Abstract
Apelin, the ligand of the G protein–coupled receptor APJ, is involved in the regulation of cardiovascular functions, fluid homeostasis, and vessel formation. Recent reports indicate that apelin secreted from endothelial cells mediates APJ regulation of blood vessel caliber size; however, the function of apelin in lymphatic vessels is unclear. Here we report that APJ was expressed by human lymphatic endothelial cells and that apelin induced migration and cord formation of lymphatic endothelial cells dose-dependently in vitro. Furthermore, permeability assays demonstrated that apelin stabilizes lymphatic endothelial cells. In vivo, transgenic mice harboring apelin under the control of keratin 14 (K14-apelin) exhibited attenuated UVB-induced edema and a decreased number of CD11b-positive macrophages. Moreover, activation of apelin/APJ signaling inhibited UVB-induced enlargement of lymphatic and blood vessels. Finally, K14-apelin mice blocked the hyperpermeability of lymphatic vessels in inflamed skin. These results indicate that apelin plays a functional role in the stabilization of lymphatic vessels in inflamed tissues and that apelin might be a suitable target for prevention of UVB-induced inflammation.
The lymphatic vascular system is composed of a dense network of thin-walled capillaries that drain protein-rich lymph from the extracellular space; its function is important for homeostasis of the circulatory and immune systems, maintenance of interstitial fluid composition and volume, and immune cell trafficking in health and in disease.1, 2 Chronic skin inflammation in mice has been associated with lymphatic endothelial cell (LEC) proliferation, and the skin disease psoriasis exhibited pronounced cutaneous lymphatic hyperplasia,3 indicating that the lymphatic vascular system participates in both acute and chronic inflammation.
Acute exposure of skin to UVB irradiation (290 to 320 nm) leads to inflammation associated with epidermal hyperplasia, erythema, vascular hyperpermeability, and edema formation.4, 5 Previous studies have demonstrated that acute UVB irradiation of both human and mouse skin promotes marked angiogenesis.6, 7 Several angiogenesis factors, including vascular endothelial growth factor-A (VEGF-A), basic fibroblast growth factor, and interleukin-8, were up-regulated in skin after UVB-irradiation.7, 8, 9 Thrombospondin-1, a potent endogenous angiogenesis inhibitor, was up-regulated.7 Moreover, targeted overexpression of VEGF-A enhanced sensitivity to UVB-induced cutaneous photodamage,10 but transgenic overexpression of thrombospondin-1 in the epidermis completely prevented UVB-induced photodamage.11 Taken together, these findings indicate that the cutaneous blood vasculature plays an important role in the mediation of photodamage. A previous study from our research group demonstrated that UVB irradiation caused enlargement of lymphatic vessels with leaky and hyperpermeable function.12 More recently, we found that activation of the VEGF-C/VEGFR-3 pathway attenuates UVB-induced inflammation by promoting lymphangiogenesis.13 These studies point to a crucial role of lymphatic vessels in UVB-induced inflammation.
Apelin is an endogenous ligand for the previously orphan G protein–coupled receptor, APJ. The apelin gene (APLN), which is located on the long arm of the human X chromosome, encodes a 77-amino-acid preproprotein that is then cleaved to shorter active peptides.14, 15 The full-length mature peptide, which was originally isolated from bovine stomach extracts, comprises 36 amino acids and is known as apelin-36; the short-length peptide is known as apelin-13. Both peptides activate APJ.16 APJ expression has been reported in the cardiovascular system and in the central nervous system.17, 18 In the brain, the apelin/APJ system plays a role in maintaining body fluid homeostasis and regulating release of vasopressin from the hypothalamus.19 In the cardiovascular system, APJ is expressed in endothelial cells, vascular smooth muscle cells, and cardiomyocytes.20, 21 Apelin/APJ in cells of endothelial lineage promotes hypotensive activity22; the activation of APJ leads to nitric oxide (NO) production by the endothelial cells,23 and this possibly plays a role in the relaxation of smooth muscle cells.
Apelin is also essential for blood vessel formation. The apelin/APJ system plays a role in the cardiovascular system of Xenopus laevis24 and of zebrafish.25 Xenopus apelin (Xapelin) is expressed in the region around the presumptive blood vessels during early embryogenesis as Xenopus APJ (Xmsr). Knockdown of Xapelin or Xmsr resulted in a defect of blood vessel formation in the posterior cardinal vein, intersomitic vessels, and vitelline vessels. The regulation of blood vessel formation by apelin in mammals has been described recently. The Apelin/APJ system was shown to be involved in downstream signaling of Ang1/Tie2 in endothelial cells and in regulation of blood vessel diameter during angiogenesis.26 However, the function of apelin in lymphatic vessels and its role in inflammation is not completely clear.
