Abstract
Potassium (K) is one of the major nutrients that is essential for plant growth and development. The majority of cellular K+ resides in the vacuole and tonoplast K+ channels of the TPK (Two Pore K) family are main players in cellular K+ homeostasis. All TPK channels were previously reported to be expressed in the tonoplast of the large central lytic vacuole (LV) except for one isoform in Arabidopsis that resides in the plasma membrane. However, plant cells often contain more than one type of vacuole that coexist in the same cell. We recently showed that two TPK isoforms (OsTPKa and OsTPKb) from Oryza sativa localize to different vacuoles with OsTPKa predominantly found in the LV tonoplast and OsTPKb primarily in smaller compartments that resemble small vacuoles (SVs). Our study further revealed that it is the C-terminal domain that determines differential targeting of OsTPKa and OsTPKb. Three C-terminal amino acids were particularly relevant for targeting TPKs to their respective endomembranes. In this addendum we further evaluate how the different localization of TPKa and TPKb impact on their physiological role and how TPKs provide a potential tool to study the physiology of different types of vacuole.
Key words: TPK channels, small vacuoles, vacuolar targeting, potassium
The roles of plant vacuolar K+ channels are diverse and include potassium homeostasis, turgor regulation and responses to abiotic stress. Vacuolar K+-selective channels belong to two-pore K+ (TPK) channel families which have been found in genomes of many plant species such as Arabidopsis, poplar, Physcomitrella, Eucalyptus, barley, potato, rice and tobacco (Fig. 1). TPKs have structural similarity to mammalian “tandem P domain” channels with a secondary structure that contains four transmembrane domains and two pore regions (Fig. 2).1–5 TPK channels have pore regions with a GYGD signature that endows K+ selectivity and a variable number of Ca2+ binding EF domains in the C terminus.3–8 One of the best characterized members of the TPK family is AtTPK1 from Arabidopsis thaliana. AtTPK1 activity is voltage independent but sensitive to cytosolic Ca2+, cytosolic pH and N-terminal phosphorylation by 14-3-3 proteins.5,6,8,9 In Arabidopsis, AtTPK1 expresses in the large lytic vacuole (LV) and plays roles in cellular K+ homeostasis, K+-release during stomatal closure and seed germination.4,5 Other members of the Arabidopsis TPK family (AtTPK2, AtTPK3, AtTPK5) have been shown to localize to the LV but also showed some expression in smaller, vesicle-like, compartments.4 However, none of these isoforms appears to form functional channels in planta although our experiments with heterologous expression of AtTPK3 and AtTPK5 in the K+ uptake deficient E. coli LB2003 demonstrates complementation of bacterial growth phenotype (Isayenkov S, et al. unpublished results). Equally intriguing, is the plasma membrane localization of the Arabidopsis TPK4 isoform, in spite of its sequence being very similar to that of other TPKs.10
Figure 1.
