HMGN1 is a novel alarmin that signals through TLR4 and is required for LPS-induced immune responses in vivo.
Abstract
Alarmins are endogenous mediators capable of promoting the recruitment and activation of antigen-presenting cells (APCs), including dendritic cells (DCs), that can potentially alert host defense against danger signals. However, the relevance of alarmins to the induction of adaptive immune responses remains to be demonstrated. In this study, we report the identification of HMGN1 (high-mobility group nucleosome-binding protein 1) as a novel alarmin and demonstrate that it contributes to the induction of antigen-specific immune responses. HMGN1 induced DC maturation via TLR4 (Toll-like receptor 4), recruitment of APCs at sites of injection, and activation of NF-κB and multiple mitogen-activated protein kinases in DCs. HMGN1 promoted antigen-specific immune response upon co-administration with antigens, and Hmgn1−/− mice developed greatly reduced antigen-specific antibody and T cell responses when immunized with antigens in the presence of lipopolysaccharide (LPS). The impaired ability of Hmgn1−/− mice to mount antigen-specific immune responses was accompanied by both deficient DC recruitment at sites of immunization and reduced production of inflammatory cytokines. Bone marrow chimera experiments revealed that HMGN1 derived from nonleukocytes was critical for the induction of antigen-specific antibody and T cell responses. Thus, extracellular HMGN1 acts as a novel alarmin critical for LPS-induced development of innate and adaptive immune responses.
Alarmins are structurally diverse endogenous mediators that can induce both recruitment and activation of APCs, such as DCs and monocytes/macrophages (Oppenheim and Yang, 2005; Bianchi, 2007; Yang et al., 2009). Alarmins are predominantly produced in peripheral tissues by infiltrating leukocytes or epithelial cells in response to tissue damage and microbial attack. All alarmins identified so far, such as defensins, cathelicidins, eosinophil-derived neurotoxin, granulysin, and HMGB1 (high-mobility group box 1) protein, have been shown to enhance inflammation, antimicrobial defense, adaptive immunity, and wound healing (Wang et al., 1999; Rovere-Querini et al., 2004; Kurosaka et al., 2005; Bianchi, 2007; Straino et al., 2008; Yang et al., 2008, 2009; Chen et al., 2009; Tewary et al., 2010). However, it remains to be shown that any alarmin is crucial for the induction of antigen-specific immune response.
HMGB1 and HMGN1 (high-mobility group nucleosome-binding protein 1) are members of the HMG superfamily of nonhistone chromatin-binding proteins (Bianchi and Agresti, 2005). HMG superfamily proteins are classified into three (HMGA, HMGB, and HMGN) subfamilies, each of which contains several members, such as HMGB1–3, HMGN1–4, etc. (Hock et al., 2007). The expression of HMG proteins is developmentally regulated. In adults, HMGB1 is highly expressed in all cell types, whereas other HMG proteins are more selectively expressed (Hock et al., 2007). For example, HMGN1 is highly expressed in proliferative tissues that undergo constant turnover, such as stem cells and some epithelial cells (Mohamed et al., 2001; Bianchi and Agresti, 2005; Furusawa et al., 2006; Hock et al., 2007). Intranuclear HMGs are major regulators of chromosome architecture and gene transcription (Calogero et al., 1999; Birger et al., 2003; Bianchi and Agresti, 2005; Hock et al., 2007). Over the past decade, HMGB1 has been shown to have multiple extracellular activities such as mediating diverse inflammatory reactions (Wang et al., 1999, 2004; Tian et al., 2007; Chen et al., 2009), induction of activation and migration of many cell types (Wang et al., 1999; Messmer et al., 2004; Rovere-Querini et al., 2004; Yang et al., 2007), promotion of wound healing (Straino et al., 2008), and acting as an alarmin (Wang et al., 1999; Messmer et al., 2004; Rovere-Querini et al., 2004; Bianchi, 2007; Yang et al., 2007, 2009; Urbonaviciute et al., 2008; Chen et al., 2009). However, it is unknown whether members of the HMGN subfamily have any extracellular alarmin activities.
In this study, we demonstrate that HMGN1 has extracellular alarmin activity and plays an important role in LPS-induced innate and adaptive immune responses. HMGN1 induces phenotypic and functional maturation of DCs in a TLR4 (Toll-like receptor 4)-, MyD88 (myeloid differentiation primary response gene 88)-, and TRIF (TIR domain–containing adaptor protein inducing IFN-β)-dependent manner. The contribution of HMGN1 to immunity was demonstrated by two complementary approaches: (1) exogenous HMGN1 enhanced antigen-specific immune responses upon administration with the antigen, and (2) HMGN1−/− mice manifested greatly reduced antigen-specific humoral and cellular immune responses in comparison with littermate-matched HMGN1+/+ mice. The reduction in immune responses in HMGN1−/− mice in comparison with HMGN1+/+ mice was accompanied by greatly decreased recruitment of leukocytes including APCs and reduced production of proinflammatory cytokines in the serum of immunized mice. Therefore, HMGN1 acts as an alarmin and plays a critical role in LPS-induced development of innate and adaptive immune responses.
RESULTS
HMGN1 promotes DC maturation
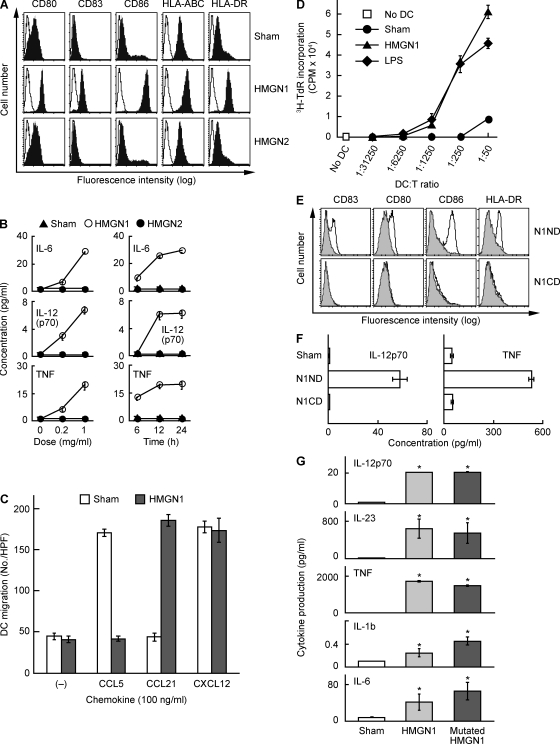
To determine whether members of the HMGN subfamily have any alarmin activities, we first investigated whether recombinant human HMGN1 or HMGN2 could induce DC maturation. DC maturation has three phenotypic hallmarks including up-regulation of certain surface marker expression, induction of proinflammatory cytokines, and acquisition of functional CCR7 (Banchereau and Steinman, 1998). Human monocyte-derived DCs (MoDCs) incubated with 1 µg/ml of recombinant HMGN1 for 48 h up-regulated the expression of co-stimulatory (CD80 and CD86), MHC (class I and II), and CD83 (a marker of DC maturation) molecules (Fig. 1 A). HMGN1 stimulated DC production of a variety of proinflammatory cytokines (IL-6, IL-8, TNF, and IL-12p70) in a dose- and time-dependent manner (Fig. 1 B). Additionally, DCs incubated with HMGN1 for 24 h down-regulated their capacity to migrate to CCL5 and up-regulated their capacity to migrate to CCL21, a ligand for CCR7 (Fig. 1 C). In contrast, DCs similarly treated with recombinant HMGN2 did not up-regulate surface co-stimulatory/MHC molecules (Fig. 1 A) or produce any of the tested cytokines (Fig. 1 B), nor did they become responsive to CCL21 (not depicted). Therefore, HMGN1 but not HMGN2 exhibited the capacity to induce phenotypic maturation of human MoDCs.
Figure 1.
HMGN1 induces phenotypic and functional maturation of DCs. (A) Human MoDCs were incubated at 5 × 105/ml in the absence (sham) or presence of recombinant HMGN1 or HMGN2 (both at a final concentration of 1 µg/ml) for 48 h before they were immunostained and analyzed by flow cytometry for the expression of the indicated surface molecules (open area, isotype-matched control). Shown are the results of one experiment representative of five. (B) Human MoDCs were incubated at 5 × 105/ml in the absence (sham) or presence of recombinant HMGN1 or HMGN2 at the indicated doses for 24 h (left) or at 1 µg/ml for the indicated times (right), and cytokine levels in the culture supernatants were quantitated by cytokine array (mean ± SD; n = 3). (C) Human MoDCs cultured at 1 × 106/ml in the absence (sham) or presence of 1 µg/ml of recombinant HMGN1 for 24 h were suspended in chemotaxis medium, and their migration in response to 100 ng/ml CCL5, CCL21, or CXCL12 was measured by chemotaxis assay. The migration of DCs was enumerated by the mean (±SD) of six high-power fields (HPF) randomly chosen from triplicate wells. Shown are the results of one experiment representative of two. (D) Human MoDCs cultured in the absence or presence of 1 µg/ml HMGN1 or LPS for 48 h were irradiated at 3,000 rad and incubated in triplicate with allogeneic human peripheral blood T cells (105/well) in 0.2 ml of medium in wells of 96-well flat-bottomed plates at the indicated DC/T ratios for 5 d. The proliferation was measured as the mean (±SD) [3H]TdR incorporation of triplicate wells. Shown are the results of one experiment representative of three. (E and F) Human MoDCs were incubated at 1 × 106/ml in the absence (sham) or presence of synthetic HMGN1 N-terminal domain (N1ND, HMGN11–52) or C-terminal domain (N1CD, HMGN153–100) at 10 µg/ml for 48 h before measurement of DC surface marker expression by flow cytometry (E, gray area: sham-treated DCs) and cytokine production in the supernatants (F; mean ± SD; n = 3). (G) Human DCs were incubated at 5 × 105/ml in medium in the absence (sham) or presence of 1 mg/ml HMGN1 or mutated HMGN1 for 24 h before obtaining supernatants for measurement of the indicated cytokines. Shown is the mean (±SD) of triplicate wells of one experiment representative of two. *, P < 0.05 by Student’s t test when compared with the sham treatment.
To determine whether HMGN1-induced maturation of DCs was reflected at the functional level, HMGN1-treated DCs were analyzed for their capacity to stimulate the proliferation of allogeneic T lymphocytes in a mixed lymphocyte reaction using sham- and LPS-treated DCs as negative and positive controls, respectively (Fig. 1 D). Sham-treated DCs did not stimulate [3H]TdR incorporation by allogeneic T cells. However, DCs treated with HMGN1 stimulated [3H]TdR incorporation by allogeneic T cells when used at a DC/T ratio higher than 1:1,250, indicating that HMGN1-treated DCs exhibited remarkably enhanced capacity to present antigen to T cells for their activation. Thus, HMGN1 is capable of inducing both phenotypic (Fig. 1, A–C) and functional (Fig. 1 D) maturation of DCs.
