Abstract
One of the major goals in ecology is to determine the mechanisms that drive the asymptotic increase in ecosystem productivity with plant species diversity. Niche complementarity, the current paradigm for the asymptotic diversity-productivity pattern, posits that the addition of species to a community increases productivity because each species specializes on different resources and thus can more thoroughly utilize the available resources. At higher diversity the increase in productivity decreases because resources become limiting, resulting in the classic asymptotic diversity-productivity pattern. An alternative but less tested explanation is that density-dependent disease from species-specific soil microbes drive the diversity-productivity relationship by increasing disease and thus decreasing productivity at low diversity. At higher diversity, productivity asymptotes because disease decreases with increasing diversity until it reaches a uniformly low level. Using a series of field experiments, we found that the classic asymptotic diversity-productivity pattern existed only when soil microbes were present. Soil microbes created the well-known pattern by depressing plant growth at low productivity though negative density dependent disease. In contrast, niche complementarity played only a weak role in explaining the diversity-productivity relationship because productivity remained high at low abundance in the absence of soil microbes. Based on our findings, the ongoing loss of species in natural ecosystems will likely increase per capita plant disease and lower ecosystem productivity. Furthermore, recent evidence suggests that negative density dependent disease maintains plant species diversity, and thus this single mechanism appears to link diversity maintenance to the diversity-productivity curve—two important ecological processes.
Key words: density dependence, diversity-productivity, negative feedback, pathogens, species richness, soil microbes
The asymptotically saturating increase in ecosystem productivity with increasing diversity is a well know pattern in nature1–4 (Fig. 1). The pattern has been used as an argument for the importance of species diversity,5 and understanding the mechanisms that drive the pattern is critical to determine the potential loss in productivity with ongoing and accelerating species loss in many ecosystems. The cause of the diversity-productivity pattern can be explained by either bottom-up control, such as plant resource competition, or top-down control from plant herbivores or pathogens. Most contemporary explanations for the pattern are centered on the bottom-up concept of niche-based resource competition, in which different species utilize different resources. The commonly accepted explanation, the niche complementarity hypothesis, states that the increase in species diversity increases productivity because each additional species uses a differ set of resources (e.g., nutrients) and thus more thoroughly utilizes whole-ecosystem resources.3,4,6 At high diversity, however, the resource requirements of additional species overlap with existing ones and thus productivity no longer increases with diversity, resulting in the asymptotic diversity-productivity pattern (Fig. 1).
Figure 1.
Theoretical relationship between species number and biomass. As diversity increases, total biomass increases asymptotically.
Top-down control from plant enemies may also produce the asymptotic diversity-productivity pattern if the enemies are species-specific and have a strong negative density-dependent effect at low diversity. One general group of enemies is plant pathogens and parasites (bacterial, fungal, viral) that live in the soil and infect plant roots (hereafter referred to as soil pathogens). The specificity of soil pathogens has been shown in various studies and is now generally accepted.1,7,8 The negative density dependent effect of plant pathogens at low diversity is likely because when diversity is low the relative abundance of each remaining species is high,9–11 which leads to most individuals growing in close proximity of conspecifics and thus a greater probability of species-specific disease transmission. Unlike other plant enemies, such as foliar pathogens or insect and mammalian herbivores, which can be broadly dispersed, soil-borne pathogens may be a particularly effective driver of negative density dependent effects because they have low mobility and thus are more likely to infect nearby conspecifics, which causes increased disease at low diversity.9–11 As diversity increases, the effect of soil-borne pathogens decreases because there is a lower likelihood of growing near a conspecific and there are lower concentrations of host-specific soil enemies.10 Consequently, soil-borne, species-specific disease may limit ecosystem productivity through top-down density-dependent regulation, even in the absence of niche-based explanations. Few studies, however, have considered the role of plant soil pathogens in driving the classic diversity-productivity relationship1 (see also ref. 2) and, until now, no study has compared the two potential drivers simultaneously.1
We used a modeling approach to first demonstrate that both niche complementarity and species-specific soil pathogens can both theoretically drive the well-known diversity-productivity pattern.1 We then used a series of complementary field experiments in grasslands in North America (Ontario, Canada and Minnesota, USA) to determine how plant disease and productivity change over a gradient of plant species richness in the presence and absence of soil microbes, and whether feedback between plants and their species-specific soil biota influenced the diversity-productivity pattern.1 We first tested whether the asymptotic diversity-ecosystem productivity relationship arose in the presence of soil pathogens (a test of the negative density dependence hypothesis) or in the absence of soil pathogens (a test of the niche complementarity hypothesis). We then confirmed that soil biota were species specific and examined the decrease in plant disease and increase in productivity with increasing plant diversity.
