Abstract
Root-knot nematodes are biotrophic parasites that invade the root apex of host plants and migrate towards the vascular cylinder where they induce the differentiation of root cells into hypertrophied multinucleated giant cells. Giant cells are part of the permanent feeding site required for nematode development into the adult stage. To date, a repertoire of candidate effectors potentially secreted by the nematode into the plant tissues to promote infection has been identified. However, the precise role of these candidate effectors during root invasion or during giant cell induction and maintenance remains largely unknown. Primarily, the identification of the destination of nematode effectors within plant cell compartment(s) is crucial to decipher their actual functions. We analyzed the fine localization in root tissues of five nematode effectors throughout the migratory and sedentary phases of parasitism using an adapted immunocytochemical method that preserves host and pathogen tissues. We showed that secretion of effectors from the amphids or the oesophageal glands is tightly regulated during the course of infection. The analyzed effectors accumulated in the root tissues along the nematode migratory path and along the cell wall of giant cells, showing the apoplasm as an important destination compartment for these effectors during migration and feeding cell formation.
Key words: plant pathogen, effector, immunocytochemistry, root-knot nematode, secretion, plant apoplasm
Nematodes Secrete Effectors within Plant Tissues to Promote Infection
During plant infection, pathogens secrete effectors that promote disease by manipulating plant cell functions or plant defenses. Similarly, root-knot nematodes (RKN), genus Meloidogyne, secrete proteins within the plant tissues to assist parasite establishment. RKN are biotrophic pathogens that display a highly specialized feeding relationship with their hosts. Infective juveniles penetrate the root apex and migrate intercellularly to the vascular cylinder. There, they settle and select a few cells that will differentiate into hypertrophied and multinucleated giant cells in response to secreted nematode effectors. Six to eight giant cells surrounded by dividing vascular parenchyma cells form the gall, or feeding site, that provides the nutrients required for completion of the nematode life cycle and offspring production. Understanding the role of nematode effectors is a crucial issue to decipher the molecular events leading to the establishment of the parasite, the differentiation of the giant cells and the manipulation of plant defenses throughout interaction.
The oesophageal glands are three cells connected to the oesophagus that produce proteins secreted during infection through a protrusible stylet at the buccal orifice. The stylet is compared to the type three secretion system (TTSS) of bacteria and functions as a syringe to inject secretions in the plant tissues and to retrieve nutrients from the cytoplasm of feeding cells.1,2 The amphids, two chemosensory organs located on the nematode head and open to the exterior via a prominent pore, are important secretory organs involved in the perception of chemotactic environment stimuli.3
To date, approximately hundred proteins potentially secreted by RKN have been identified (reviewed in ref. 4). Only few RKN proteins have been shown to be secreted within host tissues during parasitism but the end localization of these secretions is still unknown.5–8 One single study provided a clear demonstration for secretion of a RKN protein (the calreticulin Mi-CRT) in the feeding site and showed abundant protein accumulation along the cell wall of giant cells in the vicinity of the stylet tip.9 In order to get a broader view on the secretory activity of the nematode during infection, we used sera directed against five different candidate effectors from the RKN M. incognita and analyzed the localization of the secreted proteins throughout infection.
Nematode Proteins are Secreted within the Apoplasm Along the Path of Migrating Nematodes
In order to reach the vascular tissue where they establish their feeding site, nematodes need to penetrate and migrate along the root facing physical and molecular barriers imposed by the host. Genome sequencing has revealed in M. incognita an extraordinary diverse repertoire of cell wall degrading and modifying enzymes.10 We explored the localization of β-1,4-endoglucanases and expansins during RKN infection using a serum specific to the cellulose binding module (CBM2) appended to these enzymes. In addition, we analyzed the localization of a new pectate lyase from M. incognita (Mi-PEL3). Both sera showed the secretion of the enzymes through the stylet along the nematode head and in the apoplasm of root cells during migration, suggesting a role in plant cell wall loosening for root invasion (Fig. 1A and B).
Figure 1.
