Abstract
Plants have evolved general and specific defense mechanisms to protect themselves from diverse enemies, including herbivores and pathogens. To maintain fitness in the presence of enemies, plant defense mechanisms are aimed at inducing systemic resistance: in response to the attack of pathogens or herbivores, plants initiate extensive changes in gene expression to activate “systemic acquired resistance” against pathogens and “indirect defense” against herbivores. Recent work revealed that leaf infestation by whiteflies, stimulated systemic defenses against both an airborne pathogen and a soil-borne pathogen, which was confirmed by the detection of the systemic expression of pathogenesis-related genes in response to salicylic acid and jasmonic acid-signaling pathway activation. Further investigation revealed that plants use self protection mechanisms against subsequent herbivore attacks by recruiting beneficial microorganisms called plant growth-promoting rhizobacteria/fungi, which are capable of reducing whitefly populations. Our results provide new evidence that plant-mediated aboveground to belowground communication and vice versa are more common than expected.
Key words: aboveground, induced systemic resistance, pepper, plant growth-promoting rhizobacteria, underground, whitefly
As sessile organisms, plants are unable to actively avoid the attack of predators. To overcome this, plants have evolved a multilayer immune system against herbivores and pathogens.1 Plants, unlike animals, lack adaptive immunity. Instead, plants are dependent on a heritable, innate immunity based on the recognition by receptors of the presence of microbial triggers (cues) including effector proteins and microbe-associated molecular patterns.1 The perception of microbial cues leads to the induction of a broad spectrum of plant defenses called systemic acquired resistance (SAR).2 Until recently, SAR was thought to be limited to the induction of plant defenses against foliar microbial pathogens. However, recent results suggested that plants can activate signal exchanges between aboveground (AG) and belowground (BG) responses.3 Three phenomena indicate that plants can make use of cues that are systemically indicative of future enemy attack: (1) induced resistance against AG pathogens by BG microbes and vice versa, (2) indirect defenses against AG insects by AG herbivore infestation and (3) BG pathogen infection leading to root exudate-mediated recruitment of BG bacteria. First, many strains of rhizosphere microbes referred to as plant growth-promoting rhizobacteria/fungi (PGPR/PGPF) have beneficial effects by positively affecting plant growth and resistance against foliar plant pathogens—a process known as induced systemic resistance (ISR).4 Inducible defense responses triggered by the foliar pathogen Pseudomonas syringae pv. tomato DC3000 included the induction of root secretions such as L-malic acid that effectively recruited a PGPR strain, Bacillus subtilis FB17, in Arabidopsis roots.5 Second, herbivore attacks on plants trigger the induction of distinct resistance responses referred to as “indirect defenses.”6 In addition to the “direct defense” reaction mediated by the de novo production of toxic secondary compounds against enemies, plants also defend themselves by releasing volatile organic compounds (VOCs) or extrafloral nectar (EFN) to attract natural enemies (carnivores) of the herbivores AG.7 Third, as plant root exudates function as BG signaling molecules that affect the composition of rhizosphere microbial populations,8 certain rhizobacteria express antifungal-associated genes such as the 2,4-diacetylphloroglucinol biosynthesis gene phlA. The expression of these genes is in turn influenced by root exudates, which are modulated by soilborne fungal infections.9
In prior studies, only one-way signal transduction was considered, such as AG to BG, AG to AG or BG to BG (Fig. 1).10–13 The above three examples provide evidence of induced resistance against the same or a similar group of organisms, such as resistance against insects by insects, or against microbes by microbes. However, there are few studies addressing insect-microbe combinations during the elicitation of induced resistance. More specifically, indirect defenses by symbiotic root interactions AG were found, such as the volatile blends released by plants with arbuscular mycorrhizal fungi, which were more attractive to aphid parasitoids than the blends from plants without mycorrhiza.14 The BG to AG defense responses of plants are not limited to arbuscular mycorrhizal fungi against herbivores. In addition to mycorrhiza-altered insect feeding preferences, a combination of Pseudomonas spp. strains affected the development of leaffolder pest and actively enhanced resistance against leaffolder attack by triggering the synthesis of systemic defense enzymes such as chitinase and proteinase inhibitors in rice plants.15 Bacillus sp. PGPR strain treatment of tomato triggered ISR to Tomato mottle virus under natural conditions by reducing the population of the silverleaf whitefly vector.16
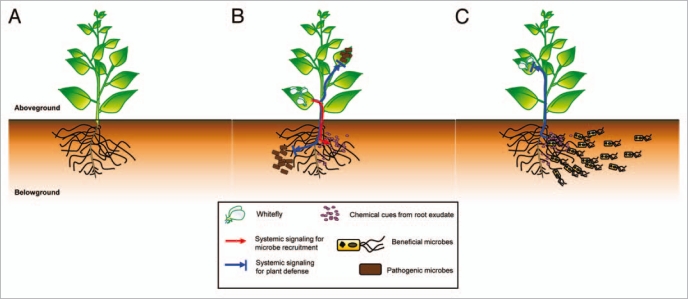
Figure 1.
