA1c (P<0.001–0.02). The group identified with the modified IADPSG criteria only had higher BMI at 28 weeks of gestation (P=0.05), higher HbA1c at both visits, tended to be taller (P=0.07) and fewer were nulliparous (P=0.04) compared with the group identified with the WHO criteria only.
Effect of ethnicity
For GDM with the WHO criteria, ethnic minority origin was an independent predictor (overall, P=0.026; South Asians, OR 2.24 (95% CI 1.26–3.97); Middle Eastern women, 2.13 (1.12–4.08)) when adjusted for age, prepregnant BMI and parity (model A; Table 3). However, when education and body height (a proxy for early life socioeconomic status) were added (model B), the increased OR for ethnic minority women was eliminated. Similar findings were made in the final model (model C) with additional adjustments for a family history of diabetes. In this model, only age (OR (95% CI): 1.12 (1.06–1.18)), parity (OR (95% CI): 2.33 (1.39–3.90)), body height (cm; OR (95% CI): 0.92 (0.88–0.96)) and family history (1.89 (1.32–2.71)) were independently associated with GDM, while education and prepregnant BMI were borderline significant predictors.
Table 3.
Multiple logistic regression models for the (GDM) groups identified with the WHO and the modified (IADPSG) criteria, showing the impact of ethnic origin on GDM after adjusting for covariates.
| Multiple model A | Multiple model B | Multiple model C | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| WHO (n=99) | ||||||
| Years of maternal age | 1.13 (1.08–1.19) | <0.001 | 1.13 (1.08–1.19) | <0.001 | 1.12 (1.06–1.18) | <0.001 |
| Body height | 0.93 (0.89–0.97) | <0.001 | 0.92 (0.88–0.96) | <0.001 | ||
| BMI prepregnant | 1.05 (1.00–1.09) | 0.044 | 1.04 (1.00–1.09) | 0.054 | 1.04 (0.99–1.09) | 0.111 |
| Parous ≥1 | 2.08 (1.29–3.36) | 0.003 | 2.29 (1.39–3.76) | 0.001 | 2.33 (1.39–3.90) | 0.001 |
| <10 years education | 1.91 (1.05–3.47) | 0.034 | 1.88 (1.01–3.49) | 0.047 | ||
| First-degree relatives with diabetes | 1.89 (1.32–2.71) | 0.001 | ||||
| Ethnic origin ref. Western Europe | 0.026 | 0.772 | 0.771 | |||
| South Asia | 2.24 (1.26–3.97) | 0.006 | 1.18 (0.62–2.27) | 0.614 | 0.76 (0.38–1.55) | 0.456 |
| Middle East | 2.13 (1.12–4.08) | 0.022 | 1.03 (0.49–2.16) | 0.944 | 0.77 (0.35–1.66) | 0.503 |
| Other minoritiesa | 1.45 (0.77–2.73) | 0.244 | 0.82 (0.41–1.65) | 0.580 | 0.69 (0.33–1.43) | 0.314 |
| IADPSG (n=239) | ||||||
| Years of maternal age | 1.03 (1.00–1.07) | 0.068 | 1.04 (1.00–1.08) | 0.054 | 1.03 (0.99–1.07) | 0.116 |
| Body height | 1.01 (0.98–1.03) | 0.641 | 1.00 (0.98–1.03) | 0.746 | ||
| BMI prepregnant | 1.10 (1.06–1.13) | <0.001 | 1.09 (1.06–1.13) | <0.001 | 1.09 (1.05–1.13) | <0.001 |
| Parous ≥1 | 1.17 (0.82–1.65) | 0.390 | 1.21 (0.85–1.73) | 0.281 | 1.15 (0.81–1.64) | 0.438 |
| <10 years education | 1.50 (0.96–2.36) | 0.078 | 1.55 (0.98–2.45) | 0.062 | ||
| First-degree relatives with diabetes | 1.10 (0.83–1.47) | 0.510 | ||||
| Ethnic origin ref. Western Europe | <0.001 | <0.001 | 0.002 | |||
| South Asia | 2.94 (1.94–4.47) | <0.001 | 2.93 (1.84–4.67) | <0.001 | 2.54 (1.56–4.13) | <0.001 |
| Middle East | 1.79 (1.10–2.93) | 0.019 | 1.58 (0.92–2.73) | 0.100 | 1.44 (0.83–2.51) | 0.196 |
| Other minoritiesa | 1.44 (0.91–2.28) | 0.120 | 1.35 (0.82–2.21) | 0.234 | 1.25 (0.76–2.06) | 0.381 |
Includes Eastern Europe, East Asia, Somalia and sub-Saharan Africa/South America.
