Abstract
Arabidopsis mutant hps1 that over-accumulates sucrose has enhanced sensitivity in almost all the aspects of plant responses to phosphate starvation. The detailed characterization of hps1 has led to the conclusion that sucrose is a global regulator of plant phosphate responses. Here, we show that hps1 is also hypersensitive to nitrogen and potassium deprivation, as well as to decreased levels of overall macronutrients. These results suggest that sucrose regulates plant deficiency responses to multiple nutrients and is part of a general response to nutrient deprivation.
Key words: nutrient deficiency, plant response, hps1, sucrose, signaling
Plants often encounter nutrient deficiency in their surrounding environments,1 and because they are sessile, plants have to develop sophisticated strategies to cope with nutritional stress. The strategies include an array of biochemical, physiological, and developmental responses.2–5 Interactions among the different nutrients have also been documented.6,7 For example, ammonium interacts with potassium (K) for uptake,8 and nitrate transporters were downregulated in K-deprived plants.9 Phosphate (P) deficiency induces expression of the K transporter gene HAK5 and causes high accumulation of iron.7,10,11 The intimate cross talk among the different nutrients suggests the existence of some common signaling components that are involved in regulating plant responses to different nutrient stresses. However, the molecular identity of these signaling components remains unknown.
Previously, we reported the characterization of an Arabidopsis mutant hps1.10 In hps1, a sucrose transporter gene, SUC2, is overexpressed, resulting in enhanced uptake of sucrose from the culture medium. As a consequence, hps1 accumulates a high level of sucrose in both its shoot and root tissues. hps1 is hypersensitive in almost all the aspects of plant responses to P starvation. In contrast, the suc2 knockout mutant displays opposite phenotypes. We also showed that a high level of sucrose in hps1 can directly induce most P starvation-responsive genes even under P sufficient condition. Under normal growth condition, hps1 contains less chlorophyll, probably due to the feedback inhibition of photosynthesis by the high level of sucrose. Combined with the results from our genomic research and early work by other groups,10,12 we conclude that sucrose is a global regulator of plant responses to P starvation. In this study we determine if sucrose is also involved in responses to other nutrients and thus is part of a general response mechanism to nutrient deprivation.
We first tested whether hps1 also shows hypersensitivity to nitrogen (N) and K deprivation. The seeds of the WT and hps1 were directly sown on MS medium in which sucrose was replaced by glucose to ensure the same growth status. Five days after seed germination, the seedling were transferred to sucrose-containing MS medium without supplementation of N, P or K, respectively, and grow for another 5 or 12 days. Compared to the WT, P-starved hps1 had smaller size of shoots and accumulated more anthocyanin (Fig. 1A and B); this was consistent with our previous findings.10 Similarly, hps1 showed enhanced sensitivity to N and K deprivation in term of primary root growth (Fig. 1A). N starved-hps1 plants also accumulated more anthocyanins than N starved-WT plants (Fig. 1B). However, there was no significant difference of the shoot size between the WT and hps1 on N and K deficiency medium.
Figure 1.
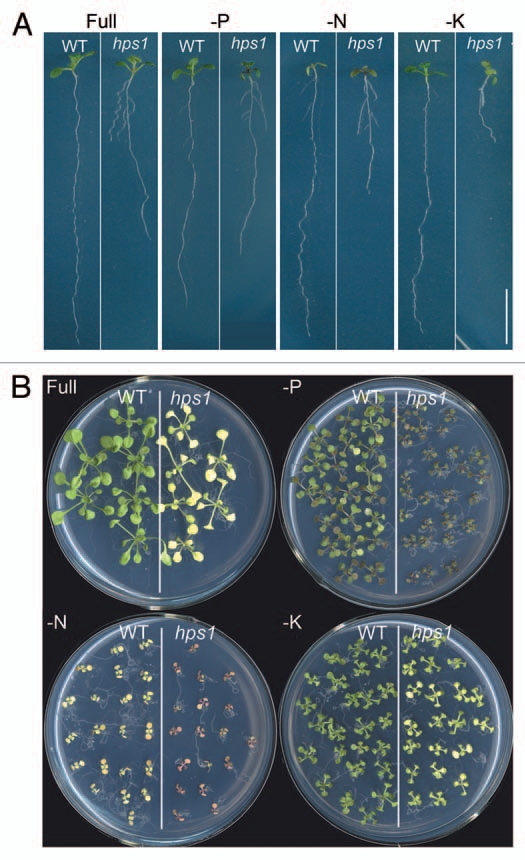
The hps1 mutant is hypersensitive to N, P and K deprivation. Five-day-old seedlings of the WT and hps1 mutant grown on glucose-containing MS medium were transferred to sucrose-containing MS medium with or without supplemented phosphate, nitrogen or potassium. The seedlings were photographed 5 days (A) and 12 days (B) after transfer. Bar = 10 mm.
