Abstract
The wound response of plants is characterized by rapid changes in gene expression, biochemistry and physiology, and is important both in its own right and as a model for studying events elicited by herbivory. We have recently identified links between light and the wound response in Arabidopsis leaves. This includes an influence of the external light environment on the molecular and biochemical response to wounding, and the observation that endogenous bioluminescence (light emission) is a consequence of tissue damage. Here, we show that this link extends to the production of reactive oxygen species. We show that wounding causes rapid, light-dependent production of superoxide and hydrogen peroxide in chloroplasts via disruption of photosynthesis, and that wound-induced bioluminescence is a consequence of the generation of singlet oxygen.
Key words: wounding, reactive oxygen, light, photosynthesis, chloroplast, bioluminescence
Light is of fundamental importance for plant biology, and is likely to influence a wide range of plant developmental processes and responses to the environment. Light has already been recognized as an important factor during plant pathogen-interactions,1 and we recently identified aspects of the wound response that were differentially regulated dependent on the external light environment.2 Prior to that, we and others had identified low level bioluminescence as an early response to wounding and herbivory.3–5 Hence, light can act as both an input and an output of the wound response in plant leaves. Spontaneous bioluminescence has been reported to occur in many groups of organisms, and is often linked with the generation of reactive oxygen species (ROS).6,7 ROS are common components of many stress responses,8 and it is widely accepted that ROS are produced in wounded plant leaves. However, the nature and origin of wound-induced ROS are relatively poorly defined. In tomato, hydrogen peroxide is produced in response to jasmonate signaling and is involved in the regulation of the later stages of the transcriptional response to wounding, including in systemic leaves.9–11 H2O2 is also produced in response to herbivory, where it has been reported to accumulate extracellularly.12,13 We were therefore interested to characterize the wound-induced production of ROS in plants in more detail.
Work by Chen et al. implicates singlet oxygen, 1O2, as a cause of luminescence produced by wounded soybean cotyledons. We performed experiments to determine whether wound-induced luminescence in Arabidopsis leaves might also be a consequence of 1O2 formation. Firstly, we used Rose Bengal as a photosensitizer to generate 1O2 in detached Arabidopsis leaves. Figure 1A shows that strong bioluminescence is emitted following illumination of Rose Bengal-treated leaves, suggesting that 1O2 production can indeed cause bioluminescence. We next examined luminescence in wounded leaves equilibrated in either 10 mM histidine, a scavenger of 1O2, or in deuterium oxide (D2O; heavy water), which extends the half-life of 1O2 around ten-fold. Consistent with the hypothesis that luminescence is a consequence of 1O2 production, the inclusion of histidine reduced the intensity of wound-induced luminescence relative to controls, whereas luminescence was increased in the presence of D2O (Fig. 1B and C). 1O2 accumulation around wound sites in Arabidopsis was also detected by Flors et al. using a fluorescent reporter.
Figure 1.
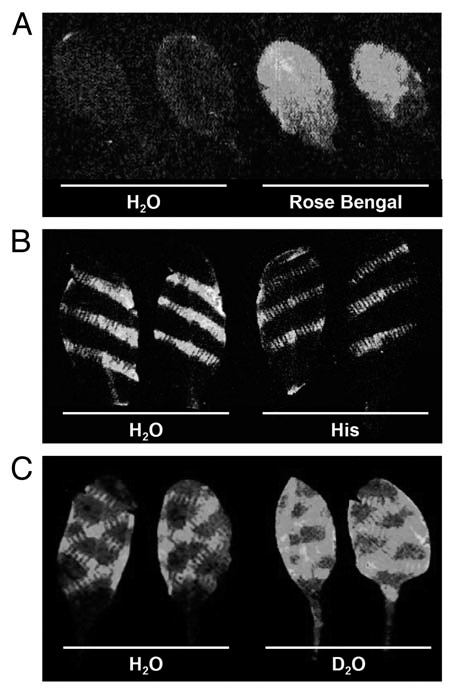
Wound-induced bioluminescence correlates with singlet oxygen production. (A) Luminescence images of Arabidopsis leaves treated with water or the photosensitizer, Rose Bengal. Leaves were vacuum-infiltrated with water or 10 µM Rose Bengal (4,5,6,7-tetrachloro-2′,4′,5′,7′-tetraiodofluorescein) and illuminated for 5 min at a PAR intensity of 750 µmolquanta m−2 sec−1. (B and C) Luminescence images of wounded Arabidopsis leaves pre-treated with either water (H2O), 10 mM L-histidine (His) or deuterium oxide (D2O). Images were taken 5 (A and C) or 10 (B) min after the respective treatments, and are 5 min exposures captured using a fibre optic-coupled CCD system as described previously (Flor-Henry, 2004).4
To further characterize the production of ROS in wounded leaves, we used histochemical staining with nitroblue tetrazolium (NBT) and 3,3′-diaminobenzidine (DAB) to detect superoxide and hydrogen peroxide respectively. Both species were found localized around sites of damage (Fig. 2A–C) and could be detected within the first few minutes following wounding. Microscopic examination of stained leaves revealed that the majority of O2•− and H2O2 were restricted to the chloroplasts of mesophyll cells (Fig. 2D–F). We recently identified a signal originating from photosynthetic electron transport (PET) that is involved in wound-induced gene expression,2 and since the most likely source of chloroplast ROS is also PET, we investigated the role of photosynthesis in wound-induced O2•− and H2O2 production. We found that NBT and DAB staining was eliminated in the dark, and that the degree of staining was proportional to the PAR intensity provided following wounding (Fig. 2A and B). Pre-treatment of leaves with the PET inhibitors DCMU (3-(3′,4′-dichlorophenyl)1,1-dimethylurea) and DBMIB(2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone), that respectively prevent the reduction and oxidation of plastoquinone (PQ) in photosystem II, indicated PQ as a likely site for the generation of O2•−, since DCMU but not DBMIB prevented wound-induced NBT staining (Fig. 2C). Together, these data suggest that chloroplast ROS are generated as a consequence of perturbation of the light reactions of photosynthesis in wounded leaves. Although O2•− and H2O2 are generated at the wound site coincident with bioluminescence, we were unable to establish any causal link between bioluminescence and either of these ROS.