In the present study, we found that the APJ receptor is expressed in lymphatic endothelial cells in vitro and in vivo, and that apelin/APJ signaling promotes stabilization of lymphatic vessels. Moreover, using apelin transgenic mice, we demonstrated that apelin attenuates UVB-induced inflammation by promoting stabilization of lymphatic and blood vessels. These results suggest that apelin might be a suitable target for prevention of UVB-induced skin inflammation and photodamage.
Materials and Methods
Cells
Human dermal LECs were isolated from neonatal human foreskins by immunomagnetic purification, as described previously.27 The lineage-specific differentiation was confirmed by real-time RT-PCR for the lymphatic vascular markers Prox1, LYVE-1, and podoplanin, as well as by immunostaining for Prox1 and podoplanin, as described previously.28 Human umbilical vein endothelial cells (HUVECs) were purchased from PromoCell (Heidelberg, Germany). Cells were cultured in endothelial basal medium (Lonza, Verviers, Belgium) supplemented with supplements provided by the suppliers for up to 11 passages.
Immunoblotting
For Western blot analyses of APJ, Akt, and p-Akt, confluent LECs and HUVECs were homogenized in lysis buffer, and protein concentrations were determined using a DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). Equal amounts of lysates (10 μg protein) were immunoblotted with a rabbit polyclonal antibody against APJ, as described previously.26 LECs were also cultured with apelin-36 (1000 ng/mL; Peptide Institute, Osaka, Japan) for 2 minutes, followed by homogenization in lysis buffer. Untreated cells were prepared as controls in the same manner. Cell lysates (100 μg total protein each) were immunoprecipitated with antibodies against p-Akt and Akt (Cell Signaling Technology, Danvers, MA). Equal loading was confirmed with an antibody against β-actin (Sigma-Aldrich, St. Louis, MO).
Migration and Cord Formation Assays
The LEC migration assay was performed as described previously,29 using 24-well FluoroBlock inserts of 8-μm pore size (Falcon; BD Biosciences, Franklin Lakes, NJ). The bottom sides of the inserts were coated with 10 μg/mL fibronectin (BD Biosciences, Bedford, MA) for 1 hour, followed by incubation with 100 μg/mL of bovine serum albumin. Cells (105 cells in 100 μL) were seeded in serum-free endothelial basal medium into the upper chambers, and were incubated for 5 hours at 37°C in the presence or absence of human recombinant apelin-13 (500 to 1000 ng/mL) or apelin-36 (500 to 1000 ng/mL). Cells on the underside of inserts were stained with Hoechst dye 33342 (Molecular Probes; Invitrogen, Carlsbad, CA). Five different digital images were captured per well, and the number of migrated cells was counted. Cord formation assays were performed as described previously.30 LECs were grown on fibronectin-coated 24-well plates until confluence. In all, 0.5 mL of neutralized isotonic bovine dermal collagen type I (Vitrogen; Celtrix Laboratories, Palo Alto, CA) in the presence or absence of apelin-13 (50 to 1000 ng/mL) or apelin-36 (50 to 1000 ng/mL) was added to the cells. After incubation at 37°C for 24 hours, cells were fixed with 4% paraformaldehyde for 30 minutes at 4°C. Before lymphatic endothelial cells form tubes in collagen gels, endothelial cells connect with each other to make cords in vitro. Representative images were captured, and the total length of cordlike structures per area was measured using IP-LAB software version 4.0. All studies were performed in triplicate. Statistical analyses were performed using the unpaired Student's t-test.
Permeability Assay
LECs were grown into confluence on the fibronectin-coated surface of tissue culture inserts of 0.4-μm pore size (Transwell; Corning, Lowell, MA) and then in serum-free endothelial basal medium for 24 hours. Apelin-13 (500 to 1000 ng/mL) was placed into the upper and lower chambers for 6 hours. Fluorescein isothiocyanate-dextran was added to the upper chambers, and the apparatus was then placed in a CO2 incubator at 37°C. After incubation for 15 minutes, a 100-µL sample was taken from the lower chamber, and the absorbance of fluorescein isothiocyanate-dextran was determined at 492 nm using a spectrophotometer (Fluoroskan Ascent; Thermo Fisher Scientific, Waltham, MA).
UVB Irradiation Regimen
Transgenic mice harboring apelin under the control of keratin 14 (K14-apelin) were generated on a C57BL/6 background, as described previously.31 A total of 10 K14-apelin mice and wild-type (WT) mice, 12 weeks old (n = 5/group) were exposed to a single dose of 200 mJ/cm2 of UVB irradiation using 10 Toshiba FL-20 SD fluorescent lamps that deliver energy in the UVB wavelength range (280 to 340 nm) with maximum energy at a wavelength of 305 nm. On day 3 or 4 after UVB irradiation, mouse ears were collected and were processed for histological analysis of frozen sections. Control mice without UVB irradiation were also analyzed. All procedures including UVB irradiation were performed under anesthesia. The study was approved by the ethics committee of Shiseido Research Center in accordance with guidelines of the U.S. National Institutes of Health (7th edition).