HMGN1 is composed of two major domains, an N-terminal nucleosomal binding domain (NBD) and a C-terminal chromatin-unfolding domain (Hock et al., 2007). To determine which domain plays a more important role in its DC-activating effect, we synthesized two peptides that correspond to the N-terminal (N1ND = HMGN11–52) and C-terminal (N1CD = HMGN153–100) half of HMGN1 and investigated the effect of the two peptides on DC maturation. N1ND containing the NBD of HMGN1 up-regulated the expression by DCs of surface markers (Fig. 1 E) and their production of IL-12p70 and TNF (Fig. 1 F), whereas N1CD containing the chromatin-unfolding domain of HMGN1 failed to do so when tested at identical concentrations (Fig. 1, E and F), suggesting that the NBD accounts for the DC-activating effect of HMGN1.
Because HMGN1 is a nucleosomal binding protein that regulates the structure of chromatin and gene transcription (Bianchi and Agresti, 2005; Hock et al., 2007), the DC-activating activity of HMGN1 could be caused by its chromatin-binding activity. To rule out this possibility, we compared the cytokine-inducing capacity of native HMGN1 with that of HMGN1[S20, 24E], a mutated HMGN1 that does not bind to nucleosomes (Prymakowska-Bosak et al., 2001). Interestingly, both the native and mutant HMGN1 proteins induced similar up-regulation of CD83, CD80, CD86, HLA-ABC, and HLA-DR on the surface of DCs (Fig. S1) and the production of comparable levels of various cytokines by DCs (Fig. 1 G), indicating that the capacity of HMGN1 to induce DC activation is independent of its chromatin-binding capability.
HMGN1 induces the recruitment of DCs
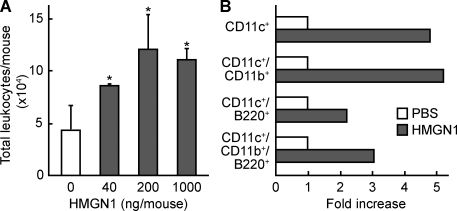
Another feature of alarmins is their capacity to recruit APCs (Oppenheim and Yang, 2005; Yang et al., 2009). HMGN1 injected into the peritoneal cavity of C57BL/6 mice by 4 h increased the total number of leukocytes infiltrating the peritoneal cavity in a dose-dependent manner (Fig. 2 A). The leukocyte subsets present in the peritoneal cavity of mice 4 h after i.p. injection of HMGN1 (1 µg/mouse) were analyzed by flow cytometry. HMGN1 caused a two- to fivefold increase in various types of mouse DCs, including myeloid (CD11c+/CD11b+) and plasmacytoid (CD11c+/B220+) DCs (Fig. 2 B). Therefore, in addition to activating DCs, HMGN1 can also recruit DCs in vivo, indicating that extracellular HMGN1 has the characteristics of an alarmin.
Figure 2.
HMGN1 induction of leukocyte recruitment. (A) 8-wk-old female C57BL/6 mice (five mice/group) were injected i.p. with PBS alone or PBS containing the indicated amount of HMGN1. After 4 h, leukocytes in the peritoneal cavity were enumerated. Shown is the mean (±SD) number of leukocytes. *, P < 0.05 by ANOVA when compared with mice injected with PBS alone. Shown are the results of one experiment representative of three. (B) Two groups of female C57BL/6 mice (10 wk old; n = 5) were injected i.p. with PBS alone or PBS containing 1 µg HMGN1. After 4 h, leukocytes were harvested from the peritoneum and immunostained with either isotype-matched control antibodies or a combination of PE-conjugated anti–mouse CD11c, FITC-conjugated anti–mouse CD11b, and APC-conjugated anti–mouse B220. The indicated subpopulations of DCs were determined by flow cytometry and calculated as fold increase induced by HMGN1 over the PBS group. Shown are the results of one experiment representative of two.
HMGN1 activates a signal transduction pathway that relies on MyD88 and TLR4
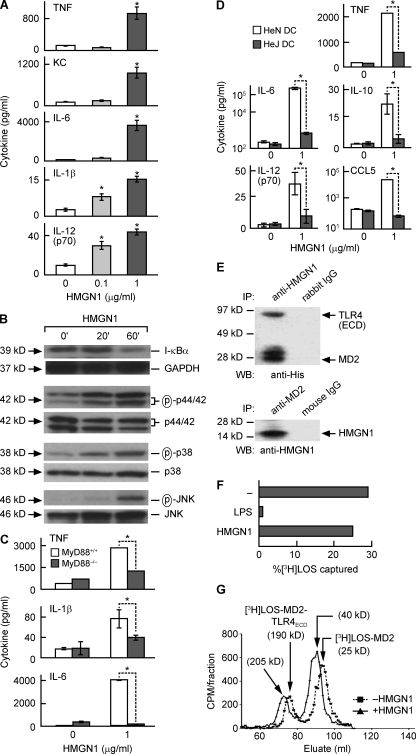
The capacity of HMGN1 to initiate signal transduction in DCs was investigated using DCs generated from mouse bone marrow progenitors. HMGN1 dose-dependently stimulated the production of various cytokines and chemokines by mouse DCs (Fig. 3 A). HMGN1 treatment resulted in a decrease in I-κBα and increase in the phosphorylated forms of p44/42, p38, and JNK in DCs in a time-dependent manner, suggesting that HMGN1 activated DC NF-κB and multiple mitogen-activated protein kinases (MAPKs) in DCs (Fig. 3 B). Interestingly, HMGN1-stimulated production of TNF, IL-1β, and IL-6 was markedly reduced in MyD88−/− DCs in comparison with MyD88+/+ DCs, indicating that HMGN1 induction of DC activation is MyD88 dependent (Fig. 3 C).
Figure 3.
HMGN1 activation of DCs is dependent on TLR4 and MyD88. (A) DCs generated from bone marrow progenitors of C57BL/6 mice were incubated at 5 × 105/ml in medium containing the indicated concentration of HMGN1 for 48 h, and cytokines in the supernatant were measured by cytokine array. Shown is the mean (±SD) of triplicate wells of one experiment representative of three. *, P < 0.05 by Student’s t test when compared with DCs cultured without HMGN1. (B) Mouse bone marrow–derived DCs were treated with 1 µg/ml HMGN1 for 20 or 60 min before they were solubilized in lysis buffer (106 DCs/0.1 ml), and the levels of I-κBα and phosphorylated MAPKs (p44/42, p38, and JNK) in DC lysates were detected by Western blot. After stripping, the membranes were reprobed with GAPDH and the unphosphorylated MAPKs antibodies. The results of one experiment representative of two are shown. (C) Bone marrow–derived DCs from MyD88+/+ and MyD88−/− mice were cultured at 5 × 105/ml without or with 1 µg/ml HMGN1 for 48 h, and cytokines were measured by cytokine array. Shown is the mean (±SD) of three independent experiments. *, P < 0.05 by ANOVA. (D) BM-derived DCs from C3HeN and C3HeJ mice were cultured at 5 × 105/ml without or with 1 µg/ml HMGN1 for 48 h, and cytokines were measured by cytokine array. Shown is the mean (±SD) of three independent experiments. *, P < 0.05 by ANOVA. (E) HMGN1 was incubated with complexes of the extracellular domain of TLR4 (TLR4 ECD) and MD2 for 30 min followed by immunoprecipitation (IP) with rabbit anti-HMGN1 (top) or mouse anti-MD2 (bottom) IgG. The immunoprecipitated proteins were separated by SDS-PAGE and Western blotted (WB) with anti-polyhistidine (top) or anti-HMGN1 (bottom) antibody. Shown are the results of one experiment representative of two. (F) 0.5 nM FLAG–TLR4ECD–MD2 was incubated with 0.8 nM [3H]LOS–CD14 in the absence or presence of endotoxin or 150 nM HMGN1. The FLAG–TLR4ECD–MD2–[3H]LOS in the reaction mixture was captured by anti-FLAG–coated beads. The radioactivity of captured [3H]LOS and uncaptured [3H]LOS was measured by scintillation spectrometry. The binding of [3H]LOS to TLR4ECD–MD2 was shown as the percentage of [3H]LOS captured. The results of one experiment representative of two are shown. (G) TLR4ECD was incubated with MD2–[3H]LOS in the absence or presence of HMGN1, and the reaction mixture was analyzed by Sephacryl HR S300 chromatography. Shown are the results of one experiment representative of two.
Among the many diverse DC-activating stimulants, only the IL-1 family of cytokines (including IL-18) and many TLR ligands activate DCs in a MyD88-dependent manner (Kaisho and Akira, 2001; Akira and Takeda, 2004). There is no obvious structural or functional resemblance between HMGN1 and cytokines. We therefore investigated whether HMGN1 uses any of the TLRs as its receptor. Screening HEK293 cells transfected to express various TLR revealed that HMGN1 could induce the production of IL-8 and TNF in HEK293 cells transfected with a combination of human TLR4, MD2, and CD14 but not in untransfected HEK293 cells (Fig. S2). Additionally, HMGN1 stimulated the production of TNF, IL-6, IL-10, IL-12p70, and CCL5 in DCs derived from HeN mice but not in DCs from HeJ mice that harbor a loss-of-function TLR4 mutation (Fig. 3 D). These data indicate that HMGN1 is an endogenous mediator that induces DC activation in a TLR4-dependent manner.