Putative model of plant-mediated aboveground to belowground communication and vice versa during the induction of systemic resistance via tritrophic (insect-plant-rhizobacteria) interactions. (A) A plant under normal condition. (B) Whitefly infestation elicits plant systemic defenses against leaf and root pathogens. Chemical cues from root exudates secreted from AG whitefly infestation trigger the recruitment of beneficial microbes including saprophytic fungi, Gram-positive bacteria and actinomycetes. (C) The induction of systemic resistance by colonization by beneficial microbes confers plant self-protection against subsequent herbivore attacks.
Recently, we found another type of induced resistance response: bidirectional signal exchanges between AG and BG (Fig. 1).17 Our study demonstrated that the phloem feeding whiteflies can induce systemic resistance against both a leaf bacterial pathogen and a soil-borne bacterial pathogen. A similar study using the whitefly as an AG feeding insect to test the induction of plant defenses only observed its effects against conspecific insect herbivore competitors AG.18 However, in our study, foliar attack by the whitefly not only elicited AG resistance against a leaf pathogenic bacterium, Xanthomonas axonopodis pv. vesicatoria, but also enhanced resistance against the soil-borne pathogenic bacterium, Ralstonia solanacearum. The induction of systemic resistance was confirmed by significant upregulation of the SA and JA defense signaling pathway marker genes, Capsicum annuum pathogenesis-related protein (CaPR)1, CaPR4, CaPR10 and Ca protease inhibitor (CaPIN) in both leaves (AG) and roots (BG) after whitefly feeding. Interestingly, AG white-fly feeding significantly increased the population density of beneficial BG microflora including Gram-positive bacteria, actinomycetes and saprophytic fungi that may induce systemic resistance (Fig 1).4 Among BG microbial groups, several Grampositive Bacillus sp. strains significantly elicited plant systemic defenses against the whitefly population in the tomato field.16 Our studies provide a new understanding of tritrophic (insect-plant-PGPR) interactions and their role in the induction of defense mechanisms. In the near future, it will be important to define plant defense signaling molecules from AG to BG and to dissect the signaling transduction pathways using “omics” technology to reveal the mechanisms by which plants protect themselves against enemy attacks.
Acknowledgments
Financial support was obtained from Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0011655), the Industrial Source Technology Development Program of the Ministry of Knowledge Economy (TGC0281011) of Korea, the 21C Frontier Microbial Genomics and Application Center Program, Ministry of Education, Science and Technology, the Next-Generation BioGreen 21 Program (SSAC, grant #PJ008170), Rural Development Administration, and the KRIBB initiative program, South Korea.
