Abstract
Phytochrome (phy) family of photoreceptors is a broad sensor of environmental light signals that promote photomorphogenic development of plants. Phytochrome interacting Factors (PiFs), bHLH family of transcription factors, repress photomorphogenesis in the dark in an overlapping manner. Phytochromes interact with PiFs in response to light and induce rapid phosphorylation, poly-ubiquitylation and degradation of PiFs through the ubiquitin/26S proteasome pathway to promote photomorphogenesis. Structure-function analyses with PiF family members revealed that multiple domains are necessary for the light-induced phosphorylation and degradation of PiFs. CK2, a ubiquitious Ser/Thr kinase, phosphorylates PiF1 independent of light. In addition, PiF1 mutants deficient in CK2 phosphorylation sites are still robustly phosphorylated but not efficiently degraded in response to light. These data suggest that multiple kinases phosphorylate PiF1 to promote light-induced degradation and photomorphogenesis.
Key words: CK2, kinase, phosphorylation, photomorphogenesis, phytochrome interacting factors, ubiquitin-mediated degradation
One of the best-characterized photoreceptors in plants is the phytochromes (phys) that tract red/far-red and blue light signals and modulate growth and development according to ambient light environment.1,2 Phytochromes (encoded by five genes in Arabidopsis: PHYA-PHYE) are cytosolic proteins in the Pr form (biologically inactive) that converts to the Pfr form (biologically active) upon red light exposure. The Pfr form can be converted back to the inactive Pr form by exposure to FR light. The Pfr form also migrates into the nucleus and initiate signaling events that lead to changes in a large number of gene expression to drive photomorphogenic development.3,4
Current model suggests that upon nuclear migration, the active Pfr form of phytochromes interact with the Phytochrome Interacting Factors (PIF1 and PIF3-7), members of the basic helix-loop-helix (bHLH) class of transcription factors that act as repressors of photomorphogenesis in the dark.5 Therefore, phytochromes and PIFs have antagonistic relationship in controlling photomorphogenesis. To remove the repressive function of PIFs, photoactivated phytochromes physically interact with PIFs and induce rapid light-induced phosphorylation, poly-ubiquitylation and degradation of PIFs to promote photomorphogenesis. However, how phytochromes biochemically induce such a rapid degradation of PIFs is still poorly understood. Several recent reviews summarized the details of early events in this signaling pathway.5–8 This minireview will highlight recent advances on understanding molecular determinants and factors necessary for light-induced degradation of PIFs with a focus on PIF1.
Structure-Function Studies Revealed Multiple Domains Necessary for Light-Induced Degradation of PIF1
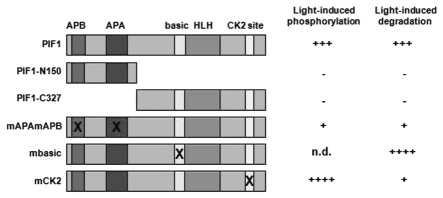
Being members of the bHLH superfamily of transcription factors, PIFs have well-characterized bHLH domain for DNA binding (basic) and dimerization (HLH).9 However, sequence alignment revealed that PIFs also have another region at their N-terminus with high sequence similarity.10,11 In vitro interaction studies using isolated domains (1 to ∼60 amino acid) showed that this region of all PIFs is sufficient to interact with the Pfr form of phyB.10,12 Site-directed mutagenesis identified critical residues within this domain that are necessary to interact with the active Pfr form of phyB.10,12 Therefore, this domain was named active phytochrome binding (APB) domain (Fig. 1). Further studies showed that the APB domain is not necessary for physical interaction with the Pfr form of phyA, as deletion of APB did not abolish interaction with phyA.12,13 In addition, site directed mutagenesis identified an adjacent region in PIF1 and PIF3, named Active PhyA Binding (APA) domain that is necessary for interaction with phyA (Fig. 1). Interestingly, although the overall position of the APA domain is conserved in PIF1 and PIF3 (two PIFs that interact with phyA), the critical residues necessary for interaction are not conserved. While F203 and F209 are critical for PIF3, L95 and N144 are critical for PIF1 interaction with phyA. This difference is possibly a reflection of the differential affinities of PIF1 and PIF3 toward phyA, where PIF1 displayed >10-fold higher affinity compared to PIF3.14 Overall, it appears that although APB domain is highly conserved among all the PIFs, APA domain has evolved with multiple critical residues for phyA interaction with differential affinities.