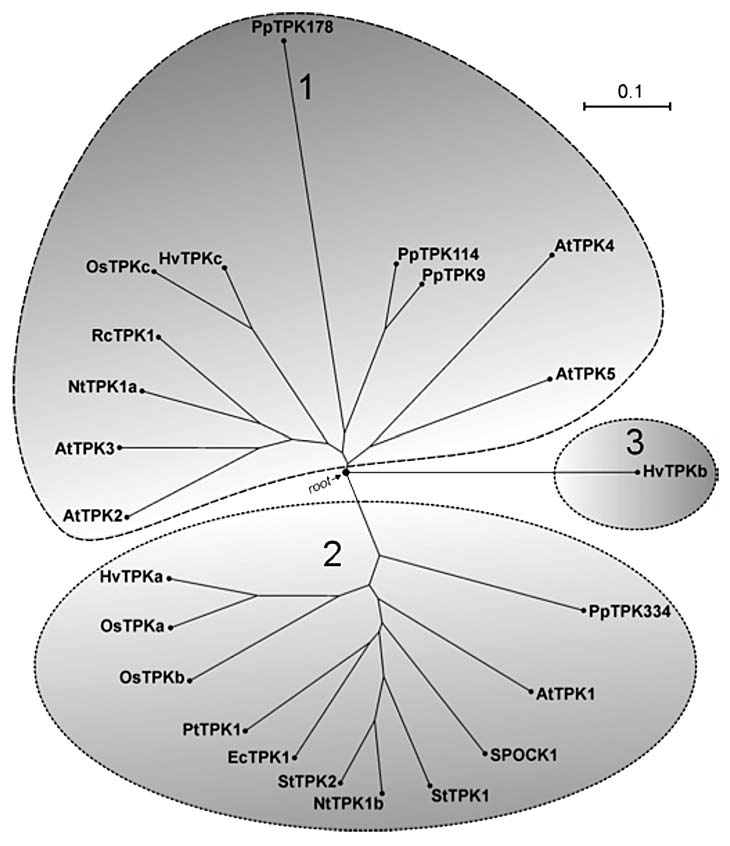
Phylogenetic tree of plant TPKs. The three main clusters of TPKs comprise: Cluster 1 with AtTPK1-like channels; Cluster 2 with AtTPK3/TPK5-like channels; Cluster 3 with barley HvTPKb. Bootstrap analysis was performed using ‘Molecular Evolutionary Genetics Analysis, MEGA4’ software available at www.megasoftware.net/mega4/mega
Figure 2.

Two-pore potassium channel secondary structure. TPK channels comprise four transmembrane domains (1–4) and two pore regions (P) per subunit. Functional channels are formed from two subunits. In most TPKs, both P regions contain a K+ selectivity signature, GYGD. However, the tobacco NtTPKa isoform has different motifs in the second P domain. In the N terminal region, TPKs have a 14-3-3 binding domain that impact on channel activity, with the binding of 14-3-3 protein leading to channel activation. C-termini of TPKs show a varying number of putative Ca2+ binding “EF hands” which may vary from zero to two.
TPKs in Tobacco
Tobacco appears to have two TPK isoforms.11,12 NtTPK1b is very similar to AtTPK1 and is likely involved in K+ release from the vacuole.11 In contrast, NtTPK1a is more similar to AtTPK5 and AtTPK3 and this isoform has motifs in the second pore that are different from the canonical GYGD sequence.11 NtTPK1a activity is sensitive to spermidine and spermine and its transcript levels are raised in hyperosmotic conditions. On the basis of these findings it was suggested that NtTPK1b could be involved in the response to hyperosmotic-shock.11
TPKs in Rice Express in Different Types of Vacuole
The rice genome encodes at least three TPK isoforms, OsTPKa, OsTPKb and OsTPKc. OsTPKa and OsTPKb proteins are highly similar to each other (63% identity) and to AtTPK1 (50–57% identity). The biophysical properties and membrane localizations of these two proteins were studied by using patch clamp analyses and fluorescent protein reporter fusions.13 The obtained data show that ion channel parameters such as conductance, current rectification and 14-3-3 activation are comparable for OsTPKa and OsTPKb. Surprisingly for two proteins that are so much alike (Fig. 1), OsTPKa localisation was predominantly in the LV whereas OsTPKb mainly expressed in small vacuoles (SVs) that have many hallmarks of proteins storage vacuoles found in reproductive tissues (Fig. 3). Co-transformation experiments showed that expression of both TPK isoforms can occur in one cell (Fig. 3). By making a large range of OsTPKa:OsTPKb chimeras and carrying out site directed mutagenesis it was discovered that three key amino acids are particularly important for the differential vacuolar targeting.13 Mutations in the three residues, L303, S313 and N326 located between the core motifs of two putative EF hands, significantly shifts trafficking to either LV or SV. In addition, assays using the Golgi disrupting compound brefeldin A showed that OsTPKb trafficking to its destination is Golgi independent as has previously been shown for certain protein storage vacuole-located TIP proteins.14
Figure 3.