To determine whether HMGN1 could interact with TLR4, HMGN1 was incubated with the TLR4–MD2 complex and subsequently analyzed by coimmunoprecipitation. Rabbit anti-HMGN1 coimmunoprecipitated both TLR4 and MD2 (Fig. 3 E, top), whereas mouse anti-MD2 coimmunoprecipitated HMGN1 (Fig. 3 E, bottom), suggesting an association of HMGN1 with the TLR4–MD2 complex. To better define the possible site of interaction between HMGN1 and MD2 and/or TLR4, effects of HMGN1 on binding of endotoxin (i.e., tritiated meningococcal lipooligosaccharide [[3H]LOS]) to preformed FLAG–TLR4ECD–MD2 complex or on binding of [3H]LOS–MD2 to TLR4ECD were measured using assays previously described (Teghanemt et al., 2008; Piazza et al., 2011). Whereas transfer of [3H]LOS from a monomeric complex of [3H]LOS–sCD14 to FLAG–TLR4ECD–MD2, as measured by co-capture of [3H]LOS by anti-FLAG agarose, was nearly completely inhibited by the addition of an ∼200-fold molar excess of unlabeled LOS, a similar molar excess of HMGN1 had little or no effect (Fig. 3 F). However, when the same concentrations of TLR4ECD ± [3H]LOS–MD2 were incubated in the absence or presence of HMGN1 and the reaction mixture was analyzed by Sephacryl HR S300 chromatography, incubation with HMGN1 resulted in an earlier elution of the recovered [3H]LOS-containing species. In the absence of HMGN1, there were two peaks of apparent Mr 190,000 (earlier elution peak) and 25,000 (later peak) corresponding to [3H]LOS–MD2–TLR4ECD and [3H]LOS–MD2, respectively (Fig. 3 G). In contrast, the reaction mixture in the presence of HMGN1 gave rise to two peaks of ∼210,000 and 40,000, respectively (Fig. 1 G). Together with the coimmunoprecipitation data, these findings strongly suggest that HMGN1 (Mr = 15,000) forms part of a complex with [3H]LOS–MD2–TLR4ECD and [3H]LOS–MD2, most consistent with binding of HMGN1 to a site in MD2 distinct from where either endotoxin or TLR4 interacts.
HMGN1 induces DC activation in a TRIF-dependent manner
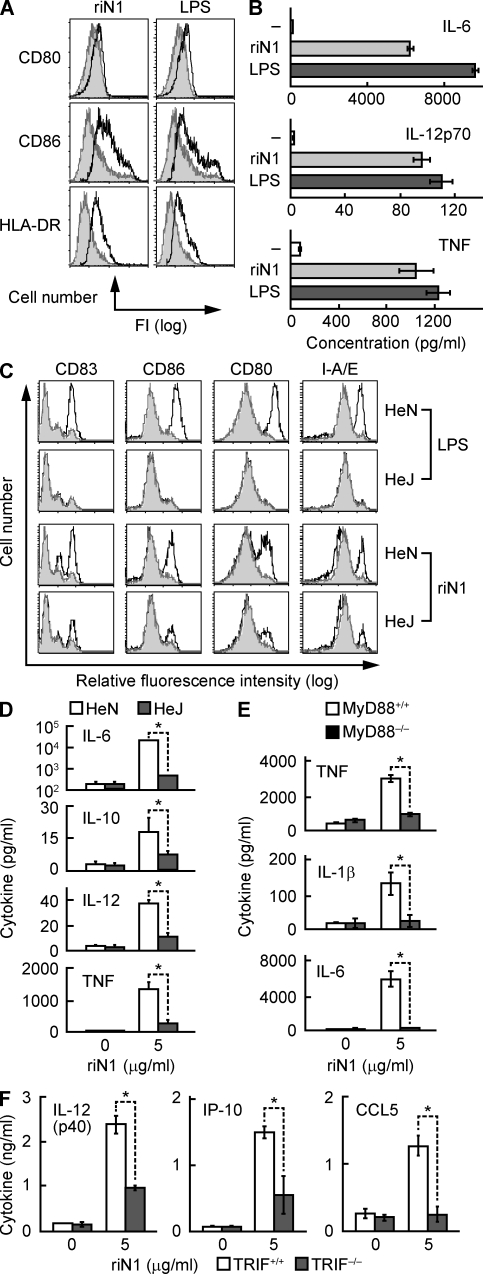
HMGN1-induced activation of DCs was dependent on TLR4, raising concern that the recombinant HMGN1 might be contaminated with endotoxin. However, the ability of unlabeled LOS but not HMGN1 at similar molar excess to compete with [3H]LOS–sCD14 for binding to the FLAG–TLR4ECD–MD2 complex (Fig. 3 F) indicated no gross contamination of recombinant HMGN1 with endotoxin. Recombinant HMGN1 displayed a single band of expected size on SDS-PAGE gel (Fig. S3) and was negative for endotoxin when tested by a limulus amebocyte lysate assay (not depicted). The capacity of recombinant HMGN1 to induce production by DCs of IL-6 and TNF was destroyed by boiling for 5 min but was not affected when digested with DNase I for 30 min before addition to DC culture (Fig. S4), suggesting that the DC-activating effect of HMGN1 preparation was neither caused by endotoxin or bacterial DNA contamination. To further exclude the possibility of endotoxin contamination, we expressed HMGN1 in insect cells, purified recombinant HMGN1 from the supernatant of insect culture (designated riN1) using affinity chromatography under sterile conditions, and investigated whether riN1 could still activate DCs. As shown in Fig. 4, riN1 enhanced DC expression of CD80, CD86, and HLA-DR (Fig. 4 A) and DC production of IL-6, IL-12p70, and TNF (Fig. 4 B). Moreover, riN1 induced remarkable up-regulation of CD83, CD80, CD86, and I-A/E in HeN DCs, but to a much lesser extent in HeJ DCs (Fig. 4 C). Furthermore, riN1-induced DC production of IL-6, IL-10, IL-12p70, and TNF was significantly reduced in the absence of functional TLR4 (Fig. 4 D). Thus, riN1-induced DC maturation is also dependent on TLR4. The ability of the synthetic N-terminal portion of HMGN1 (less likely to be contaminated with endotoxin) to activate DCs (Fig. 1, E and F) is also consistent with the view that HMGN1 by itself possesses the capacity to activate DCs in a TLR4-dependent manner.
Figure 4.
HMGN1 activates DCs in a TRIF-dependent manner. (A and B) Human MoDCs were incubated in the absence or presence of 1 µg/ml LPS or recombinant HMGN1 prepared in insect (riN1) cells at 10 µg/ml for 48 h before measurement of surface marker expression (A) or cytokine production (n = 3; B). In A, the results of one experiment representative of three are shown. Gray histograms indicate sham-treated DCs. (C) Flow cytometric analysis of the expression of DC surface molecules after treatment without or with 1 µg/ml LPS or 10 µg/ml riN1 for 48 h. Shown are the overlay histograms of C3H/HeN or C3H/HeJ DCs of one experiment representative of two (gray histograms indicate sham-treated DCs). (D) C3HeN and C3HeJ mouse DCs were cultured at 5 × 105/ml in the absence or presence of 5 µg/ml riN1 for 48 h, and cytokines were measured in the supernatants. (E) Cytokine production by MyD88+/+ or MyD88−/− DCs (5 × 105/ml) after 48-h treatment with or without riN1. (F) DCs from TRIF+/+ or TRIF−/− mice were incubated at 106/ml without or with 5 µg/ml riN1 for 48 h, and cytokines in the supernatants were measured. (D–F) Shown is the mean (±SD) of two (E and F) or three (D) independent experiments. *, P < 0.05 by ANOVA.
To confirm whether riN1-induced DC activation was also dependent on MyD88, MyD88+/+ and MyD88−/− mouse DCs were treated with riN1, and their production of TNF, IL-1β, and IL-6 was compared. Under identical conditions, riN1 promoted the production of TNF, IL-1β, and IL-6 by MyD88+/+ DCs but not by MyD88−/− DCs (Fig. 4 E). Therefore, riN1-induced DC activation was also dependent on the presence of MyD88.
To determine whether HMGN1-induced DC activation also involves TRIF, DCs generated from TRIF+/+ and TRIF−/− mouse bone marrow progenitors were treated with riN1 for the induction of mediators that rely on TRIF. TRIF+/+ DCs produced IL-12p40, IP-10, and CCL5 in response to riN1; however, TRIF−/− DCs treated under identical conditions produced much less IL-12p40, IP-10, and CCL5, indicating that activation of DCs by HMGN1 was TRIF dependent (Fig. 4 E). Comparison of LPS- and riN1-induced phenotypic maturation of HeN and HeJ DCs revealed some differences. Although LPS-induced phenotypic maturation relied on TLR4, riN1-induced phenotypic changes were not completely TLR4 dependent (Fig. 4 C). Furthermore, HMGN1 stimulation of certain DC inflammatory cytokines and chemokines (e.g., TNF, IL-1, IL-6, IL-12, and IP-10) was not completely dependent on MyD88, TLR4, or TRIF (Fig. 3, C and D; and Fig. 4 D). Therefore, HMGN1 may use an additional pathway for DC activation in addition to the major TLR4–MyD88–TRIF pathway.
HMGN1 is critical for the induction of antigen-specific immune responses
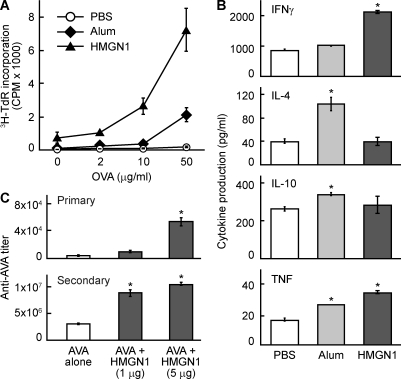
DC activation and recruitment are essential for the induction of adaptive immunity (Banchereau and Steinman, 1998; Iwasaki and Medzhitov, 2004). To investigate the potential contribution of HMGN1 to the induction of immunity, C57BL/6 mice were immunized i.p. with OVA alone or together with HMGN1 or alum (as a positive control) on day 1 and boosted with OVA on day 14. On day 21, the splenocytes of immunized mice were stimulated with various doses of OVA to determine OVA-specific proliferation and cytokine production, two parameters indicative of the development of OVA-specific cellular immune response. In comparison with mice immunized with OVA alone (PBS group), the splenocytes of mice immunized with OVA plus HMGN1 not only proliferated ex vivo more robustly in response to OVA (Fig. 5 A) but also produced more IFN-γ and TNF (Fig. 5 B), demonstrating that HMGN1 enhanced an OVA-specific cellular immune response. Alum, as expected, also enhanced the OVA-specific cellular immune response (Fig. 5, A and B) but promoted splenocyte production of IL-4, IL-10, and TNF. In contrast, HMGN1 promoted splenocyte production of IFN-γ and TNF but not IL-4, indicating that exogenous HMGN1 polarizes OVA-specific Th1 response (Fig. 5 B). To determine whether HMGN1 could also enhance the immune response to a physiologically relevant antigen, A/J mice were immunized i.p. with anthrax vaccine adsorbed (AVA), the human anthrax vaccine licensed in the US, together with HMGN1. HMGN1 dose-dependently enhanced the production of primary and secondary IgG specific for the protective antigen (PA) of anthrax toxin, demonstrating that HMGN1 can enhance immune response against a physiologically relevant antigen (Fig. 5 C). These results demonstrate that exogenous HMGN1 can act as an adjuvant to enhance antigen-specific humoral and cellular immune responses.
Figure 5.