Figure 1.
Schematic representation of various PIF1 deletion or point mutant constructs used to assess light-induced phosphorylation and degradation. These constructs were expressed as fusion proteins with either Luciferase (LUC) (for full-length PIF1, PIF1-N150, PIF1-C327, PIF1-3M, PIF1-mbHLH) or tandem affinity purification (TAP) (for full-length PIF1 and PIF1-mCK2) tags in transgenic plants. ± indicates the presence and absence of light-induced phosphorylation and degradation, respectively.12,17 The number of + indicates the degree of light-induced phosphorylation and degradation. X indicates the site(s) mutated. n.d., not done yet.
Although site directed mutagenesis showed that phytochrome interaction is necessary for light-induced degradation of PIFs,12,13 deletion studies showed that this interaction is not sufficient for degradation.12 Neither an isolated N-terminal region of PIF1 containing both APB and APA domains (1-150 aa of PIF1) nor the C-terminal region lacking the APB and APA domains (151–478 aa of PIF1) expressed as a Luciferase fusion protein in transgenic plants was degraded in response to light (Fig. 1).12 These data suggest that the C-terminal region has critical determinant(s) for light-induced degradation of PIF1. One candidate is the basic domain necessary for DNA binding, as DNA binding has previously been shown to be a prerequisite for stimulus-induced degradation of transcription factors.15,16 However, a mutant form of PIF1 lacking DNA binding displayed increased degradation compared to wild type PIF1 (Fig. 1),12 suggesting that the light-induced degradation of PIF1 is nucleoplasmic as opposed to chromatin-bound. A recent study identified the critical residues at the C-terminus of PIF1 that contribute to the light-induced degradation (Fig. 1).17 These authors have shown that CK2 phosphorylates PIF1 at multiple sites with a cluster at the C-terminal region (see below). Alanine scanning mutation of the C-terminal cluster (Ser464–466 to Ala464–466) displayed that the mutant form is highly stable compared to wild type PIF1 in response to light.17 Taken together, these studies showed that multiple residues at the N- and C-terminal regions of PIF1 are necessary for the light-induced degradation (Fig. 1).
CK2-mediated Phosphorylation is Necessary for Light-induced Degradation of PIF1
Because direct interaction with phytochromes have been shown to be necessary for light-induced phosphorylation and degradation of PIFs,12,13,18 and because phyA has been shown to possess Ser/Thr kinase activity in vitro,19 an exciting hypothesis is that phytochromes are functioning as kinases to phosphorylate PIFs in response to light. However, convincing in vivo evidence demonstrating phyA kinase activity is still missing. An alternative candidate kinase is CK2, a ubiquitous Ser/Thr kinase present in all higher organisms including plants. CK2 has been shown to phosphorylate positively acting transcription factors (e.g., HY5 and HFR1) functioning in light signaling pathways.20,21 A recent report showed that CK2 also phosphorylates negatively acting transcription factor, PIF1 at multiple Ser/Thr sites with a cluster at the C-terminal end.17 However, CK2 is not responsible for the light-induced phosphorylation of PIF1, as a mutant form of PIF1 lacking the CK2 sites is still robustly phosphorylated in response to light. Strikingly, mutant PIF1s with either six CK2 sites throughout PIF1 or three CK2 sites at the C-terminal end mutated are much more stable compared to wild type PIF1 under light, suggesting that phosphorylation by CK2 is necessary for the rapid light-induced degradation of PIF1.17 How CK2-mediated phosphorylation promotes light-induced degradation of PIF1 is still unknown. One possibility is that CK2-mediated phosphorylation primes the light-induced kinase to phosphrylate PIF1. However, this is unlikely as the mutant PIF1 with three critical CK2 sites at the C-terminal end mutated is still robustly phosphorylated in response to light.17 Alternatively, multiple kinases are acting in concert to promote PIF1 degradation. In this case, CK2 and additional unidentified light-inducible kinase(s) phosphorylate PIF1 at separate sites. This dual phosphorylated form of PIF1 is then recognized by unidentified E3 ligase(s) for ubiquitylation and degradation by the ubiquitin/26S proteasome pathway. Proteolytic removal of PIF1 and other PIFs allows photomorphogenic development to proceed in response to light (Fig. 2).
