Abstract
Background
Many patients with Parkinson’s disease (PD) develop freezing of gait (FoG), which may manifest as a hesitation or “getting stuck” when they attempt to pass through a doorway. In two experiments, we asked whether FoG is associated with (1) a deficit in internal representation of one’s body size with respect to a doorway and (2) a mismatch between imagined and actual walking times when passing through a doorway.
Method
24 subjects with PD (11 with and 13 without FoG) and 10 control subjects of similar age completed two experiments. In the Passability experiment, subjects judged the passability of doorways with different apertures scaled to their body widths. We compared passability estimates across groups. In the Imagery experiment, subjects timed themselves while: (1) imagining walking through doorways of different apertures and from different distances, and (2) actually walking in the same conditions they had just imagined. We compared imagined and actual walking durations across groups and conditions.
Results
In the Passability experiment, the estimated just-passable doorway was wider, relative to body width, in PD subjects than in control subjects, but there was no difference between PD subjects with and without FoG. In the Imagery experiment, subjects in all groups walked more slowly through narrow doorways than though wide doorways, and subjects with FoG walked much more slowly through the narrowest doorways. PD subjects with FoG showed a large discrepancy between actual and imagined time to pass through narrow doorways, unlike PD subjects without FoG and control subjects.
Conclusions
The equivalent passability judgments in PD subjects with and without FoG indicate that FoG is not specifically associated with a deficit in ability to internally represent space with reference to body size. However, the large difference in duration between actual and imagined walking through narrow doorways in subjects with FoG suggests that PD subjects with FoG did not know how much they would slow down to pass through narrow doorways. The observed discrepancy between imagined and actual walking times may point to a specific problem that contributes to the occurrence of FoG. These results also suggest that caution should be used when interpreting brain imaging results from locomotor imagery studies with PD subjects who have FoG.
Keywords: Parkinson’s disease, freezing of gait, motor imagery, body schema, internal representation, affordance
1. Introduction
Freezing of gait (FoG) is one of the most pernicious problems that people with Parkinson’s disease (PD) may encounter. This symptom, which patients often describe as a feeling that their feet are ‘glued to the floor’ when they want to walk forward, is an important cause of falls for patients who experience it, markedly limits mobility, and eventually affects up to 80% of people with PD (Hely, Reid, Adena, Halliday, & Morris, 2008). The etiology of freezing is poorly understood, but in those who are susceptible, it can be triggered by cognitive factors like distraction, anxiety, or being in a hurry; or by performing certain actions, such as initiating gait, turning, and passing through doorways or tight spaces (Bloem, Hausdorff, Visser, & Giladi, 2004; Giladi, et al., 1992). We examined two hypotheses regarding freezing in doorways to generate insights into central mechanisms underlying FoG.
It has been suggested that FoG in doorways is related to difficulty judging the passability of an opening relative to one’s own body size (Almeida & Lebold, 2010). Passability judgments necessarily depend both on the internal representation of one’s own spatial dimensions (“body schema”) and on visuospatial ability. If a deficit in either of these functions is an important contributor to freezing in doorways, one would expect subjects with PD and FoG to perform differently in judgments of doorway passability than subjects with PD without FoG. While differences in passability assessments between PD subjects with and without FoG have not been tested, two recent studies have asked PD subjects to judge the passability of different apertures. The first study found that PD subjects whose disease affected mainly the left side of their bodies (right brain) estimated that they needed a larger opening than either control subjects or subjects with primarily right-sided (left brain) disease (Lee, Harris, Atkinson, & Fowler, 2001). These authors speculated that patients with left-sided PD experience a shrinking of extrapersonal visual space. The second study found that subjects with PD and FoG made near-identical doorway passability estimates to those of control subjects (Cowie, Limousin, Peters, & Day, 2010). However, in this latter study, subjects performed the passability estimates after actually walking through doorways with different apertures; thus, they could rely on memory to complete the task. Also of note, neither study compared PD subjects with and without FoG.
In addition to a possible difficultly assessing the environment with respect to their own body dimensions, it is possible that PD subjects have a deficit in intuitively understanding their own facility for action, including how much motion a particular motor command is likely to produce and how long different actions (such as locomotion) will take to perform (Ivanenko, et al., 2011; Krakauer, Ghilardi, & Ghez, 1999). In healthy subjects, the same internal representations used for motor execution are also thought to be used for motor imagery (Decety, 1996; Jeannerod, 1995). This similarity is supported by studies showing that mental rehearsal can improve performance (Kohl & Roenker, 1980), by demonstrations of a large overlap in brain areas active for imagined and actual movement (Roth, et al., 1996), and by evidence that imagined and executed movements are of similar duration and scale similarly in response to distance and accuracy requirements (Decety & Jeannerod, 1995; Stevens, 2005).
Motor imagery depends on the supplementary motor area (SMA) (Roland, Larsen, Lassen, & Skinhøj, 1980), which, as a primary target of basal ganglia output, is underactive in people with Parkinson’s disease (Jacobs, Lou, Kraakevik, & Horak, 2009; Jahanshahi, et al., 1995; Roland, et al., 1980). Thus, it is unsurprising that motor imagery seems to be affected in PD. For instance, slow decay of motor-related potentials during motor imagery in advanced PD has been reported (Cunnington, Iansek, Johnson, & Bradshaw, 1997), and a study demonstrated that for PD patients with symptoms mainly on the right side, motor imagery of the right hand was slowed relative to motor imagery of the left hand (Helmich, de Lange, Bloem, & Toni, 2007). These results are consistent with the assumption that the internal representation used by motor imagery is the same representation used for actual movement; one would expect slower movement of the hand on the more affected side, as well as slower motor imagery. However, the primary motor deficit included in these studies is bradykinesia, which tends to be consistent across situations and repetitions. FoG, in contrast, is intermittent. It is not known whether the gait abnormalities present with FoG (including extreme slowing, variable cadence, and occasional arrests) are reflected in a common internal representation that is used both for motor imagery and for motor execution, or whether, in subjects with FoG, there is a dissociation between motor imagery and motor execution.