Figure 2.
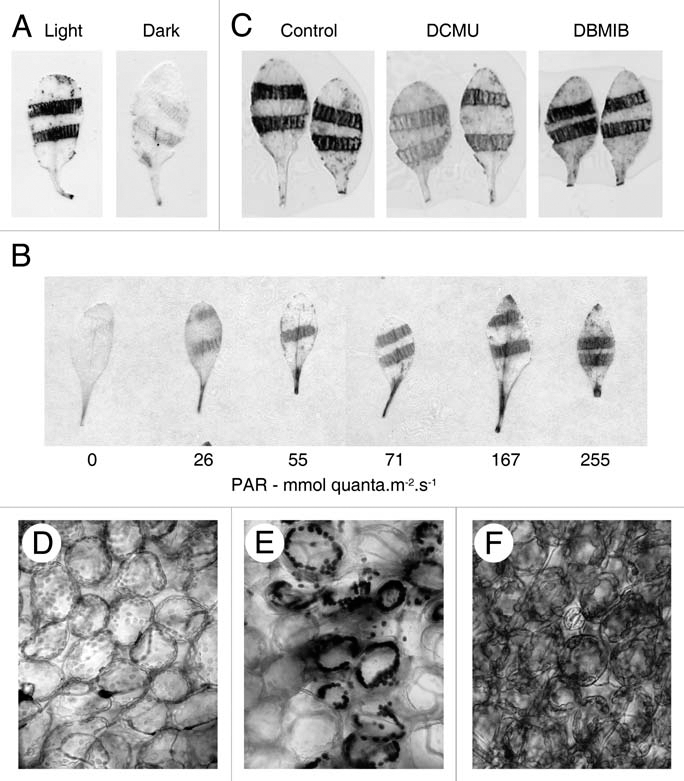
Wound-induced ROS are derived from photosynthetic electron transport. (A) Histochemical localization of superoxide using NBT under ambient light or in the dark. (B) Histochemical localization of wound-induced hydrogen peroxide using DAB at different light intensities. Photon flux densities determined at bench height are indicated below each leaf (PAR; photosynthetically active radiation). (C) Histochemical localisation of superoxide in the presence or absence (Control) of 10 µM DCMU or DBMIB. (D–F) Light micrographs of untreated Arabidopsis leaf tissue (D), and areas surrounding wound sites in leaves cleared of chlorophyll following staining with NBT (E) or DAB (F).
Taken together, our data identify rapid, localized, light-dependent wound-induced generation of O2•− and H2O2, alongside the generation of singlet oxygen from an unknown source. Furthermore, singlet oxygen is necessary and sufficient to produce luminescence at sites of wounding in plant leaves. Interestingly, Arabidopsis leaves also emit bioluminescence during an R-gene mediated pathogen resistance response, via a mechanism that is independent of O2•− and H2O2.15 One might therefore speculate that pathogen-induced bioluminescence is also a consequence of 1O2 production. Singlet oxygen is most commonly generated in chloroplasts following perturbation of PET,16 but there is no evidence for a light requirement for wound-induced bioluminescence and enzymatic lipid peroxidation is perhaps a more likely source of 1O2 in wounded leaves.4,14 However, our data indicate that wound-induced O2•− and H2O2 do originate from PET. This finding contrasts the view that wound-induced ROS are primarily produced extracellularly, by NADPH oxidase enzymes.4,17 However, this view is largely based on the use of the NADPH oxidase inhibitor, diphenyl iodonium, which also inhibits photosynthesis.18 There is clear evidence that NADPH oxidases are important in the late ROS burst produced following wounding,10,11 but whilst we cannot exclude the possibility that they also contribute to the early response, the data presented here suggest that the majority of early, localized ROS originate from electron transport in the chloroplasts. How wounding might disrupt electron transport during photosynthesis, and what roles, if any, the resultant ROS may play in wound response signaling, remains to be determined.