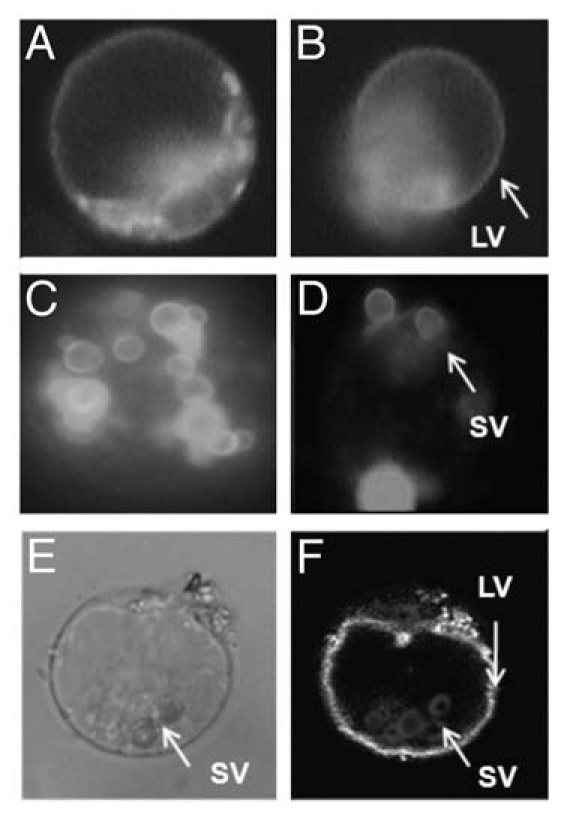
Subcellular localisation and distribution of OsTPKa and OsTPKb channels in rice protoplasts. OsTPKa channels are mainly targeted to the lytic vacuole (LV) (a and b). OsTPKa-EYFP fluorescence pattern in intact protoplast (A) and an LV released from a disrupted protoplast (B). The EYFP fluorescence in rice protoplasts transformed with OsTPKb-EYFP constructs is mainly in small vacuoles (SVs) (C and D). Fluorescence of OsTPKb-EYFP in intact protoplasts (c) and SVs released from disrupted protoplast (D). OsTPKa colocalizes with OsTPKb in one rice protoplast after cotransformation with OsTPKa-EYFP and OsTPKb-RFP. The bright field image (E) clearly shows small vacuolar structures (SVs) and central lytic vacuole (LV). Overlay image (F) shows EYFP-specific fluorescence of LV and RFP fluorescence of SVs in the same cell.
For plants, very little is known about the trafficking of integral membrane proteins in the secretory pathway, especially regarding the ultimate destination in different endocompartments. In many cases it is unlikely that plant proteins have clearly identifiable signal sequences. Rather, fairly ill defined domains in either C or N terminus may be important.2,7,15 For example, TPK C-termini have diacidic motifs (DLEs) that are necessary for ER release and may promote interaction with COPII proteins to mediate transfer from ER to Golgi.7 Some TIP aquaporins have a specific C-terminal domain (PIEPPPHH) that interacts with trafficking proteins.16 It is not clear how the identified residues in OsTPKa and OsTPKb ensure trafficking to different types of vacuole; do they direct proteins to particular ER subdomains where either vesicles for Golgi-dependent or Golgi-independent trafficking originate? Do they define binding interactions with specific trafficking proteins? Is phosphorylation of the S313 in OsTPKb required for targeting to SVs?