HMGN1 promotes antigen-specific immune responses. 8-wk-old female C57BL/6 mice were immunized i.p. with OVA (50 µg/mouse), OVA + alum (3 mg/mouse), or OVA + HMGN1 (1 µg/mouse) on day 1 and boosted i.p. with OVA on day 14. On day 21, the spleens of immunized mice were removed, and single-cell suspension was evaluated for OVA-specific cellular immune responses. (A) Splenocytes were cultured in triplicate in a 96-well plate (5 × 105/0.2 ml/well) in the presence of specified concentrations of OVA for 4 d. The culture was pulsed with 1 µCi/well [3H]TdR for the last 18 h before harvest for measurement of [3H]TdR incorporation. OVA-specific proliferation of splenocytes is shown as the mean cpm (±SD) of each group (n = 4). (B) Splenocytes were cultured in duplicate in a 48-well plate (2.5 × 106/0.5 ml/well) in the presence of 100 µg/ml OVA for 48 h, and cytokines in the supernatants were quantitated. Shown is the mean cytokine concentration (±SD) of each group (n = 4) of one experiment representative of three. *, P < 0.05 by ANOVA when compared with the PBS group. (C) A/J mice (n = 4, 10 wk old, female) were immunized i.p. with AVA (the licensed human anthrax vaccine in the US) in the absence or presence of HMGN1 at specified doses (1 or 5 µg/mouse) on day 1 and booster immunized i.p. on day 14. Sera were collected on day 10 and 20 for the measurement of primary and secondary antibody responses, respectively. The IgG specific for the PA of anthrax in the serum was quantitated by anti-PA ELISA. Shown is the geometric mean anti-PA titer (±SD) of each group of one representative experiment out of three. *, P < 0.05 by ANOVA when compared with the AVA alone group.
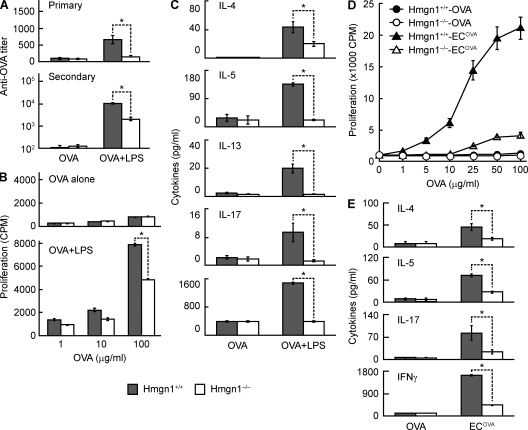
To further investigate the contribution of HMGN1 to adaptive immunity, we examined the effect of HMGN1 KO on in vivo induction of antigen-specific immune responses. Hmgn1−/− and littermate-matched Hmgn1+/+ mice were immunized i.p. with OVA alone or in the presence of an adjuvant, LPS. Immunization with OVA alone did not result in the generation of any immune response in Hmgn1−/− or Hmgn1+/+ mice (Fig. 6). Hmgn1+/+ mice immunized with OVA in the presence of LPS mounted OVA-specific humoral and cellular immune responses as indicated by elevated production of primary and secondary anti-OVA IgG antibody responses (Fig. 6 A), enhanced OVA-specific proliferation of splenocytes (Fig. 6 B), and increased OVA-specific splenocyte production of various cytokines (Fig. 6 C). In contrast, Hmgn1−/− mice produced lower levels of primary and secondary anti-OVA antibodies than Hmgn1+/+ mice (Fig. 6 A). In addition, the splenocytes of Hmgn1−/− mice immunized with OVA in the presence of LPS showed less OVA-specific proliferation than splenocytes of similarly immunized Hmgn1+/+ mice (Fig. 6 B). The reduction in OVA-specific proliferation of splenocytes of immunized Hmgn1−/− mice was not caused by an intrinsic deficiency in the capacity of Hmgn1−/− splenocytes to mount a proliferative response because splenocytes of immunized Hmgn1−/− and Hmgn1+/+ mice proliferated similarly to polyclonal stimulators including anti-CD3, IL-2, Con A, and PHA (Fig. S5). Furthermore, the splenocytes of Hmgn1−/− mice immunized with OVA in the presence of LPS produced lower levels of IL-4, IL-5, IL-13, IL-17, and IFN-γ than splenocytes of similarly immunized Hmgn1+/+ mice (Fig. 6 C). These results further demonstrate the critical role of HMGN1 in the induction of antigen-specific humoral and cellular immune responses.
Figure 6.
Reduction of antigen-specific immune responses in HMGN1 KO mice. Male HMGN1 KO (Hmgn1−/−) and littermate-matched WT (Hmgn1+/+) mice (n = 3) were immunized i.p. with OVA (50 µg/mouse) in the presence or absence of LPS (1 µg/mouse) on day 1 and boosted i.p. on day 14. On day 10 and 20, mice were bled for the separation of serum samples, which were stored at −20°C until the measurement of primary and secondary OVA-specific IgG antibody response by ELISA. (A) Primary and secondary anti-OVA titers of Hmgn1+/+ and Hmgn1−/− mice after immunization. Shown are the results of one experiment representative of three (mean ± SD). *, P < 0.05 by ANOVA. (B) OVA-specific splenocyte proliferation of splenocytes of immunized Hmgn1+/+ and Hmgn1−/− mice. Pooled splenocytes of each group were cultured in triplicate in wells of a 96-well plate (5 × 105/0.2 ml/well) in the presence of specified concentrations of OVA for 4 d. The culture was pulsed with 1 µCi/well [3H]TdR for the last 18 h before harvest for measurement of [3H]TdR incorporation. The results of one experiment representative of three are shown as the mean cpm (±SD) of triplicate wells. *, P < 0.05 by Student’s t test. (C) OVA-specific cytokine production by splenocytes of immunized Hmgn1+/+ and Hmgn1−/− mice. Splenocytes were cultured in a 48-well plate (2.5 × 106/0.5 ml/well) in the presence of 100 µg/ml OVA for 48 h before the measurement of cytokines in the supernatants. Shown is the mean concentration (±SD) of cytokines (n = 3). *, P < 0.05 by ANOVA. (D and E) Male Hmgn1−/− and littermate-matched Hmgn1+/+ mice (n = 4) were immunized i.p. with OVA (50 µg/mouse) or radiation-inactivated OVA-expressing E. coli (106/mouse) on day 1 and boosted i.p. on day 14. Splenocytes pooled from the five mice were cultured in triplicate in wells of a 96-well plate (5 × 105/0.2 ml/well) in the presence of specified concentrations of OVA for 4 d. The cultures were pulsed with 1 µCi/well [3H]TdR for the last 18 h before harvest for measurement of [3H]TdR incorporation. The results are shown as the mean cpm (±SD) of triplicate wells. *, P < 0.05 by Student’s t test. Alternatively, splenocytes were cultured in a 48-well plate (2.5 × 106/0.5 ml/well) in the presence of 100 µg/ml of OVA for 48 h before the measurement of cytokines in the supernatants. Shown is the mean concentration (±SD) of cytokines (n = 5) of one experiment representative of two. *, P < 0.05 by ANOVA.
To determine whether HMGN1 contributes to the development of immune response in a more physiologically relevant manner, we immunized mice with radiation-inactivated OVA-expressing Escherichia coli (ECOVA) and evaluated whether HMGN1 was required for the generation of antigen-specific T cell response. Splenocytes of Hmgn1+/+ mice immunized with ECOVA proliferated in response to in vitro OVA restimulation in a dose-dependent manner (Fig. 6 D) and increased production of OVA-specific T cell cytokines including IL-4, IL-5, IL-17, and predominantly IFN-γ (Fig. 6 E), suggesting that immunization with ECOVA induced the development of a robust Th1-polarized anti-OVA immune response. In contrast, Hmgn1−/− mice similarly immunized with ECOVA exhibited greatly reduced OVA-specific splenocyte proliferation and production of IL-4, IL-5, IL-17, and IFN-γ (Fig. 6, D and E). Hmgn1+/+ and Hmgn1−/− mice immunized with OVA alone, as anticipated, did not manifest a detectable level of immune response (Fig. 6, D and E). Thus, HMGN1 is critical for the development of antigen-specific immune responses in a physiologically relevant setting.
Production of HMGN1 at the site of immunization is necessary for the induction of antigen-specific immune response
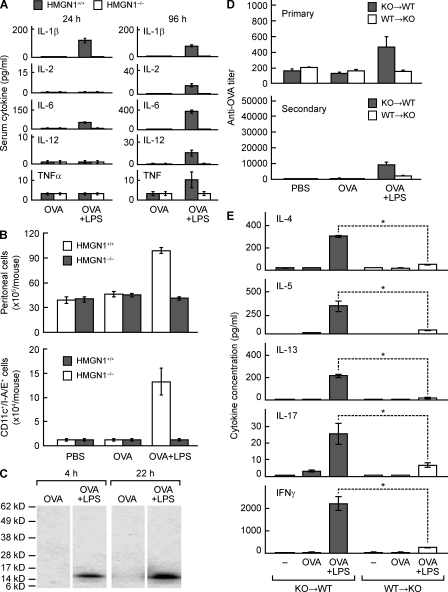
We next investigated why HMGN1 deficiency led to a reduction in antigen-specific immune responses. Although Hmgn1−/− mice have a deficiency in DNA repair processes and the differentiation of corneal epithelial cells (Birger et al., 2003, 2006), Hmgn1−/− and littermate-matched Hmgn1+/+ mice had similar spleen size and structure (not depicted) and similar number and distribution of various subsets of leukocytes (e.g., CD4+ T cells, CD8+ T cells, B cells, DC/monocytes, and granulocytes) in peripheral blood, bone marrow, lymph nodes, and spleen (Fig. S6), indicating that Hmgn1−/− mice had no obvious abnormality in the development of the immune system. Reduction of antigen-specific immune response in Hmgn1−/− mice was unlikely to be caused by malfunction of lymphocytes because Hmgn1−/− spleen lymphocytes responded similarly to polyclonal stimulations (Fig. S5). DCs derived from the bone marrow progenitors of Hmgn1−/− and littermate-matched Hmgn1+/+ mice, when loaded with OVA and subsequently matured with a cytokine cocktail consisting of IL-1β, TNF, and soluble CD40 ligand, stimulated comparable levels of OT I and OT II lymphocyte proliferation, indicating that Hmgn1−/− DCs were not intrinsically deficient in antigen uptake or presentation (Fig. S7, A and B). Hmgn1−/− DCs did not show any defect in vitro in LPS-induced activation of NF-κB and multiple MAPKs (Fig. S7 C) or LPS-induced production of a variety of cytokines and chemokines measured (Fig. S7 D). We therefore investigated whether immunized Hmgn1−/− mice were deficient in APC activation and recruitment. Immunization with OVA i.p. in the presence of LPS induced elevated serum levels of IL-1β, IL-2, IL-6, IL-12p70, and TNF at 24 and 96 h after immunization in Hmgn1+/+ but not in littermate-matched Hmgn1−/− mice (Fig. 7 A). This deficient cytokine production is presumably indicative of reduced in vivo activation of APCs in immunized Hmgn1−/− mice. Additionally, i.p. administration of OVA plus LPS resulted in a greater accumulation of total leukocytes as well as CD11c+/I-A/E+ cells than injecting with OVA alone in Hmgn1+/+ but not in littermate-matched Hmgn1−/− mice, indicating that HMGN1 deletion caused defective recruitment of APCs (Fig. 7 B). Thus, defective APC activation and recruitment presumably contributed to the reduction of antigen-specific immune responses in Hmgn1−/− mice.
