Abstract
Compared to the past 10 years, a flurry of publications, reviews and experimental papers on ABP1 have appeared in the last couple of years. Certainly, the reason is that new methods and conceptual approaches appeared to tackle the questions posed by this enigmatic auxin-binding protein. Part of the enigma is the obvious central importance of ABP1, documented by the embryo-lethal property of the homozygous T-DNA insertion into this gene.1 At the same time, this very property hindered progress in studying ABP1. Another delaying influence on ABP1 research was the fact that regulation of early auxin genes was fully explained by the mechanism provided by TRI1, the second auxin receptor.2–4
Key words: AUXIN-BINDING PROTEIN1 (ABP1), auxin transport, early auxin-regulated genes, PIN proteins, TIR1, two-receptor concept
So, what makes a binding protein a receptor? According to Jones and Sussman (reviewed in ref. 5) a receptor binds the agonist reversibly, stereospecifically, selectively, and in a defined stoichiometry. The KD should correspond to concentrations which are able to induce responses when applied in physiological experiments. Ideally, mutants of the prospective receptor should explain why they are critical to the action of the receptor. The first action of the receptor upon agonist binding should be to undergo a conformational change and trigger a change of activity in the next protein in a signal network. The 3-dimensional structure of TIR1 binding a fragment of IAA17 and auxin explains the action of TIR1 even though an induced conformational change was not directly proven.4 Recently, for the C-terminus of ABP1, a flip-flop-type of movement upon binding auxin was predicted by modeling,6 filling a long-standing gap of knowledge, even though the mobility of the C-terminus was indicated.7 The weakness of ABP1 research is that a mechanism of action cannot be derived from its structure as it is a small dimeric glycoprotein binding to the extracytosolic side of membranes.8 For signal transduction, a “docking protein” is required traversing the membrane so that the signal can induce actions on the other, the cytosolic side.9
Recent publications provide new answers to long-standing questions on ABP1 as being a receptor.10–13 First, plants suppressing ABP1 protein by ethanol-induced expression of anti-ABP1 FAB fragments of specific antibodies were developed. These plants exhibited a number of auxin physiology-related defects so that, for the first time, viable inducible abp1 mutants were generated.10 Robert et al. extended research on earlier findings that auxin rapidly inhibits endocytosis of PIN1 and PIN2 with the consequence that efflux transport of auxin catalysed by PIN proteins is enhanced. Inhibition of auxin efflux transport by auxin was too rapid to be explained by transcription and translation of new PIN proteins but required an auxin receptor which could not be TIR1.14 Robert et al. showed explicitly that endocytosis inhibition of PIN proteins is insensitive to cycloheximide so that auxin efflux enhancement does not depend on newly synthesized PIN proteins. They showed that endocytosis inhibition by auxin is independent on TIR1-like receptors in quadruple tir1/afb mutants but dependent on intact ABP1, evidenced by using mutants for both receptors. This strongly ties ABP1 to polar auxin transport regulation. Xu et al. investigated auxin-induced small G-protein signaling in epidermal cell pattern formation as another response, too rapid to be explained by TIR1. They showed that ABP1 is the receptor for this G protein-binding response that was detectable after 1 min. Again, using the comparison of wild type to abp1 mutants lead to the conclusion that ABP1 is the receptor for this response. Similarly, tip-growing root hairs require binding of small G-proteins coupled to the receptor kinase FERONIA for auxin-induced tip growth of root hairs.15 Thus, auxin signaling uses a two-receptor system which is the best known among several others in plants.16