Intravital Lymphangiography and Plasma Extravasation
WT and K14-apelin mice (n = 5/group) were anesthetized with avertin (0.4 g/kg; Sigma-Aldrich), and 1 mL of a 1% solution of Evans Blue dye in 0.9% NaCl was injected intradermally at the inner surface of the rim of the ear, using a 10-mL Hamilton syringe, to visualize the lymphatic vessels. Mouse ears were photographed at 1 and 5 minutes after dye injection. To determine blood vascular permeability, a Miles assay was performed as described previously.31 Briefly, mice were anesthetized and intravenously injected with 100 µL of a 1% solution of Evans Blue dye in 0.9% NaCl. At 60 minutes after dye injection, ears were photographed and then removed. The dye was eluted from the dissected samples with formamide at 56°C, and the optical density was measured by spectrophotometry (Biotrak II; GE Healthcare, Piscataway, NJ) at 620 nm.
Immunostaining and Computer-Assisted Morphometric Vessel Analysis
Immunofluorescence analysis was performed on cryostat sections (6 μm thick) of mouse ears using antibodies against the macrophage monocyte marker CD11b (Pharmingen; BD Biosciences, San Diego, CA), the blood vessel-specific marker Meca-32 (BD Biosciences), and the lymphatic-specific marker podoplanin (Acris Antibodies, Hiddenhausen, Germany) and using corresponding secondary antibodies labeled with Alexa Fluor 488 or Alexa Fluor 594 (Molecular Probes; Invitrogen). Routine H&E staining was also performed. Sections were examined with an Olympus AX80T microscope (Olympus, Tokyo, Japan), and images were captured with a DP controller digital camera (DP71; Olympus). Morphometric analyses were performed using IP-LAB software version 4.0, as described previously.28 Three different fields of each section were examined, and ear thickness and the number of macrophages and average vessel size in the dermis were determined. Statistical analyses were performed using the unpaired Student's t-test.
Results
Apelin Is Expressed by Lymphatic Vessels both in Vitro and in Vivo and Promotes Lymphatic Function
To investigate whether apelin functions in lymphatic endothelial cells, we analyzed the expression of apelin receptor APJ in LECs. Western blot analyses demonstrated that APJ was expressed by both LECs and HUVECs (Figure 1A). Moreover, immunofluorescence analysis of mouse ear skin using antibodies against APJ and the blood vessel marker Meca-32 or the lymphatic marker podoplanin revealed that APJ was expressed by both lymphatic vessels (Figure 1C) and blood vessels (Figure 1B) in vivo. Apelin is known to activate the phosphorylation of Akt in HUVECs.32 Treatment of LECs with 1000 ng/mL apelin-36 resulted in the increased phosphorylation of Akt, compared with untreated cells (Figure 1D). Migration assays performed to further characterize the effects of apelin on LEC revealed that both apelin-13 and apelin-36 induced LEC migration in a dose-dependent manner (Figure 1, E and F). To investigate whether apelin stimulation might promote cord formation of lymphatic endothelial cells in vitro, confluent LECs were overlaid with type I collagen. Cord formation of LECs was clearly enhanced in the presence of apelin-13 and apelin-36, compared with control cells (Figure 1, G–I). Morphometric analyses confirmed that both apelin-13 and apelin-36 induced cord formation of LECs dose-dependently (P < 0.01) (Figure 1, J and K). A permeability assay was performed to determine whether apelin contributes to the stabilization of LECs in vitro. LECs were cultured on Transwell culture inserts into confluence, and the concentration of fluorescein isothiocyanate-dextran that permeated across the culture inserts was measured with or without apelin-13. The addition of 500 or 1000 ng/mL of apelin-13 in LECs decreased the fluorescence intensity of permeated fluorescein isothiocyanate-dextran, indicating that apelin promoted the stabilization of LECs (P < 0.01) (Figure 1L).
Figure 1.