Figure 2.
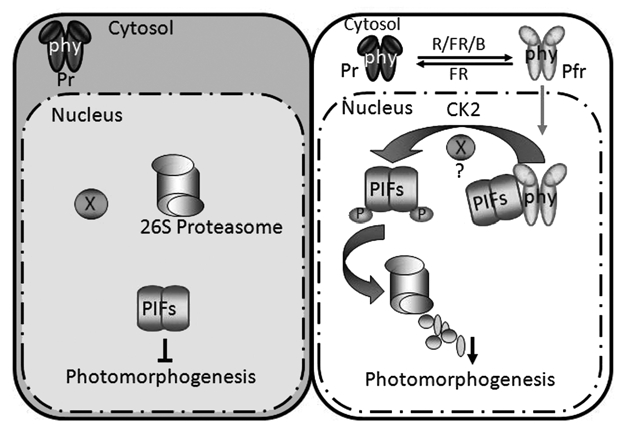
Simplified model demonstrating the antagonistic relationship between phys and PIFs. (Left) In the dark, phys are present in the cytosol as an inactive Pr form, while PIFs are constitutively localized into the nucleus repressing photomorphogenesis. (Right) phys perceive the light signal that induces photo-conversion to the active Pfr form and nuclear migration. Within the nucleus, the Pfr form of phys physically associates with PIFs and induces rapid phosphorylation of PIFs in response to light. CK2 also phosphorylates PIF1 and possibly other PIFs independent of light. The dual phosphorylation (separately by CK2- and an unknown light-inducible kinase-mediated) of PIF1 and possibly other PIFs promotes poly-ubiquitylation and 26S proteasome-mediated degradation in response to light. The light-induced degradation of PIFs relieves the repressive action to promote photomorphogenesis. X, indicates an unknown kinase that phosphorylates PIFs in response to light. P, phosphorylated form. This figure has been adapted and modified from Castillon et al.6
Conclusions and Future Perspectives
Although much has been learned about the molecular determinants and other factors regulating PIF levels, the central questions remain unanswered. For example: what is the biochemical mechanism by which phytochromes induce degradation of PIFs in response to light? What are the kinase(s) and E3 ligase(s) responsible for the light-induced phopshorylation and degradation of PIFs, respectively? Are other factors (e.g., phosphatases, 14-3-3 proteins, HLH proteins) regulating PIF levels? Understanding the details of post-translational regulation of PIFs will help decipher how phytochromes fine-tune plant growth and development in response to ambient light environment.
Acknowledgments
This work was supported by grants from NSF (IOS-0822811 and IOS-0849287) to E.H.