What is the Physiological Role of OsTPKb
There is currently considerable debate about the question of whether plants have more than one type of vacuole in one cell.13,17 Our data strongly suggest that vegetative rice protoplasts contain SVs that coexist with the central LV. However, the function of SVs is unknown; SVs may be “vegetative protein storage vacuoles” and a deposit for defence proteins such as chitinases or for storage of proteins involved in annual regrowth in perennials.18 The SV localized expression of OsTPKb makes it a potentially useful tool to study the physiology of SVs. According to our observation, loss of function mutations in OsTPKb invariably led to (semi)sterile rice plants but overexpression of OsTPKb led to increased tolerance to damaging abiotic conditions such as salt stress and osmotic stress (Mian et al. unpublished data). These data suggest that SVs may be important compartments for osmoregulation and that OsTPKb activity is a key part of this function. This idea is supported by other evidence such as the mechanosensitivity of plant TPKs,19 expression data that show increased OsTPKb transcript levels after exposure to salt or osmotic stress (Fig. 4) and the surmised role of NtTPK1b in the response to hyperosmotic-shock.10
Figure 4.
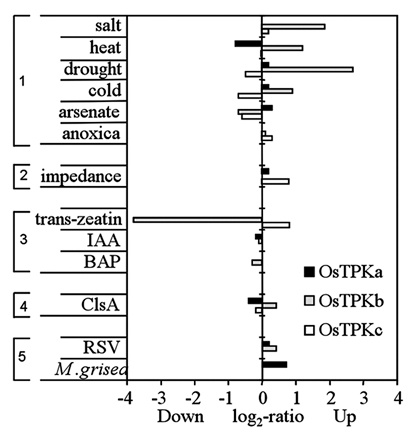
Transcriptional profile of rice TPK genes. Relative transcript abundance of rice OsTPKa, TPKb and TPKc in response to various growth conditions and stimuli. The expression profiles of OsTPKs are arranged in several groups depending on the nature of the stimuli. The first group (top) relates to various types of abiotic stress, the second group combines other types of growth conditions, the third group relates to hormonal treatments, the forth group shows changes in response to elicitors, and the last group relates to biotic stress (RSV: rice stripe virus; M grisea: rice blast fungus Magnaporthe grisea). Data are adapted from Genevestigator database available at www.genevestigator.com.
Conclusions
It is now clear that membrane localization of plant TPK channels is more diverse than previously thought and includes the plasma membrane and membrane of various endocompartments.4,9,13 The main variability between TPK isoforms occurs in the N- and C-terminal regions8 and our work with rice TPKs suggests that a relatively small number of residues in the C terminus determines protein targeting to either the central LV or SVs. The discovery that OsTPKb expresses mostly in SVs makes it a convenient marker for this compartment, a fact we hope to exploit in unravelling the physiological function of SVs in vegetative tissues.
References
- 1.Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. “TASK, a human background K+ channel to sense external pH variations near physiological pH”. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Kelly I, Butler MH, Zilberberg N, Goldstein SA. Forward transport. 14-3-3 binding overcomes retention in endoplasmic reticulum by dibasic signals. Cell. 2002;111:577–588. doi: 10.1016/s0092-8674(02)01040-1. [DOI] [PubMed] [Google Scholar]
- 3.Czempinski K, Frachisse JM, Maurel C, Barbier-Brygoo H, Mueller-Roeber B. Vacuolar membrane localization of the Arabidopsis “two-pore” K+ channel KCO1. Plant J. 2002;29:809–820. doi: 10.1046/j.1365-313x.2002.01260.x. [DOI] [PubMed] [Google Scholar]
- 4.Voelker C, Schmidt D, Mueller-Roeber B, Czempinski K. Members of the Arabidopsis AtTPK/KCO family form homomeric vacuolar channels in planta. Plant J. 2006;48:296–306. doi: 10.1111/j.1365-313X.2006.02868.x. [DOI] [PubMed] [Google Scholar]