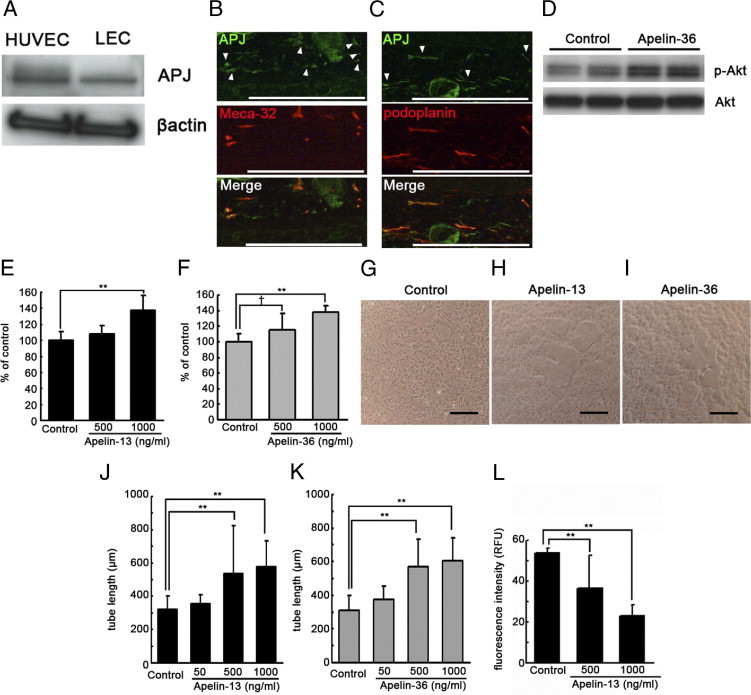
Apelin promotes formation and stabilization of lymphatic endothelial cells (LECs) in vitro. A: Immunoblot analyses confirmed that the apelin receptor APJ was expressed by both LECs and HUVECs. B and C: Double immunofluorescence analyses for APJ (green) and blood vessel marker Meca-32 (red, B) or lymphatic vessel marker podoplanin (red, C) revealed that APJ was expressed by both blood vessels and lymphatic vessels in vivo. Arrowheads show APJ expression in blood vessels (B) and lymphatic vessels (C). D: Treatment of LECs with 1000 ng/mL apelin-36 resulted in increased phosphorylation of Akt, compared with untreated cells. E and F: Apelin-13 (E) and apelin-36 (F) induced migration of LECs in a dose-dependent manner, compared with untreated control cells. G–I: Incubation of LECs with 500 ng/mL or 1000 ng/mL apelin-13 (H and J) and apelin-36 (I and K) enhanced cord formation in a dose-dependent manner after overlay with a type I collagen gel, compared with controls (G). Scale bars: 100 μm. L: The addition of 500 or 1000 ng/mL of apelin-13 decreased the fluorescence intensity of permeated fluorescein isothiocyanate-dextran from LEC, compared with controls. Data are expressed as means ± SD. **P < 0.01; †P < 0.1.
Apelin Enhances Recovery from UVB-Induced Edema Formation and Inflammation
To determine the functional role of apelin in the cutaneous vasculature in vivo, transgenic mice harboring apelin under the control of keratin-14 (K14-apelin) and WT control mice were exposed to 200 mJ cm−2 of UVB irradiation. H&E staining of skin sections at 3 days after UVB irradiation revealed characteristic features of acute photodamage in the ear skin of WT mice, including epidermal hyperplasia and edema formation in the dermis (Figure 2C). Of note, K14-apelin mouse ears irradiated with UVB were closely similar to those of non-UVB-irradiated skin (Figure 2, A, B, and D). In a physiological condition, by contrast, no obvious difference was found between WT and K14-apelin mice. The measurement of skin thickness confirmed that ear swelling was decreased in K14-apelin mouse ears, compared with WT mouse ears, after UVB irradiation (P < 0.05) (Figure 2I). Immunohistochemical staining for a monocyte macrophage marker, CD11b, demonstrated an increased number of infiltrating macrophages in the dermis of WT ears after UVB irradiation, compared with non-UVB-irradiated skin (Figure 2, E–G); however, the ear skin of UVB-irradiated K14-apelin mice exhibited decreased macrophage infiltration in the dermis (Figure 2H). Morphometric analyses confirmed a decreased number of infiltrating macrophages in the dermis of UVB-irradiated K14-apelin mouse ears, compared with WT mice after UVB-irradiation (P < 0.01) (Figure 2J).
Figure 2.
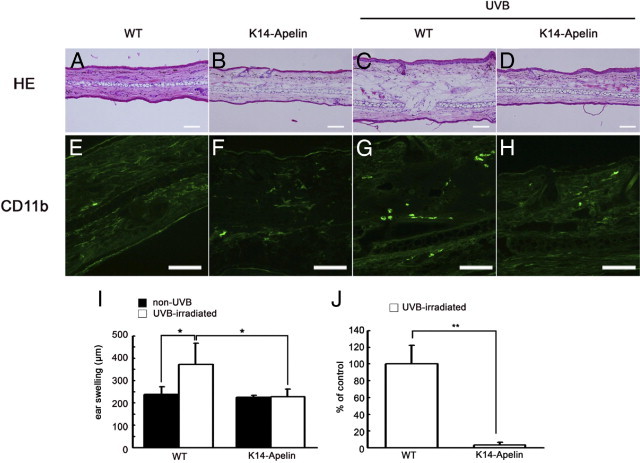
Apelin attenuates UVB-induced edema formation and inflammation. A–D: H&E staining revealed marked edema formation in the dermis of WT mouse ear skin (C), but K14-apelin mouse ear skin irradiated with UVB (D) was similar to non-UVB-irradiated skin (A and B). Scale bars: 100 μm. E–H: Immunofluorescence for CD11b (green) showed decreased macrophage infiltration in the dermis of K14-apelin mice ears (H), similar to non-UVB-irradiated mice (E and F), compared with WT mice irradiated with UVB (G). Scale bars: 100 μm. I: Skin thickness analysis indicated ear swelling in WT mice after UVB irradiation (*P < 0.05), but this swelling was attenuated in K14-apelin mice. *P < 0.05. J: The number of CD11b-positive cells was decreased in K14-apelin mice after UVB irradiation, compared with UVB-irradiated WT mice. *P < 0.01. Morphometric analyses (I and J) were performed using IP-LAB software version 4.0. Data are expressed as mean values ± SD (n = 3).