Soil Microbes, Not Niche Complementarity, Drive the Asymptotic Diversity-Productivity Pattern
Our results indicated that while both niche complementarity and the density dependent effects of soil microbes can theoretically produce the classic asymptotic diversity-productivity pattern, only soilborne microbes produced this pattern.1 When soil microbes were present, plant productivity increased asymptotically and was around 500% higher in the high diversity communities than in monocultures (circle and triangle symbols in Fig. 2). The asymptotic diversity-productivity pattern was driven primarily by the strong reduction in productivity at low plant diversity, where soil-borne disease was high, and the effects of soil microbes decreased with increasing plant diversity. We quantified the percentage of root infection by pathogenic fungi and confirmed that it was significantly lower in the high diversity plant communities compared to the monocultures. Furthermore, we confirmed that pathogens were species-specific and that their negative density dependent effect decreased with increasing diversity.1
Figure 2.
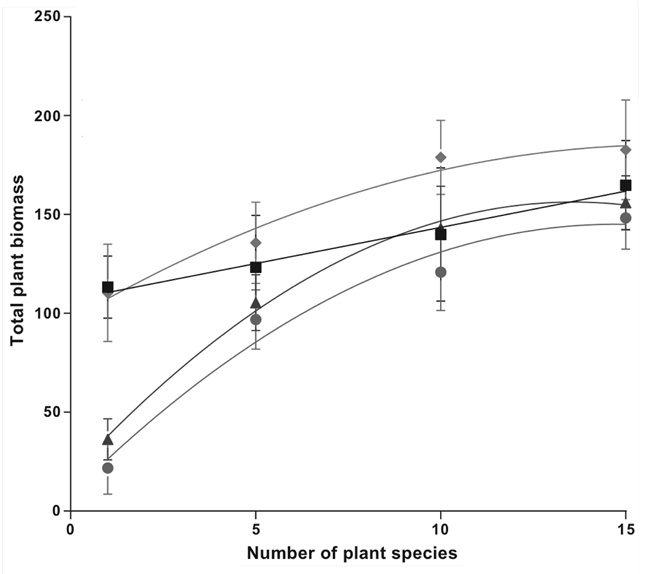
Relationship between plant diversity and productivity (in grams) in four experimental soil types. Sterile (squares), sterilized field soil; Sterile + AMF (diamonds), sterilized field soil + field-collected AMF spores; Sterile + pathogens/parasites/saprobes (circles), sterilized field soil + microbial fraction that excludes AMF (<20 microns); and Field Soil (triangles), untreated field soil. Productivity varied significantly with diversity (ANOVA F3,64 = 523.2; p = 0.0001), soil treatment (F3,64 = 66.7; p = 0.0001), and the interaction (F9,64 = 12.4; p = 0.0001). Lines represent the best fit with linear and polynomial functions (AICsterile = 0.04; AICsterile + AMF = 2.97; AICsterile + pathogens = 9.86; AICfield soil = 9.91). Error bars represent ±1 SE. Figure reprinted from Schnitzer et al.1
In contrast, in sterilized soils where soil microbes were absent, productivity remained relatively high in the low diversity treatment and we found only a weak, positive linear relationship between diversity and productivity (square symbols in Fig. 2). In a recent paper, Maron et al. also found a proportionally large increase in productivity in low diversity plant communities compared to high diversity communities after excluding fungal pathogens. Thus, our study and that of Maron et al. both indicate that density dependent disease from soil microbes drives the classic asymptotic diversity-productivity curve, and that niche complementarity plays a subordinate role. By not considering the role of soil pathogens, previous studies appear to have overestimated the role of niche complementarity as the main driver of the asymptotic diversity-productivity relationship.
Implications: The Importance of Species Diversity
Our findings confirm that plant species diversity is important for ecosystem functioning, but for reasons not previously documented. High plant species diversity limits the accumulation and transmission of host-specific soil enemies, thereby reducing the negative impacts of disease on ecosystem productivity. When diversity decreases, however, the density and frequency of the remaining species increases, and each remaining individual has a higher probability of being near a conspecific, resulting in a higher probability of infection. The loss of species remains a serious threat to native habitats, and both Schnitzer et al.1 and Maron et al. demonstrate that species diversity is important because it limits the prevalence and spread of species-specific pathogens, allowing plants to carry less disease and thus maintain productivity.
The current trend of species loss in plant communities may have additional community- and ecosystem-level ramifications, such as rendering plant communities vulnerable to invasion from non-native and weedy species.12–14 Because of their relatively small size and reduced competitive ability, diseased individuals may be more susceptible to being displaced by invasive species. Invasive species, in turn, may have a significant advantage over diseased native species because invasive species may have escaped their species-specific pathogens,15 and thus their population densities are not regulated by negative density dependence.