A different pattern was found when assessing predictors for GDM with the modified IADPSG criteria. All models revealed a significantly increased OR for GDM for ethnic minority women. Adjustment for education, body height and family history of diabetes had little impact (models B and C). In model C, only prepregnant BMI (per unit change; OR (95% CI): 1.09 (1.05–1.13)) and ethnic minority origin (overall, P=0.002; South Asians, 2.54 (1.56–4.13)) were independently associated with GDM (education borderline significant).
Discussion
To our knowledge, this is the first population-based study comparing the prevalence of GDM with the WHO and the proposed new IADPSG criteria (slightly modified) in a multi-ethnic population, based on universal screening. The main findings were the high prevalences of GDM identified with the modified IADPSG criteria in all ethnic groups studied and the considerable differences between the populations identified with the two criteria. With the WHO criteria, the excess risk for GDM related to ethnic minority origin was not apparent after adjustments for other known risk factors. However, with the modified IADPSG criteria, ethnic minority origin remained a significant predictor of GDM after adjustments for the same factors.
The prevalence of GDM with the WHO criteria in this study, overall and in Western Europeans, is higher than that reported in some (4), but not all (16, 17) studies from comparable populations. Observed differences may be attributed to secular trends for obesity, maternal age, ethnic origin of the study population and a preference for selective screening with lower sensitivity in most studies (18). In addition, some studies have used slightly modified WHO criteria (4).
In this study, there was a 2.2-to 2.8-fold increase in the GDM prevalence for the main ethnic groups when applying the modified IADPSG criteria and an equal increase has been reported by others (19, 20). The prevalence of GDM with the modified IADPSG criteria was high, but similar high rates have been reported in other high-risk ethnic groups (21). The prevalence in this study was substantially higher than that in the HAPO study (17.8%) (5); however, high-risk ethnic groups may be underrepresented in the HAPO cohort. In addition, the HAPO study was not population-based, had a lower attendance rate (53.6 vs 74%), had stricter inclusion criteria and participants had to consent to blinding for glucose values in a range that might represent possible harm (6). Only women with FPG <5.8 mmol/l and 2-h PG <11.1 mmol/l remained blinded for their glucose values throughout the study, which may have led to a selection of low-risk women in study sites with high screening rates (3). The reported GDM prevalence rates from the HAPO study were based on the blinded proportion of the cohort, and the rates differed substantially between the study sites (8.7–23.7%) (22).
The impact of socioeconomic factors for the ethnic differences in GDM prevalence rates has received scant attention (4, 11). Body height has been negatively associated with GDM (23) and may serve as a proxy for intrauterine and childhood growth in the mothers and socioeconomic determinants related to stunting (10, 23). The association between height and GDM could reflect a deleterious impact of fetal or early life deprivation on glucose metabolism in pregnancy, captured with the WHO criteria.
For the modified IADPSG criteria (predominantly driven by a lower FPG cutoff value), prepregnant BMI had a substantial effect on the prevalence of GDM. This could be related to the effect of hepatic insulin resistance on FPG (24). Furthermore, BMI may underestimate the adverse effect of obesity in Asians (25). The differential impact of early and later life exposures for developing GDM identified with the WHO and the IADPSG criteria is profound, and to our knowledge has not been addressed by others.
