Abstract
The biochemical and cellular function of NDR1 in plant immunity and defense signaling has long remained elusive. Herein, we describe a novel role for NDR1 in both pathogen perception and plant defense signaling, elucidated by exploring a broader, physiological role for NDR1 in general stress responses and cell wall adhesion. Based on our predictive homology modeling, coupled with a structure-function approach, we found that NDR1 shares a striking similarity to mammalian integrins, well-characterized for their role in mediating the interaction between the extracellular matrix and stress signaling. ndr1-1 mutant plants exhibit higher electrolyte leakage following pathogen infection, compared to wild type Col-0. In addition, we observed an altered plasmolysis phenotype, supporting a role for NDR1 in maintaining cell wall-plasma membrane adhesions through mediating fluid loss under stress.
Key words: NDR1, integrin, RGD, plant defense
NON-RACE SPECIFIC DISEASE RESISTANCE-1(NDR1) was first identified as playing an essential role in plant defense activation following the perception of the bacterial phytopathogen Pseudomonas syringae pv tomato DC3000.1 Over the past decade, the role of NDR1 in plant defense signaling has emerged through the elucidation of the genetic interactions in which NDR1 participates. This includes the activation of the coiled-coil nucleotide binding site leucine rich repeat (CC-NB-LRR) family of resistance (R) proteins. In parallel, the function of ENHANCED DISEASE SUCEPTIBILITY-1 (EDS1 2) has been extensively described through its genetic and biochemical relationship with the activation of Toll-Interleukin Receptor (TIR)-NB-LRR R-proteins.3 As central activators required for defense signaling, NDR1 and EDS1 represent critical nodes required for the activation of host resistance.
NDR1 is Associated with the Activation of PAMP- and Effector-Triggered Immunity
Two primary pathways of pathogen defense signaling have been described in reference 4. In the first, PAMP (Pathogen Associated Molecular Pattern) triggered immunity (PTI) represents one of the initial lines of defense, characterized by the activation of broad resistance signaling (i.e., reactive oxygen species, MAPK signaling, etc.,) through the recognition of conserved, often ubiquitous, pathogen signatures.5 In the second layer of resistance, effector-triggered immunity (ETI) requires the molecular genetic and biochemical perception of pathogen-derived effector molecules by cognate host R-proteins. Recent research has revealed that PTI and ETI do not function as independent, parallel modes of resistance, but rather, are intimately linked through shared signaling pathways as well as pathogen virulence targets.6
NDR1 has been best characterized by correlating the activation of ETI with the genetic requirement for NDR1.1,7,8 Initial experiments demonstrated an increase in the growth of P. syringae DC3000 in ndr1-1 mutant plants; however, the underlying mechanism(s) explaining this were not established.1 This initial observation has led to the idea that NDR1 plays a dual role in plant physiology and defense. First, ndr1-1 plants appear physiologically impaired, and thus permit increased growth of pathogen populations in compatible interactions. Second, in a recent publication,9 a role for NDR1 has been described which extends beyond the narrow activation of ETI, including the cellular and physiological impact of NDR1 in both PTI and broader stress response signaling. Analysis of mRNA expression of FLG22-INDUCED RECEPTOR-LIKE KINASE-1 (FRK1) as well as the phosphorylation status of MITOGEN-ACTIVATED PROTEIN KINASE (MAPK) 3/6, two signatures of PTI signaling, demonstrates that NDR1 is required for the full activation of PTI following inoculation with a PAMP.9 Previous work has firmly placed NDR1 into the ETI pathway,3 but we hypothesize that the role of NDR1 in defense signaling is through the activation of the early events in both PTI and ETI. Based on the PTI response identified in Knepper et al. (2011), coupled with previous studies describing NDR1's role in ETI, NDR1 is likely required for the rapid activation and amplification of the initial events following pathogen perception (Fig. 1).
Figure 1.