Acknowledgments
This work was funded by a Royal Society University Research Fellowship to M.R.R., a Lancaster University 40th Anniversary Studentship to K.H.M., and a Lancaster University Research Committee grant to M.R.R.
Abbreviations
- DAB
3,3′-diaminobenzidine
- DBMIB
2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone
- DCMU
3-(3′,4′-dichlorophenyl)1,1-dimethylurea
- NBT
nitroblue tetrazolium
- PET
photosynthetic electron transport
- PQ
plastoquinone
- ROS
reactive oxygen species
References
- 1.Roden LC, Ingle RA. Lights, Rhythms, Infection: The role of light and the circadian clock in determining the outcome of plant-pathogen interactions. Plant Cell. 2009;21:2546–2552. doi: 10.1105/tpc.109.069922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morker KH, Roberts MR. Light exerts multiple levels of influence on the Arabidopsis wound response. Plant Cell Environ. 2011;34:717–728. doi: 10.1111/j.1365-3040.2011.02276.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen WL, Xing D, Tan SC, Tang YH, He YH. Imaging of ultra-weak bio-chemiluminescence and singlet oxygen generation in germinating soybean in response to wounding. Luminescence. 2003;18:37–41. doi: 10.1002/bio.703. [DOI] [PubMed] [Google Scholar]
- 4.Flor-Henry M, McCabe TC, deBruelles GL, Roberts MR. Use of a highly sensitive two-dimensional luminescence imaging system to monitor endogenous bioluminescence in plant leaves. BMC Plant Biol. 2004;4:19. doi: 10.1186/1471-2229-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshinaga N, Kato K, Kageyama C, Fujisaki K, Nishida R, Mori N. Ultraweak photon emission from herbivory-injured maize plants. Naturwissenschaften. 2006;93:38–41. doi: 10.1007/s00114-005-0059-9. [DOI] [PubMed] [Google Scholar]
- 6.Devaraj B, Usa M, Inaba H. Biophotons: Ultraweak light emission from living systems. Curr Opin Solid State & Materials Sci. 1997;;2:188–193. [Google Scholar]
- 7.Havaux M. Spontaneous and thermoinduced photon emission: new methods to detect and quantify oxidative stress in plants. Trends Plant Sci. 2003;8:409–413. doi: 10.1016/S1360-1385(03)00185-7. [DOI] [PubMed] [Google Scholar]
- 8.Apel K, Hirt H. Reactive oxygen species: Metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 9.Orozco-Cardenas M, Ryan CA. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA. 1999;96:6553–6557. doi: 10.1073/pnas.96.11.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orozco-Cardenas ML, Narvaez-Vasquez J, Ryan CA. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin and methyl jasmonate. Plant Cell. 2001;13:179–191. [PMC free article] [PubMed] [Google Scholar]
- 11.Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, et al. Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell. 2004;16:616–628. doi: 10.1105/tpc.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leitner M, Boland W, Mithofer A. Direct and indirect defences induced by piercing-sucking and chewing herbivores in Medicago truncatula. New Phytol. 2005;167:597–606. doi: 10.1111/j.1469-8137.2005.01426.x. [DOI] [PubMed] [Google Scholar]
- 13.Maffei ME, Mithofer A, Arimura GI, Uchtenhagen H, Bossi S, Bertea CM, et al. Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol. 2006;140:1022–1035. doi: 10.1104/pp.105.071993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flors C, Fryer MJ, Waring J, Reeder B, Bechtold U, Mullineaux PM, et al. Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, Singlet Oxygen Sensor Green®. J Exp Bot. 2006;57:1725–1734. doi: 10.1093/jxb/erj181. [DOI] [PubMed] [Google Scholar]
- 15.Bennett M, Mehta M, Grant M. Biophoton imaging: A nondestructive method for assaying R gene responses. Mol Plant-Microbe Int. 2005;18:95–102. doi: 10.1094/MPMI-18-0095. [DOI] [PubMed] [Google Scholar]
- 16.Triantaphylidès C, Havaux M. Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci. 2009;14:219–228. doi: 10.1016/j.tplants.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe T, Sakai S. Effects of active oxygen species and methyl jasmonate on expression of the gene for a wound-inducible 1-aminocyclopropane-1-carboxylate synthase in winter squash (Cucurbita maxima) Planta. 1998;206:570–576. [Google Scholar]
- 18.Chang CCC, Ball L, Fryer MJ, Baker NR, Karpinski S, Mullineaux PM. Induction of ASCORBATE PEROXIDASE 2 expression in wounded Arabidopsis leaves does not involve known wound-signaling pathways but is associated with changes in photosynthesis. Plant J. 2004;38:499–511. doi: 10.1111/j.1365-313X.2004.02066.x. [DOI] [PubMed] [Google Scholar]