Strengths of our study include the population-based study cohort, the universal screening, high attendance rate, a multi-ethnic population and minimal loss to follow-up (4%). Compared with other clinical studies, we consider a response rate of 74% as rather good (HAPO study included only 53.5%), especially in a time-consuming study in which women who do not speak the majority language are included. Our efforts to adapt the study methods to the needs of women often excluded in research protocols and to reduce barriers for inclusion of illiterate or recently immigrated women therefore seem to have worked. Ethnic minority women attending the Child Health Clinics appear to be fairly representative for healthy women of childbearing age from these groups (12). Forty percent of the total population living in this residential area has an ethnic minority background. However, the ethnic minority proportion is higher among those in reproductive age as the immigrant population in Norway is fairly young, with a few people in the old age group. The proportion of ethnic minorities in this study was higher than for Norway as a whole, but we chose this residential area to improve our knowledge regarding the health of ethnic minority women and explore the possible differences compared with our reference population. As our sample of Western European women seems characteristic for urban Norwegian women (12), the results should be fairly representative for the largest ethnic groups included in the study. Migration is a global phenomenon and similar ethnic groups live in many countries, which make the results relevant outside the Norwegian context.
Limitations of the study include the relatively small numbers in some ethnic minority groups and the absence of 1-h PG measurements, as the study was conducted before the IADPSG criteria were published. With the availability of 1-h PG values, the true IADPSG prevalence rates may have been even higher.
To facilitate participation for universal OGTT screening, the OGTT was performed in the primary care setting usually responsible for antenatal care for normal pregnancies in Norway (13). We used venous EDTA samples analyzed within 5 min by HemoCue, a method authorized by the FDA (Department of Health and Human Services, USA, 2002) for diagnosing diabetes and used in epidemiological research (26). This made it possible with an on-site diagnosis of GDM for clinical care. The comparison with the measurements obtained at the hospital laboratory revealed that preanalytical glucose consumption was not completely eliminated despite short transport time. Unfortunately, we were not able to use glycolytic inhibitors that may reduce preanalytical glucose consumption, as the automated central laboratory procedures did not accept this. However, even the use of standard inhibitors like sodium fluoride, available in commercially marketed sample tubes, does not eliminate preanalytical glucose consumption (27, 28).
Implications for public health
The IADPSG represents a welcome initiative to achieve internationally accepted criteria for the diagnosis of GDM. We agree to the principle of an initial screening test early in pregnancy to identify women with undiagnosed pregestational diabetes, followed by universal screening by a standard OGTT later in pregnancy (5, 29). However, lowering of the diagnostic threshold for FPG as suggested will have a major effect on the numbers diagnosed and increase the workload on the health care system (8). It also raises concern about the consequences of labeling a large number of women, based on a test with poor reproducibility (18, 30, 31).
Positive short-time effects from intervention studies on GDM are observed (32, 33), but only a marginal effect on the prevalence of macrosomia can be expected (30), and follow-up data of offspring are so far not convincing (34, 35). This may indicate that the window of opportunity to prevent long-term predisposition to obesity is earlier in fetal life than the currently recommended time of screening for GDM (36, 37). Population-based strategies to prevent becoming overweight and improve the living conditions in early life seem to be increasingly important (37, 38).
Acknowledgements
The authors thank Akershus University Hospital and Oslo University Hospital, Ullevål, the city of Oslo (Stovner, Grorud and Bjerke districts) and its administrative leaders. Translation was made by the City Services Department, The Interpreting and Translating Service in Oslo. They also thank H S Hatlehol and the other staff at the Child Health Clinics for collecting the data; H Skaaland, Oslo University Hospital, Aker, for organizing the database; and E T Aasheim, Oslo University Hospital, Aker, for commenting on the final manuscript. Last but not the least, the authors thank the participating women.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
The Research Council of Norway has funded PhD fellowships for K Mørkrid and L Sletner, and the data collection was also supported by the South-Eastern Norway Regional Health Authority, The Norwegian Directorate of Health and collaborative partners in the city of Oslo, Stovner, Grorud and Bjerke administrative districts.
Author contribution statement
A K Jenum and K Mørkrid contributed equally to the work. A K Jenum provided overall leadership and guidance on the development of the paper, in close collaboration with K Mørkrid and K I Birkeland. A K Jenum prepared the first and the revised drafts of the paper, with major contributions from K Mørkrid and K I Birkeland. K Mørkrid did all the analyses, with major contribution and guidance about the statistical methods by M H Vårdal and I Holme. L Sletner prepared the figures. All authors contributed to the study by being part of the Steering Committee or as an international collaborative partner (C S Yajnik), critical analyses, and discussion of the data and to the revisions of the paper. All authors have read and approved the final version. The authors alone are responsible for the views expressed in the paper.