References
- 1.Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 2.Bostock RM. Signal crosstalk and induced resistance: straddling the line between cost and benefit. Ann Rev Phytopathol. 2005;43:545–580. doi: 10.1146/annurev.phyto.41.052002.095505. [DOI] [PubMed] [Google Scholar]
- 3.Bezemer TM, van Dam NM. Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol Evol. 2005;20:617–624. doi: 10.1016/j.tree.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Kloepper JW, Ryu CM. CBTNS, editor. Bacterial endophytes as elicitors of induced systemic resistance. In: Schulz B, editor. Soil biology, microbial root endophytes. Berlin Heidelberg: Springer-Verlag; 2006. pp. 33–52. [Google Scholar]
- 5.Rudrappa T, Czymmek KJ, Pare PW, Bais HP. Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol. 2008;148:1547–1556. doi: 10.1104/pp.108.127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldwin IT, Halitschke R, Paschold A, Von Dahl CC, Preston CA. Volatile signaling in plant-plant interactions: “talking trees” in the genomics era. Science. 2006;311:812. doi: 10.1126/science.1118446. [DOI] [PubMed] [Google Scholar]
- 7.Heil M, Ton J. Long-distance signalling in plant defence. Trends Plant Sci. 2008;13:264–272. doi: 10.1016/j.tplants.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Badri DV, Vivanco JM. Regulation and function of root exudates. Plant Cell Environm. 2009;32:666–681. doi: 10.1111/j.1365-3040.2008.01926.x. [DOI] [PubMed] [Google Scholar]
- 9.Jousset A, Rochat L, Lanoue A, Bonkowski M, Keel C, Scheu S. Plants respond to pathogen infection by enhancing the antifungal gene expression of root-associated bacteria. Molec Plant-Microb Interact. 2011;24:352–358. doi: 10.1094/MPMI-09-10-0208. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin IT, Kessler A, Halitschke R. Volatile signaling in plant-plant-herbivore interactions: what is real? Curr Opin Plant Biol. 2002;5:351–354. doi: 10.1016/s1369-5266(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 11.Bezemer T, Wagenaar R, Van Dam N, Van Der Putten W, Wackers F. Above-and below-ground terpenoid aldehyde induction in cotton, Gossypium herbaceum, following root and leaf injury. J Chem Ecol. 2004;30:53–67. doi: 10.1023/b:joec.0000013182.50662.2a. [DOI] [PubMed] [Google Scholar]
- 12.Heil M. Indirect defence via tritrophic interactions. New Phytologist. 2008;178:41–61. doi: 10.1111/j.1469-8137.2007.02330.x. [DOI] [PubMed] [Google Scholar]
- 13.Yi HS, Heil M, Adame-Alvarez RM, Ballhorn DJ, Ryu CM. Airborne induction and priming of plant defenses against a bacterial pathogen. Plant Physiol. 2009;151:2152–2161. doi: 10.1104/pp.109.144782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerrieri E, Lingua G, Digilio MC, Massa N, Berta G. Do interactions between plant roots and the rhizosphere affect parasitoid behaviour? Ecol Entomol. 2004;29:753–756. [Google Scholar]
- 15.Saravanakumar D, Muthumeena K, Lavanya N, Suresh S, Rajendran L, Raguchander T, et al. Pseudomonas induced defence molecules in rice plants against leaffolder (Cnaphalocrocis medinalis) pest. Pest Manag Sci. 2007;63:714–721. doi: 10.1002/ps.1381. [DOI] [PubMed] [Google Scholar]
- 16.Murphy JF, Zehnder GW, Schuster DJ, Sikora EJ, Polston JE, Kloepper JW. Plant growth-promoting rhizobacterial mediated protection in tomato against Tomato mottle virus. Plant Disease. 2000;84:779–784. doi: 10.1094/PDIS.2000.84.7.779. [DOI] [PubMed] [Google Scholar]
- 17.Yang JW, Yi HS, Kim H, Lee B, Lee S, Ghim SY, et al. Whitefly infestation of pepper plants elicits defence responses against bacterial pathogens in leaves and roots and changes the below ground microflora. J Ecol. 2011;99:46–56. [Google Scholar]
- 18.Mayer RT, Inbar M, McKenzie C, Shatters R, Borowicz V, Albrecht U, et al. Multitrophic interactions of the silverleaf whitefly, host plants, competing herbivores and phytopathogens. Arch Insect Biochem Physiol. 2002;51:151–169. doi: 10.1002/arch.10065. [DOI] [PubMed] [Google Scholar]