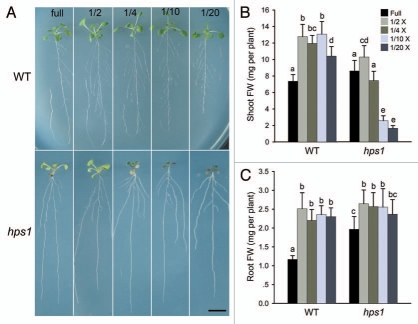
To further determine whether hps1 is hypersensitive to a general decrease in nutrient supply, we examined the responses of hps1 grown on sucrose-containing MS medium with different levels of nutrients. When grown on full-strength MS medium, shoot size and shoot fresh weight were similar for the WT and hps1 (Fig. 2). Decreasing the macronutrient concentration to 1/2 strength significantly enhanced the shoot growth of both the WT and hps1. Reducing the macro-nutrient concentration to 1/10 had no further obvious effect on the WT, but it dramatically inhibited the shoot growth of hps1 (Fig. 2A and B). Ten days after seed germination, the shoot fresh weight of hps1 grown on 1/20 MS medium was only about 15% of that for the WT. Regardless of the strength of the MS media, no accumulation of anthocyanin could be visually detected for the WT. For the hps1 mutant, however, anthocyanin accumulation was evident even on 1/4 MS medium, and the accumulation increased as the macronutrient level was further decreased (Fig. 2A). Interestingly, the hypersensitive responses of hps1 to decreased macronutrient concentration only occurred in shoot growth, but not in root growth (Fig. 2A and C).
Figure 2.
The hps1 mutant is hypersensitive to a decreased level of macronutrients. Five-day-old seedlings of the WT and hps1 mutant grown on glucose-containing MS medium were transferred to sucrose-containing MS medium with different strength of macronutrients. After transfer, the seedlings were grown for another 10 days. (A) Photographs of the WT and hps1 seedlings after growth on the MS medium with different nutrient strength. (B and C): Shoot and root fresh weight (FW) of the WT and hps1 seedlings shown in (A). Data represents means ± SE (n > 10). Values with different letters differ significantly (p < 0.01). Bar = 10 mm.
Conclusions
Our previous work demonstrated that the hps1 mutant is hypersensitive to P deficiency. In this work, we further extended our observations that hps1 is also hypersensitive to N and K deprivation, as well as to decreased levels of overall macronutrients. These results suggest that sucrose mediates plant responses to deficiencies in multiple nutrients and thus is an important component of general responses to nutrient deprivation.
Acknowledgments
We thank Dr. Nigel Crawford (University of California at San Diego) for his valuable comments on this manuscript. This work was supported by funds from the National Natural Science Foundation of China (grant no. 31071060), the Ministry of Science and Technology of China (grant no. 2007CB948200) and the Ministry of Agriculture of China (grant no. 2009ZX08009-123B).
References
- 1.Marschner H. Mineral Nutrition of Higher Plants. San Diego: Academic; 1995. [Google Scholar]
- 2.Scheible WR, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, et al. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiology. 2004;136:2483–2499. doi: 10.1104/pp.104.047019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amtmann A, Hammond JP, Armengaud P, White PJ. Nutrient sensing and singaling in plants: Potassium and phosphorus. Adv Bot Res. 2006;43:209–257. [Google Scholar]
- 4.Schachtman DP, Shin R. Nutrient sensing and signaling: NPKS. Annu Rev Plant Biol. 2007;58:47–69. doi: 10.1146/annurev.arplant.58.032806.103750. [DOI] [PubMed] [Google Scholar]
- 5.Yuan H, Liu D. Signaling components involved in plant responses to phosphate starvation. J Integr Plant Biol. 2008;50:849–859. doi: 10.1111/j.1744-7909.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- 6.de Groot CC, Marcelis LFM, van den Boogaard R, Kaiser WM, Lambers H. Interaction of nitrogen and phosphorus nutrition in determining growth. Plant and Soil. 2003;248:257–268. [Google Scholar]
- 7.Zheng LQ, Huang FL, Narsai R, Wu JJ, Giraud E, He F, et al. Physiological and Transcriptome Analysis of Iron and Phosphorus Interaction in Rice Seedlings. Plant Physiol. 2009;151:262–274. doi: 10.1104/pp.109.141051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santa-Maria GE, Danna CH, Czibener C. High-affinity potassium transport in barley roots. Ammonium-sensitive and -insensitive pathways. Plant Physiol. 2000;123:297–306. doi: 10.1104/pp.123.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armengaud P, Breitling R, Amtmann A. The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol. 2004;136:2556–2576. doi: 10.1104/pp.104.046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei M, Liu Y, Zhang B, Zhao Y, Wang X, Zhou Y, et al. Genetic and genomic evidence that sucrose is a global regulator of plant responses to phosphate starvation in Arabidopsis. Plant Physiol. 2011;156:1116–1130. doi: 10.1104/pp.110.171736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward JT, Lahner B, Yakubova E, Salt DE, Raghothama KG. The effect of iron on the primary root elongation of Arabidopsis during phosphate deficiency. Plant Physiol. 2008;147:1181–1191. doi: 10.1104/pp.108.118562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammond JP, White PJ. Sucrose transport in the phloem: integrating root responses to phosphorus starvation. J Exp Bot. 2008;59:93–109. doi: 10.1093/jxb/erm221. [DOI] [PubMed] [Google Scholar]