Activation of Apelin/APJ Pathway Inhibits UVB-Induced Inflammation by Blocking Abnormal Enlargement of Lymphatic Vessels and Blood Vessels
To investigate how activation of apelin/APJ signaling attenuates edema formation and inflammation induced by UVB irradiation, we analyzed cutaneous lymphatic vessels after UVB irradiation. To visualize lymphatic vessels, Evans Blue dye was injected intradermally into the rim of mouse ears. At 1 and 5 minutes after injection, Evans Blue dye had extravasated from lymphatic vessels in UVB-irradiated WT skin, but such leakage was attenuated in K14-apelin mice (Figure 3, A–D). Next, Miles assay was performed to determine the effects of apelin on blood vessels. UVB exposure induced marked leakage of Evans Blue dye in WT mice (Figure 3E), but such leakage was attenuated in K14-apelin mice (Figure 3F). Quantitative analysis demonstrated that the increase of dye leakage in WT mouse ears was significantly blocked in UVB-irradiated K14-apelin mice (Figure 3G).
Figure 3.
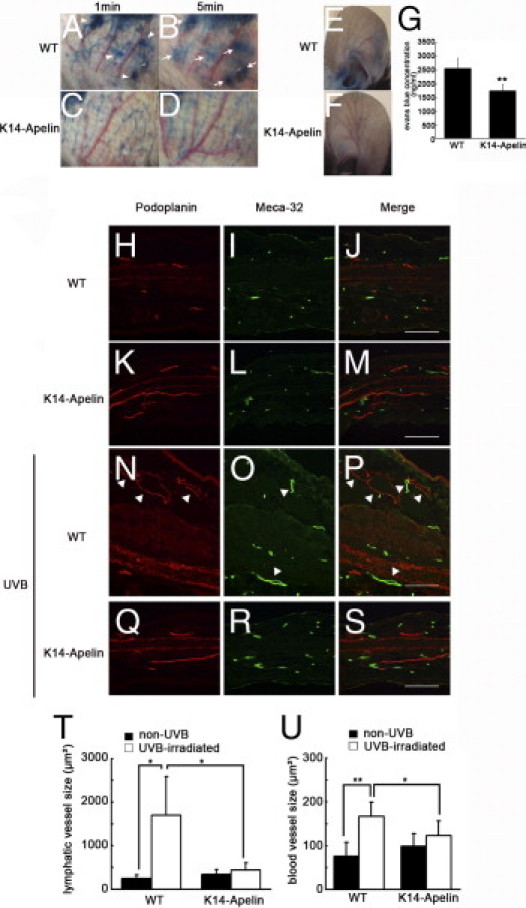
Activation of apelin/APJ signaling inhibited UVB-induced inflammation by blocking enlargement of lymphatic vessels and blood vessels. A–D: Evans Blue dye was injected intradermally into the rim of mouse ears. After 5 minutes, Evans Blue dye had extravasated from lymphatic vessels in UVB-irradiated WT mouse skin (A, arrowheads; B, arrows), but such leakage was attenuated in K14-apelin mice after UVB (C and D). E–G: Miles assay revealed that UVB exposure induced vascular hyperpermeability of WT mice (E), but this effect was markedly inhibited in K14-apelin mice (F). G: Quantitative analysis demonstrated increased Evans Blue leakage in the ear of UVB-irradiated WT mice, compared with UVB-irradiated K14-apelin mice. *P < 0.01. H–S: Double immunofluorescence staining of ear sections for podoplanin (red) and Meca-32 (green) revealed enlargement (arrowheads) of podoplanin-positive lymphatic vessels and Meca-32-positive blood vessels in UVB-irradiated WT mice (N–P), compared with nonirradiated mice (H–M). This enlargement of lymphatic vessels and blood vessels after UVB irradiation was blocked in K14-apelin mice (Q–S). Scale bars: 100 μm. T and U: According to morphometric analyses using IP-LAB software version 4.0, the average size of lymphatic vessels (T) and blood vessels (U) was significantly reduced in K14-apelin mice after UVB irradiation (*P < 0.05). In contrast, size of lymphatic vessels and blood vessels was increased in WT mice irradiated with UVB. *P < 0.05; *P < 0.01.