Our own work discovered that a heterozygous abp1/ABP1 plant is an auxin mutant in its own right.13 Heterozygous abp1/ABP1 plants are defect in a range of typical auxin responses requiring polar transport: phototropism, gravitropism, apical dominance and basipetal auxin transport in the root. Most importantly, all 12 early auxin genes that we investigated were upregulated less efficiently in the mutant than in wild type 30 min after NAA application. Moreover, ABP1 itself was found to be an early auxin-regulated gene. Early auxin gene regulation was also tested in the eir1/pin2 background, eir1 being a loss-of-function allele of the PIN2 gene. PIN2 is a major regulator of polar auxin transport in phototropism and gravitropism.17,18 Again, in eir1 almost all 12 genes of the genes tested were mis-regulated demonstrating that mis-regulation of polar auxin transport leads to changes in auxin-induced transcription. Transcription regulation is the function of TIR1 and its AFB homolog. Quite fittingly, when the mutant tir1 was discovered it was named “TRANSPORT INHIBITOR RESISTANT1” because it was screened as resistant to inhibitors of PIN-dependent auxin transport.19 Thus, we showed that mutations in ABP1 and PIN2 have similar consequences on gene regulation as a rapid response. This suggests that they are operating in the same signaling chain or network in a closely linked logical position in that network. In fact, there could be several auxin transport proteins contributing to regulation of signal strength. The general agreement is that regulation of early auxin genes is executed by TIR1 and the homologous AFB proteins by inducing proteolysis of IAA proteins, co-repressors of early auxin gene regulation. Most likely therefore, besides PIN2 other PIN proteins are mediators between ABP1 and TIR1, as indicated in the scheme in Figure 1. Noteworthy is PIN5 which is localized to the ER membranes and hypothesized to concentrate auxin first in the perinuclear ER and somehow direct auxin into the nuclear cytosol,20 where it can be sensed by TIR1. This PIN5 localization is supposed to enhance auxin concentration in the nucleus although auxin diffusion through the inner nuclear membrane was not explained20 whereas PIN1- and PIN2-directed auxin efflux would enhance auxin concentration in the cell wall,14 sensed there by ABP.11–13 TIR1, by transcriptionally regulating ABP1, would be coupled to ABP1. Regulation at the post-translational level of several PIN proteins is also described which will make the whole system even more complex.21 The coupling to receptors of other auxin transport regulating proteins, AUX1 and LAX, remains to be defined but, undoubtedly, they contribute to auxin concentration regulation.
Figure 1.
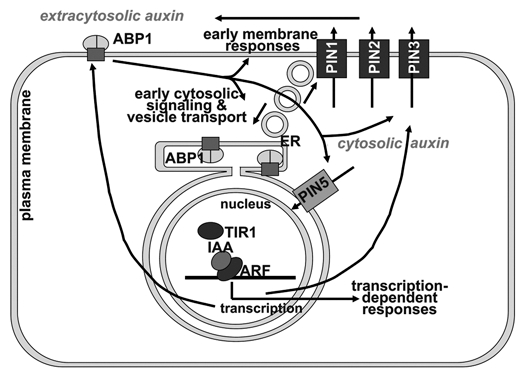