Figure 7.
Involvement of HMGN1 generated by nonleukocytes at the site of immunization in the induction of immune response. (A) HMGN1 KO (Hmgn1−/−) and littermate-matched WT (Hmgn1+/+) mice (n = 4) were i.p. immunized with OVA (50 µg/mouse) in the presence or absence of LPS (1 µg/mouse). Serum cytokines were measured at 24 and 96 h after immunization. Shown is the mean (±SD) serum cytokine concentration of each group, which is representative of three independent experiments. (B) HMGN1 KO (Hmgn1−/−) and littermate-matched WT (Hmgn1+/+) mice (n = 3) were immunized i.p. with PBS, OVA (50 µg/mouse), or OVA + LPS (1 µg/mouse). After 4 h, peritoneal leukocytes were enumerated for total leukocytes (top) or DCs (bottom; defined as CD11c and I-A/E double-positive cells). Shown is the mean (±SD) cell number of individual group of one experiment representative of two. (C) C57BL/6 mice (n = 3) were injected with OVA (50 µg/mouse) alone or OVA + LPS (1 µg/mouse). HMGN1 expression in the peritoneal cavity 4 or 22 h after injection was assessed by Western blot with anti-HMGN1 antibody. Shown are the results of one experiment representative of two. (D and E) Bone marrow chimeric mice were generated by reconstituting lethally irradiated Hmgn1−/− mice with Hmgn1+/+ bone marrow mononuclear cells (WT→KO) or vice versa (KO→WT). The chimeric mice (n = 3) were immunized i.p. with PBS containing OVA (50 µg/mouse) or OVA in the presence of LPS (1 µg/mouse) on day 1 and boosted on day 14. On day 10 and 20, OVA-specific IgG antibody responses were measured by ELISA (D). On day 21, splenocytes were cultured in a 48-well plate (2.5 × 106/0.5 ml/well) in the presence of 100 µg/ml OVA for 48 h. The cytokines in the supernatants were measured and are shown as the mean concentration (±SD) of each group (E). Shown are the results of one experiment representative of two. *, P < 0.05.
The decrease in APC activation and/or recruitment in immunized Hmgn1−/− mice might result from a deficiency in the production of extracellular HMGN1. To determine whether HMGN1 was produced upon immunization, the peritoneal lavages of immunized C57BL/6 mice were tested for the presence of HMGN1 protein by Western blot. The lavage supernatants collected 4 and 22 h after immunization with OVA in the presence of LPS contained HMGN1 protein, whereas the supernatants of mice immunized with OVA alone did not, indicating that HMGN1 was produced extracellularly upon immunization in the presence of LPS (Fig. 7 C). HMGN1 was not detected in the lysate of peritoneal cell pellets (not depicted), suggesting that either peritoneal leukocytes completely released their HMGN1 or cells other than leukocytes were responsible for the local production extracellular HMGN1.
To determine whether leukocyte- or nonleukocyte-derived HMGN1 played a more important role in the induction of immune response, we immunized WT→KO (lethally irradiated Hmgn1−/− mice reconstituted with Hmgn1+/+ bone marrow) and KO→WT (lethally irradiated Hmgn1+/+ mice reconstituted with Hmgn1−/− bone marrow) bone marrow chimeric mice and evaluated their OVA-specific immune responses. In comparison with KO→WT chimeric mice, WT→KO chimeric mice produced much less serum anti-OVA IgG antibodies upon i.p. immunization with OVA plus LPS (Fig. 7 D). Additionally, the splenocytes of immunized WT→KO chimeric mice, when stimulated with OVA, not only proliferated less robustly (Fig. S8) but also secreted much less IL-4, IL-5, IL-13, IL-17, and IFN-γ (Fig. 7 E) than the splenocytes of similarly immunized KO→WT chimeric mice. Thus, production of HMGN1 at the site of immunization, which was mostly derived from nonleukocytes, is critical for the induction of antigen-specific immune responses.
HMGN1 contributes to LPS-induced innate immune responses
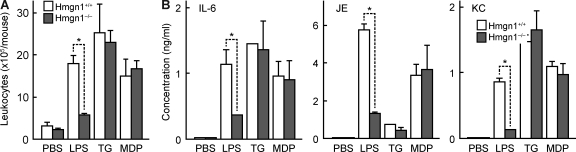
Hmgn1−/− mice showed markedly reduced leukocyte infiltration and production of inflammatory cytokines in response to i.p. administration of OVA and LPS, suggesting that LPS-induced innate immune response was dependent on the presence of HMGN1 (Fig. 7, A and B). To evaluate whether Hmgn1−/− mice have a generalized deficiency in the manifestation of innate/inflammatory response, we investigated the capacity of thioglycolate (TG) and muramyl dipeptide (MDP) to induce peritoneal inflammatory response in Hmgn1−/− and littermate-matched Hmgn1+/+ mice. Injection i.p. of LPS, TG, or MDP resulted in peritoneal infiltration of leukocytes and production of IL-6, JE, and KC in Hmgn1+/+ mice (Fig. 8, A and B). LPS-induced leukocyte infiltration and cytokine production were greatly reduced in Hmgn1−/− mice; however, TG and MDP stimulated similar levels of leukocyte infiltration and cytokine production in both Hmgn1+/+ and Hmgn1−/− mice (Fig. 8, A and B). Therefore, Hmgn1−/− mice have no generalized deficiency in the manifestation of innate response. Lack of endogenous HMGN1 compromised LPS-induced innate/inflammatory response, which accounts at least in part for the reduction of antigen-specific immune response upon immunization with OVA and LPS in Hmgn1−/− mice.
Figure 8.
HMGN1 contributes to innate immune responses induced by LPS. (A and B) Hmgn1−/− and littermate-matched Hmgn1+/+ mice (n = 5) were injected i.p. with 0.2 ml PBS or PBS containing 10 µg/ml LPS, 5% TG, or 50 µg/ml MDP. After 24 h, peritoneal exudates were analyzed for the total cell number (A) and inflammatory mediators (IL-6, JE, and KC) in the lavage fluid (B). Shown are the results of one experiment representative of two (mean ± SD). *, P < 0.05 by ANOVA.
DISCUSSION
Alarmins are defined as endogenous mediators that are capable of rapidly inducing the activation and recruitment of APCs and consequently capable of promoting the induction of innate and adaptive immune responses (Oppenheim and Yang, 2005; Bianchi, 2007; Yang et al., 2009). HMGN1, a member of the HMGN subfamily of HMG proteins which regulates chromatin structure and gene transcription, has not previously been shown to have any extracellular activity (Bianchi and Agresti, 2005; Birger et al., 2006; Furusawa et al., 2006; Hock et al., 2007). In this study, we show that HMGN1 is capable of activating (Figs. 1, 3, and 4) and recruiting (Fig. 2) human and mouse DCs, as well as promoting the development of antigen-specific Th1 polarized immune responses upon co-administration with antigens (Fig. 5), demonstrating that extracellular HMGN1 is an authentic alarmin. The critical contribution of HMGN1 to the development of antigen-specific immune responses is further substantiated by the fact that Hmgn1−/− mice manifest much weaker antigen-specific immune responses than littermate-matched Hmgn1+/+ mice when LPS was used as the adjuvant (Fig. 6). When antigen was administered in the form of OVA-expressing E. coli, a more physiologically relevant manner, antigen-specific immune response in Hmgn1−/− mice was still compromised (Fig. 6). Thus, HMGN1 is an alarmin critical for the development of antigen-specific immune responses induced by LPS.
HMGN1 can induce DC production of various inflammatory cytokines (Figs. 1, 3, and 4) and local recruitment of leukocytes (Fig. 2). Preliminary data suggest that HMGN1 can also up-regulate the expression of co-stimulatory molecules on monocytes (not depicted). LPS-induced production of systemic (serum) and local (peritoneal lavage fluid) inflammatory cytokines as well as peritoneal infiltration of leukocytes were greatly reduced in Hmgn1−/− mice in comparison with littermate-matched Hmgn1+/+ mice (Figs. 7 and 8). The reduction in LPS-induced innate/inflammatory response in Hmgn1−/− mice is unlikely to be caused by an intrinsic deficiency in the manifestation of inflammatory response because of HMGN1 deletion because TG and MDP induced similar peritoneal inflammatory response in both Hmgn1+/+ and Hmgn1−/− mice (Fig. 8, A and B). Therefore, HMGN1 is also critical for the development of LPS-induced innate as well as adaptive immune responses.
Notably, Hmgn1−/− mice did not manifest a complete deficiency in the development of LPS-induced innate and antigen-specific immune response. Upon i.p. immunization with OVA in the presence of LPS, Hmgn1−/− mice showed a partial reduction in both antibody production (Fig. 6 A) and OVA-specific splenocyte proliferation (Fig. 6 B). LPS-induced infiltration of peritoneal leukocytes as well as peritoneal production of IL-6 and JE were not completely abrogated in Hmgn1−/− mice (Fig. 8, A and B). In addition, ECOVA-induced antigen-specific immune response was dramatically reduced but not completely abrogated in Hmgn1−/− mice in comparison with littermate-matched Hmgn1+/+ mice (Fig. 6, D and E). It is possible that other alarmins such as HMGB1 may partially compensate for the deficiency of HMGN1. It is still remarkable that HMGN1 demonstrates a major nonredundant role in the development of LPS-induced innate and antigen-specific immune responses.