We performed double immunofluorescence staining for the lymphatic marker podoplanin and the blood vascular marker Meca-32. In a physiological condition, the density of blood vessels was similar between WT and K14-apelin mice, but K14-apelin mice exhibited increased size of blood vessels, as has been demonstrated previously.31 No great difference in lymphatic vessel formation was immediately evident in K14-apelin mice; however, precise histological examination of skin stained for podoplanin revealed enlarged lymphatic vessels of K14-apelin mice, compared with WT mice (Figure 3, H–M). Enlargement of lymphatic vessels and blood vessels was induced in WT mice after UVB irradiation; surprisingly, however, in K14-apelin mice the UVB-induced enlargement of lymphatic and blood vessels was inhibited (Figure 3, N–S). Morphometric analyses of sections demonstrated that the average size of lymphatic vessels and blood vessels was significantly decreased in skin of UVB-irradiated K14-apelin mice, compared with WT mice after UVB-irradiation (−74%, P < 0.05 for lymphatic vessels; −26%, P < 0.05 for blood vessels; Figure 3, T and U).
Discussion
Apelin has been recently reported to be an important regulator of blood vessel formation. The present study reveals, for the first time, that the apelin receptor APJ is expressed by human lymphatic endothelial cells and that apelin/APJ signaling plays a crucial role in UVB-induced inflammation through stabilization of blood and lymphatic vessels.
Acute photodamage of the skin is characterized by epidermal hyperplasia, erythema, and edema formation. Edema is caused by accumulation of extracellular fluid due to excess leakage from hyperpermeable blood vessels33 and by a failure of lymphatic vessels to sufficiently drain the fluid from the interstitium.12 Moreover, the dysfunction of lymphatic vessels also results in reduced clearance of macrophages from the tissue via lymphatic drainage,34 suggesting that the function of lymphatic vessels is profoundly related to the process of UVB-induced inflammation. We have previously reported that skin tissues during days 2 to 4 after UVB irradiation exhibit enlargement of lymphatic vessels and macrophage infiltration.13 Surprisingly, K14-apelin mice inhibited the enlargement and hyperpermeability of lymphatic vessels and macrophage infiltration by UVB irradiation, indicating that apelin plays a defensive role in UVB-induced inflammation.
How does apelin attenuate skin inflammation? Although the function of the apelin/APJ system in endothelial cells is known to activate endothelial nitric oxide synthase (eNOS), resulting in decreased the blood pressure with vasodilation,22 the present results indicate that apelin attenuates the abnormal enlargement of lymphatic and blood vessels in inflamed skin. Additionally, plasma extravasation was markedly decreased in K14-apelin mice, compared with WT mice after UVB irradiation, indicating a protective role of apelin in blood vessels, as described recently.31 Moreover, we have previously demonstrated that systemic blockade of lymphatic function by the VEGFR-3 pathway prolongs UVB-induced edema formation and inflammation,35 whereas intradermal injection of VEGF-C accelerates the resolution of UVB-induced edema and inflammation by inducing lymphangiogenesis.13 In contrast to such VEGF-C treatment, no major differences in the number of lymphatic vessels were observed in skin of K14-apelin mice. It was therefore of considerable interest to see the differential mechanism of attenuating inflammation by apelin. A previous study from our research group demonstrated that acute UVB irradiation increases overextension of lymphatic vessels, which leads to impaired fluid transport and so contributes to prolonged edema formation.12 Of note, the hyperpermeability of lymphatic vessels was blocked in K14-apelin mice after UVB irradiation, compared with that observed in UVB-irradiated WT mice, and the permeability assay in vitro demonstrated that apelin blocked the permeability of human lymphatic endothelial cells. Taken together, these data suggest that inhibiting hyperpermeability by enhancing apelin expression could facilitate transport of tissue fluid, resulting in rapid resolution of edema and the related inflammation induced by UVB.
The molecular events that regulate blood vessel formation, especially the caliber size determination of blood vessels by apelin, have been recently suggested. A remarkable study showed that the apelin/APJ system is involved in downstream signaling of Ang1/Tie2 in blood vessel formation.26 With the present study, we have demonstrated that apelin induces migration and cord formation of LECs and that lymphatic vascular size in K14-apelin mice is greater than in WT mice. Given that apelin induces expression of the junctional proteins claudin-5 and vascular endothelial cadherin (VE-cadherin) in blood vessels, resulting in abundant cell-to-cell contact and regulation of endothelial cell assembly, it is possible that apelin inhibits hyperpermeability of lymphatic vessels and inflammation by UVB-irradiation via the regulation of the junctional protein in lymphatic endothelial cells.26 Further studies would be needed to clarify a molecular regulation of lymphatic integrity by apelin and to determine whether apelin is involved in downstream Ang1/Tie2 signaling in lymphatic vessels.