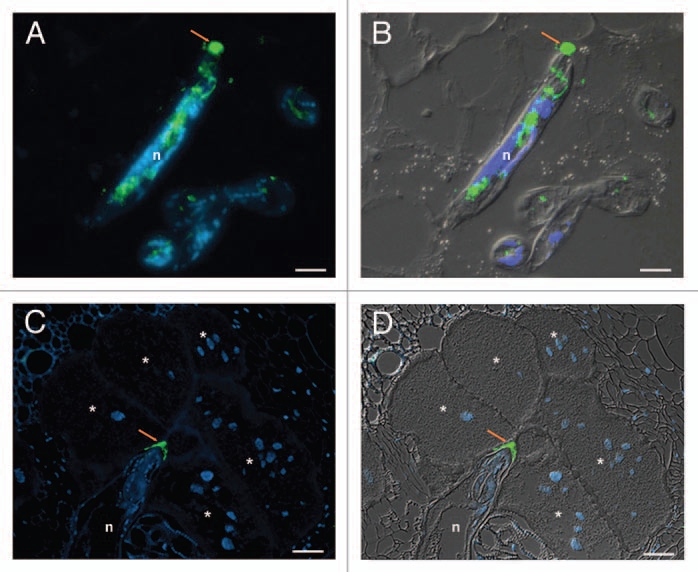
Immunodetection of Meloidogyne incognita secreted proteins during parasitism. (A and B) Localization of secreted CBM2-bearing proteins in the apoplasm (arrow) during nematode migration in Arabidopis thaliana root sections. (C and D) Localization of secreted MAP-1 in the apoplasm accumulating along the giant cell wall (arrow) by sedentary nematode in tomato (Solanum lycopersicum) root sections. Micrographs (A and C) are overlays of an Alexa-488 fluorescence (green) and DAPI-stained nuclei (blue). Micrographs (B and D) are overlays of an Alexa-488 fluorescence (green), DAPI-stained nuclei (blue) and differential interference contrast (grey). n, nematode. *, giant cells. Scale bars = 10 µm. Micrographs (A and B) are from Vieira et al. 2011.23
Similarly, we observed abundant and continuous secretion through the stylet of an aspartyl protease-like protein in the apoplasm at the forefront of migrating juveniles. The potential role of this new aspartyl protease-like protein as an effector was further supported by its phylogenetic position in a cluster clearly distinct from all other eukaryotes, including other nematodes, suggesting that it could have evolved independently towards a specific function related to plant parasitism. Enzymatic assays will be necessary to further unravel its role in infection.
A Meloidogyne candidate avirulence protein (MAP-1) produced in the amphids of pre-parasitic juveniles was identified in avirulent nematode lines that trigger Mi-1 resistance in tomato.11 At the time of its discovery, map-1 did not show any significant similarity in databases.11 However, based on the whole-genome sequence of the nematode,10 a wide phylogenetic analysis recently positioned M. incognita map-1 homologues into a cluster of genes encoding expansin-like proteins,12 thus confirming individual homologies found with related genes from other nematode species.13,14 Our immunolocalization study showed secretion of MAP-1 from the amphids of migrating juveniles, supporting a role of the protein during the early steps of infection. Whether this role is related to cell wall loosening is still to be determined.
Nematode Proteins are Secreted during Parasitism within the Nematode-Feeding Site
The formation of the nematode feeding site is determined by the molecular dialog between the nematode and its susceptible plant host. Our localization studies have demonstrated that two proteins, one produced by the amphids (MAP-1) and one secreted through the stylet (6D4), accumulate at the plant cell wall during giant cell formation and maintenance (Fig. 1C and D), suggesting that the intercellular space may be an important interface during nematode feeding site induction and maintenance. Our finding here suggests that MAP-1 is not only secreted in planta by migrating juveniles but could have an additional role during the initiation of the feeding site. The protein 6D4 was identified by screening a panel of antibodies that reacted with the secretory oesophageal glands of the nematode.15 Unfortunately, this protein could not be purified and its sequence is still unknown. Interestingly, we only observed residual secretion of CBM2-bearing enzymes and pectate lyase by sedentary nematodes, suggesting that these proteins have no role in the cell wall expansion and thickening associated with giant cell formation. Rather, the plant cell wall degrading enzymes that are induced during infection16,17 could be responsible for the cell wall modifications occurring in the already hypertrophied giant cells.