HMGN1 used in some experiments of the present study was expressed using a prokaryotic expression system and purified from E. coli cultures, which raised the issue of potential endotoxin contamination, particularly because HMGN1 was found to activate DCs in a TLR4-dependent manner. However, multiple lines of evidence indicate that HMGN1 is responsible for the DC-activating effect rather than endotoxin contamination. First of all, the HMGN1 preparation was quite pure (a single band under SDS-PAGE) and contained undetectable level of endotoxin, and its DC-activating effect was destroyed by boiling. HMGN2 preparation expressed and purified in a similar manner as HMGN1 did not activate DCs (Fig. 1), suggesting the methods used for the preparation of HMGN1 and HMGN2 can yield endotoxin-free protein preparations. Although HMGN2 has limited homology (∼50%) with HMGN1 and is only 10 aa shorter than HMGN1, it is unclear why HMGN2 does not possess a DC-activating effect. Additionally, synthetic N1ND peptide corresponding to the N-terminal domain of HMGN1 (less likely to be contaminated with endotoxin) also demonstrated a DC-activating effect (Fig. 1, E and F). Furthermore, HMGN1 expressed in insects and purified under sterile conditions induced DC activation in a manner that was dependent on TLR4, MyD88, and TRIF (Fig. 4). These data clearly indicate that HMGN1 itself, rather than endotoxin contamination, is responsible for the observed immunoenhancing effect.
Recombinant HMGN1 produced in insects (riN1) was less active than that generated in E. coli in terms of inducing cytokine production by DCs (Figs. 1, 3, and 4). This was probably because of the addition to riN1 of a C-terminal tag consisting of both c-Myc and poly-His motifs, which were engineered for easy detection and purification of riN1. Consequently, riN1 was ∼20% bigger than HMGN1 generated in E. coli, and thus a higher mass concentration was needed to achieve similar molar concentration and biological effect. In addition, the C-terminal c-Myc–poly-His tag might influence the structure of riN1 and therefore negatively affect its potency to activate DCs.
How does HMGN1 contribute to the development of LPS-induced antigen-specific immune response? HMGN1 gene deletion does not result in deficiency in the development of lymphoid organs or the reactivity and function of lymphocytes and DCs. Therefore, it is likely that HMGN1 generated at the site of immunization, in turn, promotes both the mobilization of DCs (peritoneal cavity in the present study) and the activation of innate immune response, both of which are important for the induction of subsequent adaptive immune responses. Indeed, HMGN1 is produced at the size of immunization (Fig. 7 C). Hmgn1−/− mice had greatly reduced production of serum inflammatory cytokines such as IL-1β, IL-6, IL-12, and TNF after i.p. immunization with a mixture of OVA and LPS (Fig. 7 A). Additionally, LPS induction of peritoneal innate/inflammatory response (leukocyte infiltration and production of inflammatory mediators) was greatly decreased in HMGN1 KO mice (Figs. 7 and 8). Thus, lack of endogenous HMGN1 markedly compromises LPS-induced innate/inflammatory responses. Furthermore, the reduction in antigen-specific immune responses in Hmgn1−/− mice was accompanied by a lack of DC accumulation in the peritoneum, which was intact in the peritoneum of immunized Hmgn1+/+ mice (Fig. 7 B). Promotion of DC activation by extracellular HMGN1 depends on the presence of MyD88 (Fig. 3 C), which is also in agreement with the essential role of MyD88 in the induction of antigen-specific immune response (Adachi et al., 1998; Feng et al., 2003; Su et al., 2005).
HMGN1 activates DCs by triggering a TLR4–MyD88–TRIF-dependent signaling pathway, independent of its chromatin-binding capacity (Figs. 1, 3, and 4; and Figs. S1 and S2), and thus appears to be an endogenous TLR4 agonist. Other reported endogenous TLR4 agonists include mouse β-defensin 2, HMGB1, heat-shock proteins, granulysin, S100A8/9, and certain degraded extracellular matrix such as low molecular weight hyaluronan and fibronectin extra domain A (Ohashi et al., 2000; Okamura et al., 2001; Biragyn et al., 2002; Termeer et al., 2002; Park et al., 2004; Apetoh et al., 2007; Vogl et al., 2007; Tewary et al., 2010). The capacity of TLR4 to respond to these diverse endogenous mediators remains mysterious. However, the contribution of endogenous HMGN1 to immunity is quite unique because other endogenous TLR4 agonists did not compensate for the lack of endogenous HMGN1 in Hmgn1−/− mice in promoting the development of LPS-induced innate and antigen-specific immune responses (Figs. 6, 7, and 8). It is certain that endogenous HMGN1 is required for LPS-induced innate and adaptive immune responses; however, how endogenous HMGN1 contributes to this process is less clear. The ability of HMGN1 to bind to the TLR4ECD–MD2 complex as judged by coimmunoprecipitation (Fig. 3 F) and to both [3H]LOS–MD2–TLR4ECD and [3H]LOS–MD2 as judged by gel-sieving chromatography (Fig. 3 G) is most consistent with HMGN1 binding to a site in MD2 that is distinct from both the site in MD2 binding to LPS as well as the site engaging TLR4 as needed to form an MD2–TLR4 heterodimer. Therefore, one possibility would be that full in vivo activation of TLR4 requires both endogenous HMGN1 and endotoxin. Further studies are also needed to determine whether HMGN1 is critical for the development of immune responses induced by adjuvants other than LPS.
Overall, our data demonstrate that HMGN1 acts as a critical alarmin for LPS-induced innate and antigen-specific immune responses. The findings in this study may have broader ramifications. First of all, it has been observed that aged Hmgn1−/− mice have a higher incidence of multiple malignant and metastatic tumors than littermate-matched Hmgn1+/+ controls (Birger et al., 2005). The increased spontaneous occurrence of malignant tumors in Hmgn1−/− mice is thought to be based on reduced DNA repair caused by the absence of nuclear HMGN1 (Birger et al., 2005; Hock et al., 2007). However, a reduction in antitumor immunity caused by HMGN1 deficiency may also be contributory because the activation of TLR4 is important for the generation of antitumor immunity (Apetoh et al., 2007). Second, one may use exogenous HMGN1 as a defined potent molecular adjuvant for promoting the efficiency of vaccines, or conversely, one can identify antagonists to target undesirable pathological inflammatory or immune reactions by suppressing the activity or release of extracellular HMGN1. Finally, HMGN1 may provide a means for nonleukocytes to respond to danger by rapidly alerting the host defense.
MATERIALS AND METHODS
Reagents and mice.
Recombinant human or mouse GM-CSF, IL-4, IL-1β, TNF, soluble CD40 ligand, CXCL12, CCL5, and CCL21 were purchased from PeproTech. Con A, PHA, LPS (E. coli O55:B5), and alum were obtained from Sigma-Aldrich. EndoGrade OVA with extremely low endotoxin levels (<1 EU/mg) was purchased from PROFOS. AVA was obtained from BioPort. Recombinant HMGN1, HMGN2, and mutated HMGN1 were generated in E. coli and purified as previously described (Lim et al., 2004). The purity of the HMGN preparation was analyzed by Coomassie brilliant blue staining of an SDS-PAGE. The potential endotoxin contamination was monitored using the Chromogenic LAL Assay kit (Cambrex). Peptides corresponding to HMGN11–52 (N1ND) and HMGN153–100 (N1CD) with a purity of >95% were chemically synthesized by New England Peptide, Inc. To produce human HMGN1 in insect cells, HMGN1 cDNA was cloned into pFastBac/HBM-TOPO vector (Invitrogen). To facilitate detection and purification, both c-Myc and poly-His motifs were cloned in-frame after HMGN1 cDNA so that the HMGN1 would be expressed as a protein with a C-terminal c-Myc–poly-His tag. After sequence confirmation, the construct was transformed into Max Efficiency DH10Bac competent E. coli (Invitrogen) to generate recombinant bacmid. The bacmid was transfected into Sf9 insect cells (Invitrogen) to make recombinant baculovirus. The 48-h culture supernatant of High Five cells infected by the recombinant baculovirus (at a multiplicity of infection of 10) was used for the purification of secreted HMGN1 (termed riN1) by affinity chromatography under sterile condition using nickel affinity columns. C57BL/6 and A/J mice were obtained from Charles River. OT I and OT II mice were purchased from the Jackson Laboratory. Hmgn1−/− and littermate-matched Hmgn1+/+ mice were generated by genotyping the offspring of breeders consisting of Hmgn1+/− dam and sire. MyD88+/+ and MyD88−/− mice were generated by breeding MyD88+/− dams and sires. TRIF+/+ and TRIF−/− mice were generated by breeding TRIF+/− dams and sires. All mice were kept under specific pathogen–free conditions with water and food given ad libitum. All experiments with mice were performed in compliance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals and were approved by the National Cancer Institute at Frederick Animal Care and Use Committee.
DC generation and treatment.
Human peripheral blood monocytes and T lymphocytes were isolated 90–95% pure as previously described (Yang et al., 2008). Human DCs were generated by culturing purified monocytes at 5 × 105/ml in complete RPMI 1640 medium (RPMI 1640 supplemented with 10% FBS [Hyclone], 2 mM glutamine, 25 mM Hepes, 100 U/ml penicillin, 100 µg/ml streptomycin, and 50 µM of 2-ME) containing 50 ng/ml hGM-CSF and 50 ng/ml hIL-4 at 37°C in a humidified CO2 (5%) incubator for 5–6 d. Mouse DCs were generated by culturing mouse hematopoietic progenitors isolated from the femurs and tibias of various KO and littermate-matched WT mice in complete RPMI 1640 containing 20 ng/ml mGM-CSF for 6 d as previously reported (Yang et al., 2008). Human and mouse DCs were incubated at specified concentrations for 20 min to 48 h in the absence or presence of the indicated concentration of activators (e.g., HMGN1, LPS, etc.) before being analyzed for phenotype, function, or signaling. In some experiments, mouse DCs were pulsed with antigen by incubating with 100 µg/ml OVA for 24 h before induction of maturation.
Assessment of DC phenotype and function.
Multiple cytokines and chemokines in the culture supernatants of human and mouse DCs were measured using SearchLight cytokine array (Thermo Fisher Scientific). DC migration was assessed using a 48-well microchemotaxis chamber assay as described previously (Kurosaka et al., 2005). Expression of surface molecules on human DCs was determined by flow cytometry using a FACScan (BD) after staining with FITC- or PE-conjugated mouse monoclonal antibodies (all from BD) against human CD11c (IgG1, κ, clone B-Ly6), CD80 (IgG1, κ, clone L307.4), CD83 (IgG1, κ, clone HB15e), CD86 (IgG1, κ, clone 2331), HLA-ABC (IgG1, κ, clone G46-2.6), HLA-DR (IgG2a, κ, clone G46-6), or isotype-matched control antibodies. The capacity of DCs to stimulate the proliferation of allogeneic T cells was evaluated by mixed lymphocyte reaction in which purified allogeneic T cells or OT I/II lymphocytes (105/well) were cultured with different numbers of differentially treated and γ-irradiated (3,000 rad) DCs in a 96-well flat-bottom plate for a specified period of time at 37°C in humidified air with 5% CO2. The proliferative response of T cells was examined by pulsing the culture with 1 µCi/well [3H]TdR (DuPont) for the last 18 h before harvest. [3H]TdR incorporation was measured with a microbeta counter (Wallac).