Model of auxin signal transduction. The receptor ABP1 is depicted as a dimer in complex with a transmembrane “docking protein.” ABP1 triggers a number of typical signaling pathways in the cytosol (reviewed in ref. 16). These responses include the phosphorylation status of PIN proteins (not shown in drawing) and control of endocytosis/exocytosis balance.21 Several PIN proteins, including PIN 1, PIN 2 and PIN 3 are integrated into cell polarity and auxin efflux transport to regulate extracytosolic auxin concentration and, thereby, polar auxin transport and tropisms. Indirectly, they may also regulate cytosolic auxin concentration as is assumed for PIN 5 which is localized to the ER. This localization is postulated to increase nuclear auxin concentration where it is sensed by the receptor TIR 1. Formation of the ternary complex [TIR 1 × auxin × IAA] leads to ubiquitination of IAA proteins and their hydrolysis by the proteasome. At least PIN 2 and PIN 3 are regulated by phosphoryation and rapid transcriptional responses. TIR 1 is also assumed to be the relevant receptor for ABP1 transcriptional regulation so that ABP1, PIN s and TIR 1 are a completely interlocking system of two receptors linked by auxin transport. Other systems of two or more receptors for one signal where one receptor is closely associated to regulation of proteasomal activity are known or likely in plants.16
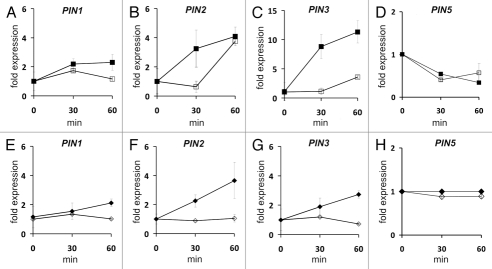
Previously, we had not tested expression of PIN genes as potential early auxin genes. PIN2 and PIN3 are rapidly upregulated by auxin whereas PIN1 is only weakly upregulated during 1 h in wild type seedlings (Fig. 2). In heterozygous abp1/ABP1 and in eir1 mutant plants these genes are not upregulated within 1 h after NAA application or less upregulated like PIN2 in abp1/ABP1 (Fig. 2B and C). This transcriptional response is aberrantly slow as compared to the respective wild types so that abp1/ABP1 and eir1 plants are both multiply damaged in auxin transport. PIN5 was downregulated by about 50% by auxin in the Ws wild type but not in the Col wild type but the respective mutants abp1/ABP1 (Ws) and eir1 (Col) did not differ significantly from wild types in PIN5 transcription in the presence of auxin (Fig. 2D and H). Our results on transcription generally agree with previous ones.20 How slight downregulation of PIN5 at the ER and upregulation of PIN2 and PIN3 at the plasma membrane is coordinating extracytosolic and cytosolic auxin concentration remains open. PIN2 and PIN3 are of major importance for directing polar auxin transport in tropisms and PIN5 may regulate nuclear auxin concentration more directly. Together however, this underscores how tightly interwoven the regulatory circuit of ABP1, PINs and TIR1/AFBs is as a network where none of the components is independent of the other (Fig. 1). Coupling and interlocking of these components happens at the transcriptional level, protein phosphorylation level, PIN protein transport in membrane vesicles by endocytosis and exocytosis, and coordinated polar auxin transport throughout the plant body.13,21
Figure 2.
Rapid regulation of PIN genes by auxin in the heterozygous abp1/ABP1 receptor mutant and the PIN2 mutant eir1. (A–D) Quantification by real time PCR of transcription in wild-type Ws (filled squares) and abp1/ABP1 seedlings (open squares). (E–H) Quantification by real time PCR of transcription in wild-type Col (filled diamonds) and eir1 seedlings (open diamonds). Methods were described in reference 16. Two biological treatments with three technical repeats each were used for calculation of average and SD. List of primers: PIN1-forw: GGA GAC TTA AGT AGG AGC TCA GCA; PIN 1-rev: CCA AAA GAG GAA ACA CGA ATG; PIN 2-forw: TAT CAA CAC TGC CTA ACA CG; PIN 2-rev: GAA GAG ATC ATT GAT GAG GC; PIN 3-forw: GAG TTA CCC GAA CCT AAT CA; PIN 3-rev: TTA CTG CGT GTC GCT ATA GT; PIN5-forw: ACC CTG CCG CTC TTC ACC A; PIN5-rev: GCC CAC AAC GCT AAG ACC G.