In summary, the present results indicate that apelin plays a functional role in the stabilization of lymphatic vessels in inflamed tissues. Apelin might be a new suitable target for prevention of UVB-induced skin inflammation.
Acknowledgment
We thank Fumika Miyohashi for her technical assistance.
Footnotes
M.S. and H.K. contributed equally to the present work.
References
- 1.Cueni L.N., Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126:2167–2177. doi: 10.1038/sj.jid.5700464. [DOI] [PubMed] [Google Scholar]
- 2.Tammela T., Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 3.Kunstfeld R., Hirakawa S., Hong Y.K., Schacht V., Lange-Asschenfeldt B., Velasco P., Lin C., Fiebiger E., Wei X., Wu Y., Hicklin D., Bohlen P., Detmar M. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–1057. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- 4.Kligman A.M. The treatment of photoaged human skin by topical tretinoin. Drugs. 1989;38:1–8. doi: 10.2165/00003495-198938010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Kripke M.L. Ultraviolet radiation and immunology: something new under the sun—presidential address. Cancer Res. 1994;54:6102–6105. [PubMed] [Google Scholar]
- 6.Yano K., Kadoya K., Kajiya K., Hong Y.K., Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol. 2005;152:115–121. doi: 10.1111/j.1365-2133.2005.06368.x. [DOI] [PubMed] [Google Scholar]
- 7.Yano K., Kajiya K., Ishiwata M., Hong Y.K., Miyakawa T., Detmar M. Ultraviolet B-induced skin angiogenesis is associated with a switch in the balance of vascular endothelial growth factor and thrombospondin-1 expression. J Invest Dermatol. 2004;122:201–208. doi: 10.1046/j.0022-202X.2003.22101.x. [DOI] [PubMed] [Google Scholar]
- 8.Krämer M., Sachsenmaier C., Herrlich P., Rahmsdorf H.J. UV irradiation-induced interleukin-1 and basic fibroblast growth factor synthesis and release mediate part of the UV response. J Biol Chem. 1993;268:6734–6741. [PubMed] [Google Scholar]
- 9.Strickland I., Rhodes L.E., Flanagan B.F., Friedmann P.S. TNF-alpha and IL-8 are upregulated in the epidermis of normal human skin after UVB exposure: correlation with neutrophil accumulation and E-selectin expression. J Invest Dermatol. 1997;108:763–768. doi: 10.1111/1523-1747.ep12292156. [DOI] [PubMed] [Google Scholar]
- 10.Hirakawa S., Fujii S., Kajiya K., Yano K., Detmar M. Vascular endothelial growth factor promotes sensitivity to ultraviolet B-induced cutaneous photodamage. Blood. 2005;105:2392–2399. doi: 10.1182/blood-2004-06-2435. [DOI] [PubMed] [Google Scholar]
- 11.Yano K., Oura H., Detmar M. Targeted overexpression of the angiogenesis inhibitor thrombospondin-1 in the epidermis of transgenic mice prevents ultraviolet-B-induced angiogenesis and cutaneous photo-damage. J Invest Dermatol. 2002;118:800–805. doi: 10.1046/j.1523-1747.2002.01752.x. [DOI] [PubMed] [Google Scholar]
- 12.Kajiya K., Hirakawa S., Detmar M. Vascular endothelial growth factor-a mediates ultraviolet B-induced impairment of lymphatic vessel function. Am J Pathol. 2006;169:1496–1503. doi: 10.2353/ajpath.2006.060197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kajiya K., Sawane M., Huggenberger R., Detmar M. Activation of the VEGFR-3 pathway by VEGF-C attenuates UVB-induced edema formation and skin inflammation by promoting lymphangiogenesis. J Invest Dermatol. 2009;129:1292–1298. doi: 10.1038/jid.2008.351. [DOI] [PubMed] [Google Scholar]
- 14.Hosoya M., Kawamata Y., Fukusumi S., Fujii R., Habata Y., Hinuma S., Kitada C., Honda S., Kurokawa T., Onda H., Nishimura O., Fujino M. Molecular and functional characteristics of APJ: Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem. 2000;275:21061–21067. doi: 10.1074/jbc.M908417199. [DOI] [PubMed] [Google Scholar]
- 15.Tatemoto K., Hosoya M., Habata Y., Fujii R., Kakegawa T., Zou M.X., Kawamata Y., Fukusumi S., Hinuma S., Kitada C., Kurokawa T., Onda H., Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 16.Masri B., Knibiehler B., Audigier Y. Apelin signalling: a promising pathway from cloning to pharmacology. Cell Signal. 2005;17:415–426. doi: 10.1016/j.cellsig.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Devic E., Rizzoti K., Bodin S., Knibiehler B., Audigier Y. Amino acid sequence and embryonic expression of msr/apj, the mouse homolog of Xenopus X-msr and human APJ. Mech Dev. 1999;84:199–203. doi: 10.1016/s0925-4773(99)00081-7. [DOI] [PubMed] [Google Scholar]