Linking Diversity-Productivity to the Maintenance of Species Diversity
The finding that the negative density dependent effect of soil enemies can drive the diversity-productivity relationship provides a strong link to another major concept in ecology—the maintenance of species diversity. Host-specific enemies have long been hypothesized to maintain species diversity in many ecosystems.16,17 More recently, soil pathogens have been found to be a major determinant of the maintenance of species diversity in tropical forests8 and in grasslands.13 To date, however, both the diversity-productivity relationship and the maintenance of species diversity via density dependent effects of host-specific enemies and have been treated separately. Our findings provide evidence that these two important ecological processes may both be driven largely by the same mechanism: the negative density dependent effects of species-specific soil pathogens.1
References
- 1.Schnitzer SA, Klironomos J, Hille Ris Lambers J, Kinkel LL, Reich PB, Xiao K, et al. Soil-borne microbes drive the classic asymptotic diversity-productivity pattern. Ecology. 2011;92:296–303. doi: 10.1890/10-0773.1. [DOI] [PubMed] [Google Scholar]
- 2.Maron J, Marler M, Klironomos J, Cleveland C. Soil fungal pathogens and the relationship between plant diversity and productivity. Ecol Lett. 2011;14:36–41. doi: 10.1111/j.1461-0248.2010.01547.x. [DOI] [PubMed] [Google Scholar]
- 3.Tilman D, Naeem S, Knops JMH, Reich PB, Siemann E, Wedin D, et al. Biodiversity and ecosystem properties. Science. 1997;278:1866–1867. [Google Scholar]
- 4.Tilman D, Reich PB, Knops JMH, Wedin D, Mielke T, Lehman C. Diversity and productivity in a long-term grassland experiment. Science. 2001;294:843–845. doi: 10.1126/science.1060391. [DOI] [PubMed] [Google Scholar]
- 5.Naeem S, Thompson LJ, Lawler SP, Lawton JH, Woodfin RM. Declining biodiversity can alter the performance of ecosystems. Nature. 1994;368:734–737. [Google Scholar]
- 6.Fargione J, Tilman D, Dybzinski R, Hille Ris Lambers J, Clark C, Harpole WS, et al. From selection to complementarity: shifts in the causes of biodiversity-productivity relationships in a long-term biodiversity experiment. Proc Roy Soc B. 2007;274:871–876. doi: 10.1098/rspb.2006.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Packer A, Clay K. Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature. 2000;440:278–281. doi: 10.1038/35005072. [DOI] [PubMed] [Google Scholar]
- 8.Mangan SA, Schnitzer SA, Herre EA, Mack K, Valencia M, Sanchez E, Bever JD. Negative feedback predicts relative species abundance in a tropical forest. Nature. 2007;466:752–756. doi: 10.1038/nature09273. [DOI] [PubMed] [Google Scholar]
- 9.Knops JMH, Tilman D, Haddad NM, Naeem S, Mitchell CE, Haarstad J, et al. Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundances and diversity. Ecol Lett. 1999;2:286–293. doi: 10.1046/j.1461-0248.1999.00083.x. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell CE, Tilman D, Groth JV. Effects of grassland plant species diversity, abundance and composition on foliar fungal disease. Ecology. 2002;83:1713–1726. [Google Scholar]
- 11.Mitchell CE. Trophic control of grassland production and biomass by pathogens. Ecol Lett. 2003;6:147–155. [Google Scholar]
- 12.Kennedy T, Naeem S, Howe K, Knops JMH, Tilman D, Reich PB. Biodiversity as a barrier to ecological invasion. Nature. 2002;417:636–638. doi: 10.1038/nature00776. [DOI] [PubMed] [Google Scholar]
- 13.Klironomos JN. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature. 2002;417:67–70. doi: 10.1038/417067a. [DOI] [PubMed] [Google Scholar]
- 14.Callaway RM, Thelen GC, Rodriguez A, Holben WE. Soil biota and exotic plant invasion. Nature. 2004;427:731–733. doi: 10.1038/nature02322. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell CE, Power AG. Release of invasive plants from fungal and viral pathogens. Nature. 2003;421:625–627. doi: 10.1038/nature01317. [DOI] [PubMed] [Google Scholar]
- 16.Janzen DH. Herbivores and the number of tree species in tropical forests. Am Nat. 1970;104:501–528. [Google Scholar]
- 17.Carson WP, Anderson J, Leigh EG, Schnitzer SA. Challenges associated with testing and falsifying the Janzen-Connell hypothesis: A review and critique. In: Carson WP, Schnitzer SA, editors. Tropical Forest Community Ecology. Oxford: Wiley-Blackwell Publishing; 2008. pp. 210–241. [Google Scholar]