References
- O'Sullivan JB, Gellis SS, Dandrow RV, Tenney BO. The potential diabetic and her treatment in pregnancy. Obstetrics and Gynecology. 1966;27:683–689. [PubMed] [Google Scholar]
- Leary J, Pettitt DJ, Jovanovic L. Gestational diabetes guidelines in a HAPO world. Best Practice and Research. Clinical Endocrinology and Metabolism. 2010;24:673–685. doi: 10.1016/j.beem.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Reece EA, Leguizamon G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet. 2009;373:1789–1797. doi: 10.1016/S0140-6736(09)60515-8. [DOI] [PubMed] [Google Scholar]
- Galtier F. Definition, epidemiology, risk factors. Diabetes and Metabolism. 2010;36:628–651. doi: 10.1016/j.diabet.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva A, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc10-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Diabetic Medicine. 1999;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7%3C539::AID-DIA668%3E3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Holt RI, Coleman MA, McCance DR. The implications of the new International Association of Diabetes and Pregnancy Study Groups (IADPSG) diagnostic criteria for gestational diabetes. Diabetic Medicine. 2011;28:382–385. doi: 10.1111/j.1464-5491.2011.03236.x. [DOI] [PubMed] [Google Scholar]
- Davey Smith G. Learning to live with complexity: ethnicity, socioeconomic position, and health in Britain and the United States. American Journal of Public Health. 2000;90:1694–1698. doi: 10.2105/AJPH.90.11.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenum AK, Holme I, Graff-Iversen S, Birkeland K. Ethnicity and sex are strong determinants of diabetes in an urban Western society: implications for prevention. Diabetologia. 2005;48:435–439. doi: 10.1007/s00125-005-1668-8. [DOI] [PubMed] [Google Scholar]
- Petry CJ. Gestational diabetes: risk factors and recent advances in its genetics and treatment. British Journal of Nutrition. 2010;104:775–787. doi: 10.1017/S0007114510001741. [DOI] [PubMed] [Google Scholar]
- Jenum AK, Sletner L, Voldner N, Vangen S, Morkrid K, Andersen LF, Nakstad B, Skrivarhaug T, Rognerud-Jensen OH, Roald B, Birkeland KI. The STORK Groruddalen research programme: a population-based cohort study of gestational diabetes, physical activity, and obesity in pregnancy in a multiethnic population. Rationale, methods, study population, and participation rates. Scandinavian Journal of Public Health. 2010;38:60–70. doi: 10.1177/1403494810378921. [DOI] [PubMed] [Google Scholar]
- Holan S, Mathiesen M & Petersen K. A National Clinical Guideline for Antenatal Care. Short version. Oslo, Norway: Directorate for Health and Social Affairs, 2005.
- Bhopal RS. Ethnicity, Race, and Health in Multicultural Societies: Foundations for Better Epidemiology, Public Health and Health Care, Oxford: Oxford University Press, 2007.
- Directorate for Health and Social Affairs. National Guidelines. Diabetes. Prevention, Diagnosis and Treatment. Oslo, Norway: Directorate for Health and Social Affairs 2009.
- Lamberg S, Raitanen J, Rissanen P, Luoto R. Prevalence and regional differences of gestational diabetes mellitus and oral glucose tolerance tests in Finland. European Journal of Public Health. 2012 doi: 10.1093/eurpub/ckq193. In press. [DOI] [PubMed] [Google Scholar]
- McCance DR. Gestational diabetes mellitus. In The Evidence Base for Diabetes Care, 1st edn. pp 245–284. Eds R Williams, W Herman, AL Kinmonth & N Wareham. Hoboken, NJ: J Wiley & Sons, 2002.