Discussion
Recent yeast two-hybrid screens have identified cytoplasmic proteins as potential plant targets for nematode secreted proteins (reviewed in ref. 4). However, our in planta observations of five different RKN proteins secreted from three distinct secretory organs of the nematode, i.e., amphids, subventral and dorsal glands, reveal that the plant apoplasm constitutes an important compartment where these proteins are delivered during distinct phases of parasitism. Although we cannot exclude that the tiny amount of secreted proteins or epitope modifications might prevent the detection of the proteins into the plant cells, we clearly demonstrate that these proteins accumulate in the plant apoplasm during migratory and sedentary phases of infection. Flourishing data on oomycete and fungus effectors demonstrate that the extracellular space is the first destination compartment for secreted effectors that are subsequently translocated into the cytoplasm of recipient plant cells.18 No such translocation system has been evidenced so far for nematodes and such a hypothesis would be conflicting with the stylet acting as a syringe able to directly inject nematode effectors within plant cells. However, a role for nematode effectors in the apoplasm of feeding cells has recently been proposed by Wang et al.19 who have shown the ability of a CLE-like peptide secreted from the cyst nematode Heterodera glycines to traffic outside plant cells from the cytoplasm. Once in the apoplasm, the nematode CLE-like peptides act as ligand mimics of plant CLE peptides and interact with receptors at the plasma membrane.20 Except for plant cell wall loosening during infection, no function has been described so far for RKN effectors in the apoplasm of plant cells. Yet, the observed accumulation of nematode secreted proteins in the apoplasm seems significant enough to be perceived by the plant. Indeed, the plant plasma membrane is a sensor for pathogen-associated molecular patterns (PAMPs) and effector proteins that are perceived by plant pattern-recognition receptors or resistance proteins (reviewed in ref. 21 and 22). As a consequence, accumulation of RKN secreted proteins in the apoplasm could affect the interplay between nematode effectors and plant responses.
References
- 1.Williamson V, Hussey RS. Nematode pathogenesis and resistance in plants. Plant Cell. 1996;8:1735–1745. doi: 10.1105/tpc.8.10.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sobczak M, Golinowski W, Grundler F. Ultrastructure of feeding plugs and feeding tubes formed by Heterodera schachtii. Nematology. 1999;1:363–374. [Google Scholar]
- 3.Perry R. Chemoreception in plant-parasitic nematode. Annu Rev Phytopathol. 1996;34:181–199. doi: 10.1146/annurev.phyto.34.1.181. [DOI] [PubMed] [Google Scholar]
- 4.Rosso M, Hussey R, Davis E, Smant G, Baum T, Abad P, et al. Nematode effector proteins: Targets and functions in plant parasitism. In: Martin F, Kamoun S, editors. Effectors in Plant-Microbe Interactions. Hoboken, NJ: John Wiley & Sons, Inc.; 2011. In Press. [Google Scholar]
- 5.Doyle E, Lambert K. Cloning and characterisation of an esophageal-gland specific pectate lyase from the root-knot nematode Meloidogyne javanica. Mol Plant Microbe Interact. 2002;15:549–556. doi: 10.1094/MPMI.2002.15.6.549. [DOI] [PubMed] [Google Scholar]
- 6.Curtis R. Identification and in situ and in vitro characterization of secreted proteins produced by plant-parasitic nematodes. Parasitology. 1996;113:589–597. doi: 10.1017/s0031182000067640. [DOI] [PubMed] [Google Scholar]
- 7.Lopez de Mendoza M, Abrantes I, Rowe J, Gowen S, Curtis R. Immunolocalisation in planta of secretions from parasitic stages of Meloidogyne incognita and M. hispanica. Int J Nematol. 2002;12:149–154. [Google Scholar]