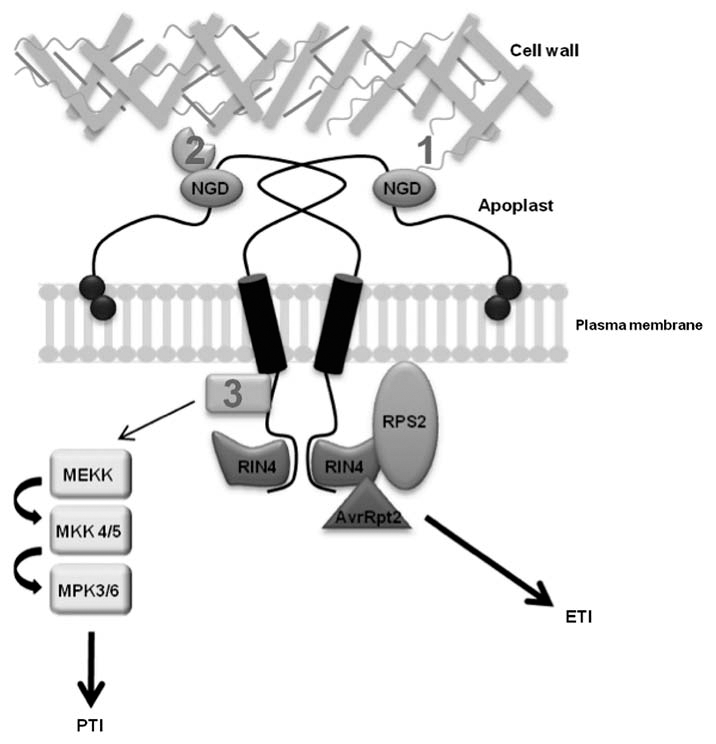
NDR1 plays multiple roles in cell physiology and pathogen defense signaling. NDR1 (NON-RACE SPECIFIC DISEASE RESISTANCE-1) is a plasma membrane localized, GPI-anchored protein that is capable of forming homodimers. NDR1 contains an NGD (Asn-Gly-Asp) motif located in the putative apoplastic-localized region of the protein, which has been shown to be important for maintaining cell wall-plasma membrane adhesion points9 though either direct (1) or indirect (2) interaction with components of the cell wall. Additional cellular components mediating this connection are as of yet unknown. The role of NDR1 in ETI had been further strengthened through the identification of an interaction between NDR1 and RIN4 (RPM1 INTERACTING PROTEIN 4).8 RIN4 has been shown to associate with theR-protein, RPS2 (RESISTANCE TO PSEUDOMONAS SYRINGAE2),26 and it is through RIN4's cleavage by the P. syringae DC3000 effector protein AvrRpt2 and the subsequent recognition of this event by RPS2, that ETI is activated. Recently, NDR1 has been directly linked to the initiationof the MAPK (MITOGEN ACTIVATED PROTEIN KINASE) pathway and PTI (PAMP Triggered Immunity). Although the specific interaction of NDR1 in the upstream components of PTI signaling is unknown (3), NDR1 is required for proper activation of the MAPK signal cascade. ETI, effector triggered immunity; PTI, PAMP triggered immunity.
NDR1 Shares Similarities with Integrins
Homology modeling of NDR1 revealed significant similarities to a Late Embryogenesis Abundant protein, and by extension, integrins.9–11 While no true integrins are known to be present in plants, several structural and functional similarities have been identified,12–14 suggesting that plants do in fact possess similar processes as those employing integrins in mammalian signal transduction.
An NGD motif (i.e., Asn-Gly-Asp) was identified in NDR1 and it was demonstrated that this short sequence was critical for proper adhesion between the plasma membrane and cell wall.9 Interestingly, this motif is similar to the RGD (i.e., Arg-Gly-Asp) motif known to be involved in ligand binding in plants15 as well as mediating the interaction between mammalian integrins and extracellular matrix (ECM) components such as fibronectin.16 Our working hypothesis is that this places NDR1 alongside integrins as a protein required to physically link interior and exterior regions of a cell, possibly as a bridge to link extra- and inter-cellular signaling processes. In mammalian innate immune signaling, integrins interact with members of the ECM, and are able to transmit signals through the plasma membrane into the cell interior.17 The activation requires the formation of integrin dimers, mediated through the transmembrane domains of the α and β subunits.18 Interestingly, recent work has demonstrated that NDR1 also forms dimers, in vivo.9 In mammalian cells, the ECM adhesion of integrins may lead to an increase in the signals generated by growth factor receptors through the bringing together of kinases and their substrates into close proximity.17 While it is currently unknown if dimerization of NDR1 is involved with, or required for, the activation of defense signaling in plants, it is interesting to speculate such a role (Fig. 1).