References
- 1.Bae G, Choi G. Decoding of light signals by plant phytochromes and their interacting proteins. Annu Rev Plant Biol. 2008;59:281–311. doi: 10.1146/annurev.arplant.59.032607.092859. [DOI] [PubMed] [Google Scholar]
- 2.Quail PH. Phytochromes. Curr Biol. 2010;20:504–507. doi: 10.1016/j.cub.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quail PH. Phytochrome-regulated gene expression. J Integr Plant Biol. 2007;49:11–20. [Google Scholar]
- 4.Jiao Y, Lau OS, Deng XW. Light-regulated transcriptional networks in higher plants. Nat Rev Genet. 2007;8:217–230. doi: 10.1038/nrg2049. [DOI] [PubMed] [Google Scholar]
- 5.Leivar P, Quail PH. PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci. 2011;16:19–28. doi: 10.1016/j.tplants.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castillon A, Shen H, Huq E. Phytochrome interacting factors: central players in phytochrome-mediated light signaling networks. Trends Plant Sci. 2007;12:514–521. doi: 10.1016/j.tplants.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Henriques R, Jang IC, Chua NH. Regulated proteolysis in light-related signaling pathways. Curr Opin Plant Biol. 2009;12:49–56. doi: 10.1016/j.pbi.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Hoecker U. Regulated proteolysis in light signaling. Curr Opin Plant Biol. 2005;8:469–476. doi: 10.1016/j.pbi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Toledo-Ortiz G, Huq E, Quail PH. The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell. 2003;15:1749–1770. doi: 10.1105/tpc.013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanna R, Huq E, Kikis EA, Al-Sady B, Lanzatella C, Quail PH, et al. A novel molecular recognition motif necessary for targeting photoactivated phytochrome signaling to specific basic helix-loop-helix transcription factors. Plant Cell. 2004;16:3033–3044. doi: 10.1105/tpc.104.025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duek PD, Fankhauser C. bHLH class transcription factors take centre stage in phytochrome signalling. Trends Plant Sci. 2005;10:51–54. doi: 10.1016/j.tplants.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Shen H, Zhu L, Castillon A, Majee M, Downie B, Huq E. Light-induced phosphorylation and degradation of the negative regulator PHYTOCHROME INTERACTING FACTOR 1 depends upon its direct physical interactions with photoactivated phytochromes. Plant Cell. 2008;20:1586–1602. doi: 10.1105/tpc.108.060020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Sady B, Ni W, Kircher S, Schäfer E, Quail PH. Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol Cell. 2006;23:439–446. doi: 10.1016/j.molcel.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Huq E, Al-Sady B, Hudson M, Kim C, Apel K, Quail PH. Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science. 2004;305:1937–1941. doi: 10.1126/science.1099728. [DOI] [PubMed] [Google Scholar]
- 15.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 16.Muratani M, Tansey WP. How ubiquitin-proteasome system controls transcription. Nat Rev Mol Cell Biol. 2003;4:1–10. doi: 10.1038/nrm1049. [DOI] [PubMed] [Google Scholar]
- 17.Bu Q, Zhu L, Dennis MD, Yu L, Lu SX, Person MD, et al. Phosphorylation by CK2 enhances the rapid light-induced degradation of PIF1. J Biol Chem. 2011;286:12066–12074. doi: 10.1074/jbc.M110.186882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castillon A, Shen H, Huq E. Blue light induces degradation of the negative regulator Phytochrome Interacting Factor 1 to promote photomorphogenic development of Arabidopsis seedlings. Genetics. 2009;182:161–171. doi: 10.1534/genetics.108.099887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh KC, Lagarias JC. Eukaryotic phytochromes: light-regulated serine/threonine protein kinases with histidine kinase ancestry. Proc Natl Acad Sci USA. 1998;95:13976–13981. doi: 10.1073/pnas.95.23.13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardtke CS, Gohda K, Osterlund MT, Oyama T, Okada K, Deng XW. HY5 stability and activity in arabidopsis is regulated by phosphorylation in its COP1 binding domain. EMBO J. 2000;19:4997–5006. doi: 10.1093/emboj/19.18.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park HJ, Ding L, Dai M, Lin R, Wang H. Multisite phosphorylation of Arabidopsis HFR1 by casein kinase II and a plausible role in regulating its degradation rate. J Biol Chem. 2008;283:23264–23273. doi: 10.1074/jbc.M801720200. [DOI] [PubMed] [Google Scholar]