- 18.O'Dowd B.F., Heiber M., Chan A., Heng H.H., Tsui L.C., Kennedy J.L., Shi X., Petronis A., George S.R., Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–360. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- 19.De Mota N., Reaux-Le Goazigo A., El Messari S., Chartrel N., Roesch D., Dujardin C., Kordon C., Vaudry H., Moos F., Llorens-Cortes C. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc Natl Acad Sci USA. 2004;101:10464–10469. doi: 10.1073/pnas.0403518101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devic E., Paquereau L., Vernier P., Knibiehler B., Audigier Y. Expression of a new G protein-coupled receptor X-msr is associated with an endothelial lineage in Xenopus laevis. Mech Dev. 1996;59:129–140. doi: 10.1016/0925-4773(96)00585-0. [DOI] [PubMed] [Google Scholar]
- 21.Katugampola S.D., Maguire J.J., Matthewson S.R., Davenport A.P. [(125)I]-(Pyr(1))Apelin-13 is a novel radioligand for localizing the APJ orphan receptor in human and rat tissues with evidence for a vasoconstrictor role in man. Br J Pharmacol. 2001;132:1255–1260. doi: 10.1038/sj.bjp.0703939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishida J., Hashimoto T., Hashimoto Y., Nishiwaki S., Iguchi T., Harada S., Sugaya T., Matsuzaki H., Yamamoto R., Shiota N., Okunishi H., Kihara M., Umemura S., Sugiyama F., Yagami K., Kasuya Y., Mochizuki N., Fukamizu A. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem. 2004;279:26274–26279. doi: 10.1074/jbc.M404149200. [DOI] [PubMed] [Google Scholar]
- 23.Tatemoto K., Takayama K., Zou M.X., Kumaki I., Zhang W., Kumano K., Fujimiya M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept. 2001;99:87–92. doi: 10.1016/s0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- 24.Cox C.M., D'Agostino S.L., Miller M.K., Heimark R.L., Krieg P.A. Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev Biol. 2006;296:177–189. doi: 10.1016/j.ydbio.2006.04.452. [DOI] [PubMed] [Google Scholar]
- 25.Scott I.C., Masri B., D'Amico L.A., Jin S.W., Jungblut B., Wehman A.M., Baier H., Audigier Y., Stainier D.Y. The G protein-coupled receptor Agtrl1b regulates early development of myocardial progenitors. Dev Cell. 2007;12:403–413. doi: 10.1016/j.devcel.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Kidoya H., Ueno M., Yamada Y., Mochizuki N., Nakata M., Yano T., Fujii R., Takakura N. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. 2008;27:522–534. doi: 10.1038/sj.emboj.7601982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirakawa S., Hong Y.K., Harvey N., Schacht V., Matsuda K., Libermann T., Detmar M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162:575–586. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kajiya K., Hirakawa S., Ma B., Drinnenberg I., Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong Y.K., Lange-Asschenfeldt B., Velasco P., Hirakawa S., Kunstfeld R., Brown L.F., Bohlen P., Senger D.R., Detmar M. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J. 2004;18:1111–1113. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- 30.Kajiya K., Huggenberger R., Drinnenberg I., Ma B., Detmar M. Nitric oxide mediates lymphatic vessel activation via soluble guanylate cyclase alpha1beta1: impact on inflammation. FASEB J. 2008;22:530–537. doi: 10.1096/fj.07-8873com. [DOI] [PubMed] [Google Scholar]
- 31.Kidoya H., Naito H., Takakura N. Apelin induces enlarged and nonleaky blood vessels for functional recovery from ischemia. Blood. 2010;115:3166–3174. doi: 10.1182/blood-2009-07-232306. [DOI] [PubMed] [Google Scholar]
- 32.Masri B., Morin N., Pedebernade L., Knibiehler B., Audigier Y. The apelin receptor is coupled to Gi1 or Gi2 protein and is differentially desensitized by apelin fragments. J Biol Chem. 2006;281:18317–18326. doi: 10.1074/jbc.M600606200. [DOI] [PubMed] [Google Scholar]
- 33.Persson C.G. Role of plasma exudation in asthmatic airways. Lancet. 1986;2:1126–1129. doi: 10.1016/s0140-6736(86)90533-7. [DOI] [PubMed] [Google Scholar]
- 34.Kataru R.P., Jung K., Jang C., Yang H., Schwendener R.A., Baik J.E., Han S.H., Alitalo K., Koh G.Y. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113:5650–5659. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- 35.Kajiya K., Detmar M. An important role of lymphatic vessels in the control of UVB-induced edema formation and inflammation. J Invest Dermatol. 2006;126:919–921. doi: 10.1038/sj.jid.5700126. [DOI] [PubMed] [Google Scholar]