NDR1 plays a Broad Role in Host Physiology and Stress-Induced Signaling
Since NDR1 was first identified, its role in defense signaling has been examined solely as a genetic component of defense activation. Based on NDR1's striking similarity with integrins, shown using homology modeling,9 a possible link between NDR1 and the cell wall was examined. In short, the ndr1-1 mutant exhibited altered plasmolysis compared to wild type Col-0.9 The analysis of altered plasmolysis phenotypes has been previously established as a reliable way to characterize proteins involved in plasma membrane-cell wall adhesion.12,19–21 In addition, the NGD site of NDR1 was identified as being required for this adhesion phenotype through mutation analysis and exogenous peptide studies.9 In mammalian cells, integrins function in the transmission of signals through the plasma membrane, presumably as part of a mechano-sensing apparatus.22 In Arabidopsis, the mediation of adhesion could potentially point to a similar role for NDR1 in signaling perturbations associated with both biotic and abiotic stresses.
The structural similarity of NDR1 to a member of the LEA family warranted the investigation of a possible connection between NDR1 and functions previously associated with LEA proteins.23–25 ndr1-1 mutant plants were found to have increased electrolyte leakage in response to inoculation with P. syringae DC3000 expressing the effector AvrRpt2.9 The demonstration of a role for NDR1 in plasma membrane-cell wall adhesion, coupled with this increased fluid loss provided the first evidence of a physiological role for NDR1 in Arabidopsis. We hypothesize that NDR1 functions as both a regulator of fluid loss through mediation of plasma membrane-cell wall adhesion, as well as signaling both biotic and abiotic stress responses.9
Final Remarks
While the requirement for NDR1 in pathogen defense signaling has been known for some time,1 the biochemical and cellular function of NDR1 remains elusive. The identification of NDR1 as a putative integrin-like protein, with roles in plasma membrane-cell wall adhesion and stress signaling,9 raises the interesting possibility that NDR1 has evolved to function in a broader role as a general response element associated with biotic and abiotic stress responses in plants.
Acknowledgments
We would like to thank members of the Day lab for critical reading of the manuscript. The work described herein was supported by a grant from the National Science Foundation (IOS-0641319) to B.D.
References
- 1.Century K, Holub EB, Staskawicz BJ. NDR1, a locus of Arabidopsis thaliana that is required for disease resistance to both a bacterial and fungal pathogen. Proc Natl Acad Sci USA. 1995;92:6597–6601. doi: 10.1073/pnas.92.14.6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker JE, Holub EB, Frost LN, Falk A, Gunn ND, Daniels MJ. Characterization of eds1, a mutation in Arabidopsis suppressing resistance to Peronosporaparasitica specified by several different RPP genes. Plant Cell. 1996;8:2033–2046. doi: 10.1105/tpc.8.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aarts N, Metz M, Holub E, Staskawicz BJ, Daniels MJ, Parker JE. Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc Natl Acad Sci USA. 1998;95:10306–10311. doi: 10.1073/pnas.95.17.10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knepper C, Day B. From perception to activation: the molecular-genetic and biochemical landscape of disease resistance signaling in plants. The Arabidopsis Book. 2010;8:012. doi: 10.1199/tab.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 6.Thomma BP, Nurnberger T, Joosten MH. Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell. 2006;23:4–15. doi: 10.1105/tpc.110.082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coppinger P, Repetti PP, Day B, Dahlbeck D, Mehlert A, Staskawicz BJ. Overexpression of the plasma membrane-localized NDR1 protein results in enhanced bacterial disease resistance in Arabidopsis thaliana. Plant J. 2004;40:225–237. doi: 10.1111/j.1365-313X.2004.02203.x. [DOI] [PubMed] [Google Scholar]