- 5.Gobert A, Isayenkov S, Voelker C, Czempinski K, Maathuis FJ. The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc Natl Acad Sci USA. 2007;104:10726–10731. doi: 10.1073/pnas.0702595104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latz A, Becker D, Hekman M, Müller T, Beyhl D, Marten I, et al. TPK1, a Ca2+-regulated Arabidopsis vacuole two-pore K+ channel is activated by 14-3-3 proteins. Plant J. 2007;52:449–459. doi: 10.1111/j.1365-313X.2007.03255.x. [DOI] [PubMed] [Google Scholar]
- 7.Dunkel M, Latz A, Schumacher K, Müller T, Becker D, Hedrich R. Targeting of vacuolar membrane localized members of the TPK channel family. Mol Plant. 2008;1:938–949. doi: 10.1093/mp/ssn064. [DOI] [PubMed] [Google Scholar]
- 8.Voelker C, Gomez-Porras JL, Becker D, Hamamoto S, Uozumi N, Gambale F, et al. Roles of tandem-pore K plus channels in plants—a puzzle still to be solved. Plant Biol. 2010;12:56–63. doi: 10.1111/j.1438-8677.2010.00353.x. [DOI] [PubMed] [Google Scholar]
- 9.Bihler H, Eing C, Hebeisen S, Roller A, Czempinski K, Bertl A. TPK1 is a vacuolar ion channel different from the slow-vacuolar cation channel. Plant Physiol. 2005;139:417–424. doi: 10.1104/pp.105.065599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker D, Geiger D, Dunkel M, Roller A, Bertl A, Latz A, et al. AtTPK4, an Arabidopsis tandem-pore K+ channel, poised to control the pollen membrane voltage in a pH- and Ca2+-dependent manner. Proc Natl Acad Sci USA. 2004;101:15621–15626. doi: 10.1073/pnas.0401502101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamamoto S, Marui J, Matsuoka K, Higashi K, Igarashi K, Nakagawa T, et al. Characterization of a tobacco TPK-type K+ channel as a novel tonoplast K+ channel using yeast tonoplasts. J Biol Chem. 2008;283:1911–1920. doi: 10.1074/jbc.M708213200. [DOI] [PubMed] [Google Scholar]
- 12.Sano T, Kutsuna N, Becker D, Hedrich R, Hasezawa S. Outward-rectifying K+ channel activities regulate cell elongation and cell division of tobacco BY-2 cells. Plant J. 2009;57:55–64. doi: 10.1111/j.1365-313X.2008.03672.x. [DOI] [PubMed] [Google Scholar]
- 13.Isayenkov S, Isner JC, Maathuis FJ. Rice Two-Pore K+ channels are expressed in different types of vacuoles. Plant Cell. 2011;23:756–768. doi: 10.1105/tpc.110.081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park M, Kim SJ, Vitale A, Hwang I. Identification of the protein storage vacuole and protein targeting to the vacuole in leaf cells of three plant species. Plant Physiol. 2004;134:625–639. doi: 10.1104/pp.103.030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada K, Osakabe Y, Mizoi J, Nakashima K, Fujita Y, Shinozaki K, et al. Functional analysis of an Arabidopsis thaliana abiotic stress-inducible facilitated diffusion transporter for monosaccharides. J Biol Chem. 2010;285:1138–1146. doi: 10.1074/jbc.M109.054288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oufattole M, Park JH, Poxleitner M, Jiang L, Rogers JC. Selective membrane protein internalization accompanies movement from the endoplasmic reticulum to the protein storage vacuole pathway in Arabidopsis. Plant Cell. 2005;17:3066–3080. doi: 10.1105/tpc.105.035212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jauh GY, Phillips T, Rogers JC. Tonoplast intrinsic protein isoforms as markers for vacuole functions. Plant Cell. 1999;11:1499–1508. doi: 10.1105/tpc.11.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Sansebastiano GP, Paris N, Marc-Martin S, Neuhaus JM. Regeneration of a lytic central vacuole and of neutral peripheral vacuoles can be visualised by GFP targeted to either type of vacuoles. Plant Physiol. 2001;126:78–86. doi: 10.1104/pp.126.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maathuis FJM. Vacuolar two-pore K+ channels act as vacuolar osmosensors. New Phytol. 2011;191:84–91. doi: 10.1111/j.1469-8137.2011.03664.x. [DOI] [PubMed] [Google Scholar]