Analysis of in vivo cell recruitment and HMGN1 production.
Male or female mice (C57BL/6, Hmgn1+/+, and Hmgn1−/−) of 8–12 wk old were injected i.p. with HMGN1, OVA, TG, MDP, and LPS alone or in combination at specified doses. After 4–22 h, the mouse peritoneal cavity was lavaged with 5 ml of ice-cold Ca2+- and Mg2+-free PBS containing 20 U/ml heparin. The lavage fluid was centrifuged (300 g) for 5 min to separate the supernatant and cells. HMGN1 in the supernatant or cells was detected by Western blot as described in the following section. Total leukocytes were enumerated with a hemocytometer, whereas cytokines in the lavage fluid were measured by cytokine array. The percentage of total DCs (as CD11c+/I-A/E+ cells) was analyzed by flow cytometry after staining total leukocytes with PE-conjugated anti-mouse CD11c (IgG1, λ2, clone HL3; BD) and FITC-conjugated anti–mouse I-A/E (IgG2a, κ, clone 2G9; BD). The subsets of DCs recruited into the peritoneal cavity were analyzed after staining the total leukocytes with FITC-conjugated anti–mouse CD11b (IgG2b, κ, clone M1/70; BD), PE-conjugated anti–mouse CD11c, and APC-conjugated anti–mouse B220 (IgG2a, κ, clone RA3-6B2; BD). The results were calculated as the number of cells per mouse peritoneal cavity.
Coimmunoprecipitation and Western blot.
Recombinant HMGN1 was incubated with the hTLR4–MD2 complex (R&D Systems) in PBS at a concentration of 10 µM for 30 min at room temperature. The mixture was then mixed with Dynabeads (Invitrogen) that were precoated with rabbit anti-HMGN1 IgG, mouse anti-hMD2 IgG, normal rabbit IgG, or normal mouse IgG and incubated at room temperature for 20 min. Subsequently, the Dynabeads were separated from the free reactants with a magnet and washed three times with wash buffer as recommended by the manufacturer. Finally, the Dynabeads were suspended in 18 µl of 1× SDS-PAGE sample buffer (62.5 mM Tris-HCl, pH 6.8 at 25°C, 2% wt/vol SDS, 10% glycerol, 50 mM dithiothreitol, and 0.01% bromophenol blue) and boiled for 5 min, and the supernatants were collected for SDS-PAGE separation and Western blot analysis.
DCs with HMGN1 as specified and leukocytes recovered from the peritoneal lavage of treated mice were washed and lysed in SDS-PAGE sample buffer at 107/ml. Protein pellets of trichloroacetic acid–precipitated supernatant (3 ml) of peritoneal lavage were dissolved in 20 µl SDS-PAGE sample buffer. Samples (10–20 µl/lane) were separated on a 4–12% NuPAGE Bis-Tris Gel using 1× NuPAGE MES SDS Running buffer as the electrode buffer. After transfer onto a piece of Immobilon membrane (Millipore), the levels of target molecules were detected by blotting using the following first antibody: mouse anti-polyhistidine, rabbit anti-HMGN1 (ProteinTech), rabbit anti–I-κBα (Cell Signaling Technology), rabbit anti-GAPDH (Cell Signaling Technology), rabbit anti–phospho-p44/42 (Cell Signaling Technology), anti-p44/42 (Cell Signaling Technology), rabbit anti–phosphorylated p38 (Cell Signaling Technology), rabbit anti-p38 (Cell Signaling Technology), rabbit anti–phosphorylated JNK (Cell Signaling Technology), and rabbit anti-JNK (Cell Signaling Technology). Horseradish peroxidase–conjugated anti–mouse IgG (GE Healthcare) or anti–rabbit IgG (Cell Signaling Technology) was used as the second antibody. The blot was developed using the ECL Plus Western Blotting Detection System (GE Healthcare) and exposed to a piece of BioMax x-ray film (Kodak).
Assay of HMGN1 binding to the TLR4–MD2 complex.
[3H]LOS with a specific radioactivity of 25,000 cpm/pmol was prepared as previously described (Giardina et al., 2001). The preparation of FLAG–TLR4ECD, TLR4ECD–MD2, [3H]LOS–MD2, and [3H]LOS–CD14 and the binding analysis were performed as previously reported (Teghanemt et al., 2008; Piazza et al., 2011). In brief, 0.5 nM FLAG–TLR4ECD–MD2 was incubated with 0.8 nM [3H]LOS–CD14 in the absence or presence of LPS or 150 nM HMGN1 in PBS containing 0.1% HSA for 30 min at 37°C. Transfer of [3H]LOS from sCD14 to FAG-TLR4ECD was measured by scintillation counting after capturing TLR4ECD–MD2–[3H]LOS by anti-FLAG–coated beads and shown as percentage of total [3H]LOS added. Alternatively, 0.5 nM TLR4ECD was incubated with 0.8 nM [3H]LOS–MD2 in the absence or presence of 150 nM HMGN1 for 30 min at 37°C, and the reaction mixture was analyzed by gel filtration liquid chromatography to resolve different [3H]LOS-containing products of different Mr. The Sephacryl HR S300 column (1.6 cm × 70 cm) used for determination of apparent Mr of the [3H]LOS-containing products were calibrated with the following Mr standards: blue dextran (2 × 106, V0), thyroglobulin (650,000), ferritin (440,000), catalase (232,000), IgG (158,000), HSA (66,000), OVA (44,500), myoglobin (17,500), and vitamin B12 (1,200, Vi). The reaction mixture was applied to the Sephacryl HR S300 column preequilibrated in PBS, pH 7.4, and 0.1% HSA and eluted in the same buffer at a flow-rate of 0.5 ml/min at room temperature using AKTA Purifier or Explorer 100 fast protein liquid chromatography (GE Healthcare), with the collection of 1-ml fractions. Radioactivity in collected fractions was analyzed by liquid scintillation spectroscopy (LS liquid scintillation counter; Beckman Coulter).
Generation of bone marrow chimeric mice.
For the generation of WT→KO chimeric mice, lethally irradiated (1,000 rad) HMGN1 KO (Hmgn1−/−) mice (8 wk old) were reconstituted by intravenous injection of bone marrow mononuclear cells (5 × 106/mouse) isolated from sex- and littermate-matched WT (Hmgn1+/+) mice. For the generation of KO→WT chimeric mice, Hmgn1−/− bone marrow mononuclear cells were injected into Hmgn1+/+ mice. After reconstitution, mice were housed under specific pathogen–free conditions with amoxicillin in the drinking water for 8 wk to allow the development of chimeric mice before use.
Immunization and detection of antigen-specific immune responses.
C57BL/6, Hmgn1−/− or littermate-matched Hmgn1+/+, A/J, or chimeric mice (8–15 wk old, male or female, three to six mice/group) were immunized i.p. with PBS, antigen (OVA or AVA) alone or in the presence of alum (2–3 mg/mouse), HMGN1 (1–5 µg/mouse), or LPS (1 µg/mouse) on day 1 or irradiation-inactivated ECOVA and i.p. booster immunized on day 14. Serum samples were taken on day 10 and 20 for the measurement of primary and secondary antibody responses. In some experiments, serum was taken at 24 and 96 h after immunization for the detection of in vivo cytokine generation. On day 21, immunized mice were euthanized to remove spleens for the measurement of antigen-specific splenocyte proliferation and cytokine production. OVA-specific IgG antibody was measured by ELISA as previously described (Yang et al., 2008). The titer of IgG specific for the PA of anthrax toxin in the serum of AVA-immunized A/J mice was quantitated by anti-PA ELISA as previously reported (Xie et al., 2005).
OVA-specific splenocyte proliferation was measured by incubating splenocytes (5 × 105/well) in triplicate in wells of 96-well round-bottomed plates in complete RPMI 1640 medium (0.2 ml/well) in the presence or absence of the indicated concentration of OVA at 37°C in a CO2 incubator for 72 h. The cultures were pulsed with 1 µCi/well for the last 18 h before harvest for the quantitation of [3H]TdR incorporation by microbeta counting. For measuring OVA-specific cytokine production, splenocytes were cultured in complete RPMI 1640 in 48-well plates (2.5 × 106/0.5 ml/well) with the indicated concentrations of OVA for 48 h. Cytokines produced by splenocytes in the culture supernatants were quantitated by SearchLight cytokine array (Thermo Fisher Scientific).
Statistical analysis.
Unless otherwise specified, all experiments were performed at least three times, and the results of one representative experiment or the mean of multiple experiments are shown. Differences in the production of antigen-specific IgG and cytokines of treated mice were determined by analysis of variance (ANOVA), whereas differences between other sham and experimentally treated groups were evaluated by Student’s t test.
Online supplemental material.
Fig. S1 shows that mutated HMGN1 incapable of chromatin binding was similarly effective at up-regulating the expression of DC surface marker molecules. Fig. S2 shows that HMGN1 induction of cytokines depends on the TLR4–MD2 signaling complex. Fig. S3 shows the preparation and purity of recombinant HMGN1 protein. Fig. S4 shows that the capacity of HMGN1 to induce DC production of IL-6 and TNF was destroyed by heating but not by DNase I digestion. Fig. S5 shows that the splenocytes of immunized WT and HMGN1 KO mice proliferated similarly in response to various polyclonal stimulators. Fig. S6 shows that Hmgn1−/− and littermate-matched Hmgn1+/+ mice have similar distribution of various subsets of leukocytes. Fig. S7 shows that DCs of Hmgn1−/− and littermate-matched Hmgn1+/+ mice had comparable antigen-uptake and antigen-presenting capacity as well as signaling. Fig. S8 shows OVA-specific splenocyte proliferation of immunized chimeric mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20101354/DC1.