- 8.Day B, Dahlbeck D, Staskawicz BJ. NDR1 interaction with RIN4 mediates the differential activation of multiple disease resistance pathways in Arabidopsis. Plant Cell. 2006;18:2782–2791. doi: 10.1105/tpc.106.044693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knepper C, Savory EA, Day B. Arabidopsis NDR1is an integrin-like protein with a role in fluid loss and plasma membrane-cell wall adhesion. Plant Physiol. 2011;156:286–300. doi: 10.1104/pp.110.169656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh S, Cornilescu CC, Tyler RC, Cornilescu G, Tonelli M, Lee MS, Markley JL. Solution structure of a late embryogenesis abundant protein (LEA14) from Arabidopsis thaliana, a cellular stress-related protein. Prot Sci. 2005;14:2601–2609. doi: 10.1110/ps.051579205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canut H, Carrasco A, Galaud J, Cassan C, Bouyssou H, Vita N, et al. High affinity RGD-binding sites at the plasma membrane of Arabidopsis thaliana link the cell wall. Plant J. 1998;16:63–71. doi: 10.1046/j.1365-313x.1998.00276.x. [DOI] [PubMed] [Google Scholar]
- 13.Faik A, Laboure AM, Gulino D, Mandaron P, Falconet D. A plant surface protein sharing structural properties with animal integrins. Eur J Biochem. 1998;253:552–559. doi: 10.1046/j.1432-1327.1998.2530552.x. [DOI] [PubMed] [Google Scholar]
- 14.Lu B, Chen F, Gong Z, Xie H, Liang J. Integrin-like protein is involved in the osmotic stress-induced Abscisic Acid biosynthesis in Arabidopsis thaliana. J Integr Plant Biol. 2007;49:540–549. [Google Scholar]
- 15.Manning VA, Hamilton SM, Karplus PA, Ciuffetti LM. The Arg-Gly-Asp-containing, solvent-exposed loop of Ptr ToxA is required for internalization. Mol Plant Microbe Interact. 2008;21:315–325. doi: 10.1094/MPMI-21-3-0315. [DOI] [PubMed] [Google Scholar]
- 16.van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 17.Huveneers S, Truong H, Danen EHJ. Integrins: signaling, disease and therapy. Int J Radiat Biol. 2007;83:743–751. doi: 10.1080/09553000701481808. [DOI] [PubMed] [Google Scholar]
- 18.Li R, Gorelik R, Nanda V, Law PB, LEAR JD, DeGrado WF, Bennett JS. Dimerization of the transmembrane domain of integrin alphaIIb subunit in cell membranes. J Biol Chem. 2004;279:26666–26673. doi: 10.1074/jbc.M314168200. [DOI] [PubMed] [Google Scholar]
- 19.Mellersh DG, Heath MC. Plasma membrane-cell wall adhesion is required for expression of plant defense responses during fungal penetration. Plant Cell. 2001;13:413–424. doi: 10.1105/tpc.13.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senchou V, Weide R, Carrasco A, Bouyssou H, Pont-Lezica R, Govers F, Canut H. High affinity recognition of a Phytophthora protein by Arabidopsis via an RGD motif. Cell Mol Life Sci. 2004;61:502–509. doi: 10.1007/s00018-003-3394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gouget A, Senchou V, Govers F, Sanson A, Barre A, Rouge P, et al. Lectin receptor kinases participate in protein-protein interactions to mediate plasma membrane-cell wall adhesions in Arabidopsis. Plant Physiol. 2006;140:81–90. doi: 10.1104/pp.105.066464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;1:769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 23.DeMuetter J, Robertson L, Parcy F, Mena M, Fenoll C, Gheysen G. Differential activation of ABI3 and LEA genes upon plant parasitic nematode infection. Mol Plant Pathol. 2005;6:321–325. doi: 10.1111/j.1364-3703.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 24.Goyal K, Walton LJ, Tunnacliffe A. LEA proteins prevent protein aggregation due to water stress. Biochem J. 2005;388:151–157. doi: 10.1042/BJ20041931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Battaglia M, Olvera-Carrillo Y, Garciarrubio A, Campos F, Covarrubias AA. The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 2008;148:6–24. doi: 10.1104/pp.108.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunkel BN, Bent AF, Dahlbeck D, Innes RW, Staskawicz BJ. RPS2, an Arabidopsis disease resistance locus specifying recognition of Pseudomonas syringae strains expressing the avirulence gene avrRpt2. Plant Cell. 1993;5:865–875. doi: 10.1105/tpc.5.8.865. [DOI] [PMC free article] [PubMed] [Google Scholar]