- Flack JR, Ross GP, Ho S, McElduff A. Recommended changes to diagnostic criteria for gestational diabetes: impact on workload. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2010;50:439–443. doi: 10.1111/j.1479-828X.2010.01218.x. [DOI] [PubMed] [Google Scholar]
- Cundy T. Proposed new diagnostic criteria for gestational diabetes – a pause for thought? Diabetic Medicine. 2012 doi: 10.1111/j.1464-5491.2011.03407.x. In press. [DOI] [PubMed] [Google Scholar]
- Morikawa M, Yamada T, Yamada T, Akaishi R, Nishida R, Cho K, Minakami H. Change in the number of patients after the adoption of IADPSG criteria for hyperglycemia during pregnancy in Japanese women. Diabetes Research and Clinical Practice. 2010;90:339–342. doi: 10.1016/j.diabres.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Agarwal MM, Dhatt GS, Shah SM. Gestational diabetes mellitus: simplifying the international association of diabetes and pregnancy diagnostic algorithm using fasting plasma glucose. Diabetes Care. 2010;33:2018–2020. doi: 10.2337/dc10-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden DR, Metzker ML, Lowe LP, Dyer AR, Coustan DR, Hod M, Oats JJ, Persson B, Trimble ER. Hyperglycemina and Adverse Pregnancy Outcome (HAPO) Study: frequency of gestational diabetes mellitus (GDM) at collaborating centers based on IADPSG consensus panel recommende criteria. Diabetologia. 2010;53:S9. doi: 10.2337/dc11-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogonowski J, Miazgowski T. Are short women at risk for gestational diabetes mellitus? European Journal of Endocrinology. 2010;162:491–497. doi: 10.1530/EJE-09-0992. [DOI] [PubMed] [Google Scholar]
- Vaag A, Alford F, Henriksen FL, Christopher M, Beck-Nielsen H. Multiple defects of both hepatic and peripheral intracellular glucose processing contribute to the hyperglycaemia of NIDDM. Diabetologia. 1995;38:326–336. doi: 10.1007/BF00400638. [DOI] [PubMed] [Google Scholar]
- WHO expert consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- Sandbaek A, Griffin SJ, Rutten G, Davies M, Stolk R, Khunti K, Borch-Johnsen K, Wareham NJ, Lauritzen T. Stepwise screening for diabetes identifies people with high but modifiable coronary heart disease risk. The ADDITION study. Diabetologia. 2008;51:1127–1134. doi: 10.1007/s00125-008-1013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns DE, Knowler WC. Stabilization of glucose in blood samples: why it matters. Clinical Chemistry. 2009;55:850–852. doi: 10.1373/clinchem.2009.126037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AY, Swaminathan R, Cockram CS. Effectiveness of sodium fluoride as a preservative of glucose in blood. Clinical Chemistry. 1989;35:315–317. [PubMed] [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–S69. doi: 10.2337/dc11-S062). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EA. Diagnosing gestational diabetes. Diabetologia. 2011;54:480–486. doi: 10.1007/s00125-010-2005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses RG. New consensus criteria for GDM: problem solved or a pandora's box? Diabetes Care. 2010;33:690–691. doi: 10.2337/dc09-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Thorp JM, Jr, Sciscione A, Catalano P, Harper M, Saade G, Lain KY, Sorokin Y, Peaceman AM, Tolosa JE, Anderson GB. A multicenter, randomized trial of treatment for mild gestational diabetes. New England Journal of Medicine. 2009;361:1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman MW, Oakey H, Baghurst PA, Volkmer RE, Robinson JS, Crowther CA. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care. 2010;33:964–968. doi: 10.2337/dc09-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt DJ, McKenna S, McLaughlin C, Patterson CC, Hadden DR, McCance DR. Maternal glucose at 28 weeks of gestation is not associated with obesity in 2-year-old offspring: the Belfast Hyperglycemia and Adverse Pregnancy Outcome (HAPO) family study. Diabetes Care. 2010;33:1219–1223. doi: 10.2337/dc09-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajnik CS. Fetal programming of diabetes: still so much to learn! Diabetes Care. 2010;33:1146–1148. doi: 10.2337/dc10-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378:169–181. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34:1249–1257. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]