Acknowledgments
We thank S.W. Stull, B.A. Demon, and A.L. Trivett for excellent technical assistance.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. This research was supported in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. This work was also supported in part by a grant (2012CB932503) from the National Key Basic Research Program of China.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- [3H]LOS
- tritiated meningococcal lipooligosaccharide
- ANOVA
- analysis of variance
- AVA
- anthrax vaccine adsorbed
- MAPK
- mitogen-activated protein kinase
- MDP
- muramyl dipeptide
- MoDC
- monocyte-derived DC
- NBD
- nucleosomal binding domain
- PA
- protective antigen
- TG
- thioglycolate
- TRIF
- TIR domain–containing adaptor protein inducing IFN-β
References
- Adachi O., Kawai T., Takeda K., Matsumoto M., Tsutsui H., Sakagami M., Nakanishi K., Akira S. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 9:143–150 10.1016/S1074-7613(00)80596-8 [DOI] [PubMed] [Google Scholar]
- Akira S., Takeda K. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511 10.1038/nri1391 [DOI] [PubMed] [Google Scholar]
- Apetoh L., Ghiringhelli F., Tesniere A., Obeid M., Ortiz C., Criollo A., Mignot G., Maiuri M.C., Ullrich E., Saulnier P., et al. 2007. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 13:1050–1059 10.1038/nm1622 [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252 10.1038/32588 [DOI] [PubMed] [Google Scholar]
- Bianchi M.E. 2007. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81:1–5 10.1189/jlb.0306164 [DOI] [PubMed] [Google Scholar]
- Bianchi M.E., Agresti A. 2005. HMG proteins: dynamic players in gene regulation and differentiation. Curr. Opin. Genet. Dev. 15:496–506 10.1016/j.gde.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Biragyn A., Ruffini P.A., Leifer C.A., Klyushnenkova E., Shakhov A., Chertov O., Shirakawa A.K., Farber J.M., Segal D.M., Oppenheim J.J., Kwak L.W. 2002. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science. 298:1025–1029 10.1126/science.1075565 [DOI] [PubMed] [Google Scholar]
- Birger Y., West K.L., Postnikov Y.V., Lim J.H., Furusawa T., Wagner J.P., Laufer C.S., Kraemer K.H., Bustin M. 2003. Chromosomal protein HMGN1 enhances the rate of DNA repair in chromatin. EMBO J. 22:1665–1675 10.1093/emboj/cdg142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birger Y., Catez F., Furusawa T., Lim J.H., Prymakowska-Bosak M., West K.L., Postnikov Y.V., Haines D.C., Bustin M. 2005. Increased tumorigenicity and sensitivity to ionizing radiation upon loss of chromosomal protein HMGN1. Cancer Res. 65:6711–6718 10.1158/0008-5472.CAN-05-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birger Y., Davis J., Furusawa T., Rand E., Piatigorsky J., Bustin M. 2006. A role for chromosomal protein HMGN1 in corneal maturation. Differentiation. 74:19–29 10.1111/j.1432-0436.2006.00054.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calogero S., Grassi F., Aguzzi A., Voigtländer T., Ferrier P., Ferrari S., Bianchi M.E. 1999. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat. Genet. 22:276–280 10.1038/10338 [DOI] [PubMed] [Google Scholar]
- Chen G.Y., Tang J., Zheng P., Liu Y. 2009. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 323:1722–1725 10.1126/science.1168988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C.G., Scanga C.A., Collazo-Custodio C.M., Cheever A.W., Hieny S., Caspar P., Sher A. 2003. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J. Immunol. 171:4758–4764 [DOI] [PubMed] [Google Scholar]
- Furusawa T., Lim J.H., Catez F., Birger Y., Mackem S., Bustin M. 2006. Down-regulation of nucleosomal binding protein HMGN1 expression during embryogenesis modulates Sox9 expression in chondrocytes. Mol. Cell. Biol. 26:592–604 10.1128/MCB.26.2.592-604.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina P.C., Gioannini T., Buscher B.A., Zaleski A., Zheng D.S., Stoll L., Teghanemt A., Apicella M.A., Weiss J. 2001. Construction of acetate auxotrophs of Neisseria meningitidis to study host-meningococcal endotoxin interactions. J. Biol. Chem. 276:5883–5891 10.1074/jbc.M009273200 [DOI] [PubMed] [Google Scholar]
- Hock R., Furusawa T., Ueda T., Bustin M. 2007. HMG chromosomal proteins in development and disease. Trends Cell Biol. 17:72–79 10.1016/j.tcb.2006.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A., Medzhitov R. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987–995 10.1038/ni1112 [DOI] [PubMed] [Google Scholar]
- Kaisho T., Akira S. 2001. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 22:78–83 10.1016/S1471-4906(00)01811-1 [DOI] [PubMed] [Google Scholar]
- Kurosaka K., Chen Q., Yarovinsky F., Oppenheim J.J., Yang D. 2005. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J. Immunol. 174:6257–6265 [DOI] [PubMed] [Google Scholar]
- Lim J.H., Catez F., Birger Y., Postnikov Y.V., Bustin M. 2004. Preparation and functional analysis of HMGN proteins. Methods Enzymol. 375:323–342 10.1016/S0076-6879(03)75021-6 [DOI] [PubMed] [Google Scholar]
- Messmer D., Yang H., Telusma G., Knoll F., Li J., Messmer B., Tracey K.J., Chiorazzi N. 2004. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J. Immunol. 173:307–313 [DOI] [PubMed] [Google Scholar]
- Mohamed O.A., Bustin M., Clarke H.J. 2001. High-mobility group proteins 14 and 17 maintain the timing of early embryonic development in the mouse. Dev. Biol. 229:237–249 10.1006/dbio.2000.9942 [DOI] [PubMed] [Google Scholar]
- Ohashi K., Burkart V., Flohé S., Kolb H. 2000. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 164:558–561 [DOI] [PubMed] [Google Scholar]
- Okamura Y., Watari M., Jerud E.S., Young D.W., Ishizaka S.T., Rose J., Chow J.C., Strauss J.F., III 2001. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 276:10229–10233 10.1074/jbc.M100099200 [DOI] [PubMed] [Google Scholar]
- Oppenheim J.J., Yang D. 2005. Alarmins: chemotactic activators of immune responses. Curr. Opin. Immunol. 17:359–365 10.1016/j.coi.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Park J.S., Svetkauskaite D., He Q., Kim J.Y., Strassheim D., Ishizaka A., Abraham E. 2004. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 279:7370–7377 10.1074/jbc.M306793200 [DOI] [PubMed] [Google Scholar]
- Piazza M., Damore G., Costa B., Gioannini T.L., Weiss J.P., Peri F. 2011. Hemin and a metabolic derivative coprohemin modulate the TLR4 pathway differently through different molecular targets. Innate Immun. 17:293–301 10.1177/1753425910369020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prymakowska-Bosak M., Misteli T., Herrera J.E., Shirakawa H., Birger Y., Garfield S., Bustin M. 2001. Mitotic phosphorylation prevents the binding of HMGN proteins to chromatin. Mol. Cell. Biol. 21:5169–5178 10.1128/MCB.21.15.5169-5178.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovere-Querini P., Capobianco A., Scaffidi P., Valentinis B., Catalanotti F., Giazzon M., Dumitriu I.E., Müller S., Iannacone M., Traversari C., et al. 2004. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 5:825–830 10.1038/sj.embor.7400205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straino S., Di Carlo A., Mangoni A., De Mori R., Guerra L., Maurelli R., Panacchia L., Di Giacomo F., Palumbo R., Di Campli C., et al. 2008. High-mobility group box 1 protein in human and murine skin: involvement in wound healing. J. Invest. Dermatol. 128:1545–1553 10.1038/sj.jid.5701212 [DOI] [PubMed] [Google Scholar]
- Su S.B., Silver P.B., Grajewski R.S., Agarwal R.K., Tang J., Chan C.C., Caspi R.R. 2005. Essential role of the MyD88 pathway, but nonessential roles of TLRs 2, 4, and 9, in the adjuvant effect promoting Th1-mediated autoimmunity. J. Immunol. 175:6303–6310 [DOI] [PubMed] [Google Scholar]
- Teghanemt A., Widstrom R.L., Gioannini T.L., Weiss J.P. 2008. Isolation of monomeric and dimeric secreted MD-2. Endotoxin.sCD14 and Toll-like receptor 4 ectodomain selectively react with the monomeric form of secreted MD-2. J. Biol. Chem. 283:21881–21889 10.1074/jbc.M800672200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termeer C., Benedix F., Sleeman J., Fieber C., Voith U., Ahrens T., Miyake K., Freudenberg M., Galanos C., Simon J.C. 2002. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J. Exp. Med. 195:99–111 10.1084/jem.20001858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewary P., Yang D., de la Rosa G., Li Y., Finn M.W., Krensky A.M., Clayberger C., Oppenheim J.J. 2010. Granulysin activates antigen-presenting cells through TLR4 and acts as an immune alarmin. Blood. 116:3465–3474 10.1182/blood-2010-03-273953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Avalos A.M., Mao S.Y., Chen B., Senthil K., Wu H., Parroche P., Drabic S., Golenbock D., Sirois C., et al. 2007. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 8:487–496 10.1038/ni1457 [DOI] [PubMed] [Google Scholar]
- Urbonaviciute V., Fürnrohr B.G., Meister S., Munoz L., Heyder P., De Marchis F., Bianchi M.E., Kirschning C., Wagner H., Manfredi A.A., et al. 2008. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J. Exp. Med. 205:3007–3018 10.1084/jem.20081165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl T., Tenbrock K., Ludwig S., Leukert N., Ehrhardt C., van Zoelen M.A., Nacken W., Foell D., van der Poll T., Sorg C., Roth J. 2007. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 13:1042–1049 10.1038/nm1638 [DOI] [PubMed] [Google Scholar]
- Wang H., Bloom O., Zhang M., Vishnubhakat J.M., Ombrellino M., Che J., Frazier A., Yang H., Ivanova S., Borovikova L., et al. 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 285:248–251 10.1126/science.285.5425.248 [DOI] [PubMed] [Google Scholar]
- Wang H., Liao H., Ochani M., Justiniani M., Lin X., Yang L., Al-Abed Y., Wang H., Metz C., Miller E.J., et al. 2004. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 10:1216–1221 10.1038/nm1124 [DOI] [PubMed] [Google Scholar]
- Xie H., Gursel I., Ivins B.E., Singh M., O’Hagan D.T., Ulmer J.B., Klinman D.M. 2005. CpG oligodeoxynucleotides adsorbed onto polylactide-co-glycolide microparticles improve the immunogenicity and protective activity of the licensed anthrax vaccine. Infect. Immun. 73:828–833 10.1128/IAI.73.2.828-833.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Chen Q., Yang H., Tracey K.J., Bustin M., Oppenheim J.J. 2007. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J. Leukoc. Biol. 81:59–66 10.1189/jlb.0306180 [DOI] [PubMed] [Google Scholar]
- Yang D., Chen Q., Su S.B., Zhang P., Kurosaka K., Caspi R.R., Michalek S.M., Rosenberg H.F., Zhang N., Oppenheim J.J. 2008. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J. Exp. Med. 205:79–90 10.1084/jem.20062027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., de la Rosa G., Tewary P., Oppenheim J.J. 2009. Alarmins link neutrophils and dendritic cells. Trends Immunol. 30:531–537 10.1016/j.it.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]