In addition to regulation of auxin fluxes or auxin concentration in compartments, coupling of ABP1 to TIR1 could be achieved by mechanisms of biochemical signal transduction to regulate the enzymatic activity of TIR1 by a post-translational mechanism. Presently, such a possibility remains completely vague and is not supported by data. However, both ways to regulate genes via TIR1-mediated mechanism would not mutually exclude each other and need a receptor.14 The function of ABP1 is presumed to trigger biochemical (post-translational) mechanisms of protein activity-regulation on several signaling pathways and the function of TIR1 is to regulate gene activity. This model of a two-receptor system for one signal is apparently also found to be realized for other plant-typical signals.16
Acknowledgments
This work was supported by a grant from the BMWI (DLR nr. 50 WB 0933). We thank R. Wimalasekera for critically reading the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Chen JG, Ullah H, Young JC, Sussman MR, Jones AM. ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev. 2001;15:902–911. doi: 10.1101/gad.866201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–455. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- 3.Kepinski S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- 4.Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, et al. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- 5.Jones AM, Sussman MR. A binding resolution. Plant Physiol. 2009;150:3–5. doi: 10.1104/pp.109.136606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertoša B, Kojic-Prodic B, Wade RC, Tomic S. Mechanism of auxin interaction with Auxin Binding Protein (ABP1): a molecular dynamics simulation study. Biophys J. 2008;94:27–37. doi: 10.1529/biophysj.107.109025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David K, Carnero-Diaz E, Leblanc N, Monestiez M, Grosclaude J, Perrot-Rechenmann C. Conformational dynamics underlie the activity of the auxin-binding protein, Nt-abp1. J Biol Chem. 2001;276:34517–34523. doi: 10.1074/jbc.M102783200. [DOI] [PubMed] [Google Scholar]
- 8.Woo EJ, Marshall J, Bauly J, Chen JG, Venis M, Napier RM, et al. Crystal structure of auxin-binding protein 1 in complex with auxin. EMBO J. 2002;21:2877–2885. doi: 10.1093/emboj/cdf291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klämbt D. A view about the function of auxin-binding proteins at plasma membranes. Plant Mol Biol. 1990;14:1045–1050. doi: 10.1007/BF00019401. [DOI] [PubMed] [Google Scholar]
- 10.Braun N, Wyrzykowska J, Muller P, David K, Couch D, Perrot-Rechenmann C, et al. Conditional repression of AUXIN BINDING PROTEIN1 reveals that it coordinates cell division and cell expansion during postembryonic development in Arabidopsis and Tobacco. Plant Cell. 2008;20:2746–2762. doi: 10.1105/tpc.108.059048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robert S, Kleine-Vehn J, Barbez E, Sauer M, Paciorek T, Baster P, et al. ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell. 2010;143:111–121. doi: 10.1016/j.cell.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu T, Wen M, Nagawa S, Fu Y, Chen JG, Wu MJ, et al. Cell surface- and Rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell. 2010;143:99–110. doi: 10.1016/j.cell.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Effendi Y, Rietz S, Fischer U, Scherer GFE. The heterozygous abp1/ABP1 insertional mutant has defects in functions requiring polar auxin transport and in regulation of early auxin-regulated genes. Plant J. 2011;65:282–294. doi: 10.1111/j.1365-313X.2010.04420.x. [DOI] [PubMed] [Google Scholar]
- 14.Paciorek T, Zazimalova E, Rudthardt N, Petrasek J, Stierhof YD, Klein-Vehn J, et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- 15.Duana Q, Kitaa D, Lia C, Cheung AY, Wua HM. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci USA. 2010;107:17821–17826. doi: 10.1073/pnas.1005366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scherer GFE. AUXIN-BINDING-PROTEIN1, the second auxin receptor: what is the significance of a two-receptor concept in plant signal transduction? J Exp Bot. 2011;62:3339–3357. doi: 10.1093/jxb/err033. [DOI] [PubMed] [Google Scholar]
- 17.Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A, et al. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998;17:6903–6911. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M. The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast grr1p. Genes Dev. 1998;12:198–207. doi: 10.1101/gad.12.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mravec J, Skupa P, Bailly A, Hoyerová K, Krecek P, Bielach A, et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature. 2009;459:1136–1140. doi: 10.1038/nature08066. [DOI] [PubMed] [Google Scholar]
- 21.Kleine-Vehn J, Friml J. Polar targeting and endocytic recycling in auxin-dependent plant development. Ann Rev Cell Dev Biol. 2008;24:447–473. doi: 10.1146/annurev.cellbio.24.110707.175254. [DOI] [PubMed] [Google Scholar]