- 8.Doyle E, Lambert K. Meloidogyne javanica chorismate mutase 1 alters plant cell development. Mol Plant Microbe Interact. 2003;16:123–131. doi: 10.1094/MPMI.2003.16.2.123. [DOI] [PubMed] [Google Scholar]
- 9.Jaubert S, Milac A, Petrescu J, de Almeida-Engler J, Abad P, Rosso M. In planta secretion of a calreticulin by migratory and sedentary stages of root-knot nematode. Mol Plant Microbe Interact. 2005;18:1277–1284. doi: 10.1094/MPMI-18-1277. [DOI] [PubMed] [Google Scholar]
- 10.Abad P, Gouzy J, Aury JM, Castagnone-Sereno P, Danchin EG, Deleury E, et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol. 2008;26:909–915. doi: 10.1038/nbt.1482. [DOI] [PubMed] [Google Scholar]
- 11.Semblat J, Rosso M, Hussey R, Abad P, Castagnone-Sereno P. Molecular cloning of a cDNA encoding an amphid-secreted putative avirulence protein from the root-knot nematode Meloidogyne incognita. Mol Plant Microbe Interact. 2001;14:72–79. doi: 10.1094/MPMI.2001.14.1.72. [DOI] [PubMed] [Google Scholar]
- 12.Danchin E, Rosso M, Vieira P, de Almeida-Engler J, Coutino P, Henrissat B, et al. Multiple lateral gene transfers and duplications have promoted plant parasitism ability in nematode. Proc Natl Acad Sci USA. 2010;107:17651–17656. doi: 10.1073/pnas.1008486107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roze E, Hanse B, Mitreva M, Vanholme B, Bakker J, Smant G. Mining the secretome of the root-knot nematode Meloidogyne chitwoodi for candidate parasitism genes. Mol Plant Pathol. 2008;9:1–10. doi: 10.1111/j.1364-3703.2007.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haegeman A, Kyndt T, Gheysen G. The role of pseudo-endoglucanases in the evolution of nematode cell wall-modifying proteins. J Mol Evol. 2010;70:441–452. doi: 10.1007/s00239-010-9343-1. [DOI] [PubMed] [Google Scholar]
- 15.Davis E, Aron L, Pratt L, Hussey R. Novel immunization procedures used to develop monoclonal antibodies that bind to specific structures in Meloidogyne spp. Phytopathology. 1992;82:1244–1250. [Google Scholar]
- 16.Jammes F, Lecomte P, de Almeida-Engler J, Bitton F, Martin-Magniette M, Renou J, et al. Genome-wide expression profiling of the host response to root-knot nematode infection in Arabidopsis. Plant J. 2005;44:447–458. doi: 10.1111/j.1365-313X.2005.02532.x. [DOI] [PubMed] [Google Scholar]
- 17.Barcala M, García A, Cabrera J, Casson S, Lindsey K, Favery B, et al. Early transcriptomic events in microdissected Arabidopsis nematode-induced giant cells. Plant J. 2010;61:698–712. doi: 10.1111/j.1365-313X.2009.04098.x. [DOI] [PubMed] [Google Scholar]
- 18.Dodds P, Rafiqi M, Gan P, Hardham A, Jones D, Ellis J. Effectors of biotrophic fungi and oomycetes: pathogenicity factors and triggers of host resistance. New Phytol. 2009;183:993–1000. doi: 10.1111/j.1469-8137.2009.02922.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Lee C, Replogle A, Joshi S, Korkin D, Hussey R, et al. Dual roles for the variable domain in protein trafficking and host-specific recognition of Heterodera glycines CLE effector protein. New Phytol. 2010;187:1003–1017. doi: 10.1111/j.1469-8137.2010.03300.x. [DOI] [PubMed] [Google Scholar]
- 20.Replogle A, Wang J, Bleckmann A, Hussey R, Baum T, Sawa S, et al. Nematode CLE signaling in Arabidopsis requires CLAVATA2 and CORYNE. Plant J. 2011;65:430–440. doi: 10.1111/j.1365-313X.2010.04433.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones J, Dangl J. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 22.Segonzac C, Zipfel C. Activation of plant pattern-recognition receptors by bacteria. Curr Opin Microbiol. 2011;14:54–61. doi: 10.1016/j.mib.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Vieira P, Danchin EG, Neveu C, Crozat C, Jaubert S, Hussey RS, et al. The plant apoplasm is an important recipient compartment for nematode secreted proteins. J Exp Bot. 2011;62:1241–1253. doi: 10.1093/jxb/erq352. [DOI] [PMC free article] [PubMed] [Google Scholar]