By carrying out two experiments with the same group of subjects, we tested two separate hypotheses about FoG in doorways. In the Passability experiment, we asked healthy older subjects and subjects with PD (with and without FoG) to assess the passability of various door widths. If FoG is associated with difficulty in evaluating the constraints in the environment with respect to the body schema, or with perceptual crowding of extrapersonal space, subjects with FoG should estimate that they need wider doorways than subjects without FoG.
In the Imagery Experiment, we asked the same subjects to time themselves while they imagined walking through doorways of different widths. As a control for ability to imagine themselves walking, we also asked subjects to time themselves while they imagined walking different distances to and through a doorway. We compared the duration of motor imagery of walking to the duration of actual walking in the same conditions. If FoG is associated with a general mismatch between motor imagery and motor execution, then PD subjects with FoG should have trouble imagining how long it would take them to actually walk in a variety of situations. If FoG is associated with a specific failure to accurately represent one’s own motor facility in situations provoking freezing of gait, then PD subjects with FoG should have particular trouble imagining how long it would take them to walk through narrow doorways.
2. Methods
2.1 Subjects
Thirty-four subjects (11 diagnosed with idiopathic PD who reported freezing of gait, 13 diagnosed with idiopathic PD who did not report freezing of gait, and 10 healthy older controls), participated in the study after signing an informed consent form approved by the Institutional Review Board of Oregon Health & Science University (OHSU). See Table 1 for demographic and clinical details. Exclusion criteria were: dementia or other neurological diseases, vestibular disorders, musculoskeletal gait impairment, and inability to stand and walk for 20 minutes. Subjects with PD were recruited from the Parkinson’s Center of Oregon and the Portland VA Medical Center. Control subjects were recruited through OHSU’s online subject recruitment service or at public lectures given by one of the investigators. Subjects with PD were tested in the morning in a practical OFF state, having taken their most recent PD medication 15 or more hours before the experiment began. Subjects participated in two experiments in a single session.
Table 1.
Demographic features of all subjects.
| PD+FoG | PD−FoG | Control | pval (+ vs −) | pval (+ vs C) | pval (− vs C) | |
|---|---|---|---|---|---|---|
| Gender (M/F) | 9/2 | 10/3 | 10/0 | > .50 | .48 | .23 |
| Age (years) | 68 ± 8 | 67 ± 6 | 67 ± 7 | > .50 | > .50 | > .50 |
| Body Width (cm) | 50 ± 4 | 51 ± 4 | 53 ± 5 | >.50 | .41 | .44 |
“+” indicates PD subjects with FoG; “−” indicates PD subjects without FoG; “C” indicates control subjects.
2.2 Procedure
2.21 Equipment
Figure 1 shows the doorway that we built out of white foam board, weighted down with bricks. The doorway was constructed in two pieces, each 2.44 m high and 18 cm thick: the stationary piece was 61 cm wide, and the movable piece was 107 cm wide. The custom doorway was installed in an intersection in the center of a 15-meter long, 168 cm-wide hallway, at a right angle to the direction of traffic. The movable piece could be slid against the stationary piece to completely close the hall, and it could be slid away from the stationary piece such that the opening could vary from 0 to 107 cm wide. Pieces of tape were affixed to the hallway floor at distances of 1.5, 3, and 6 m from one side of the doorway for starting lines, and a line of tape was placed at .5 m from the other side for a stop line.
Figure 1. Experimental Setup.
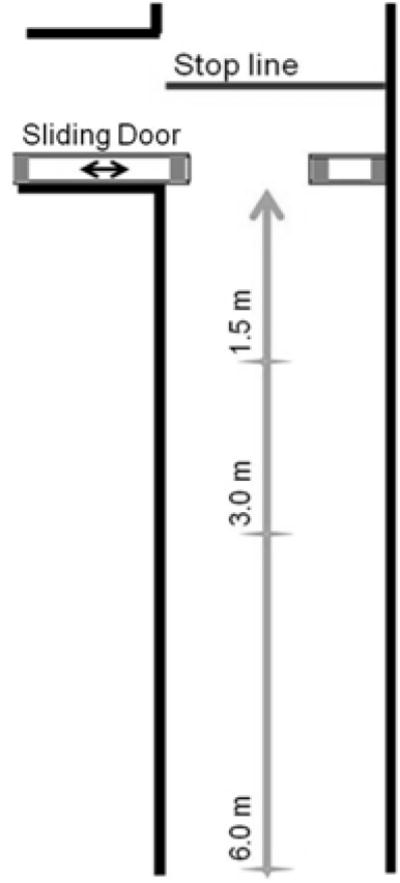
Overhead view of hallway with three start locations, one stop line, and sliding door.
2.22 Common Protocol
Following completion of informed consent, the Unified Parkinson’s Disease Rating Scale (UPDRS; (Fahn & Elton, 1987) and the Freezing of Gait Questionnaire (FOGQ; (Giladi, et al., 2000) were administered, and Hoehn and Yahr score was determined (Hoehn & Yahr, 1967). Each subjects’ actual width was measured between the foam walls by asking, subjects to stand with one arm touching the stationary foam wall, with their arms relaxed at their sides and their eyes closed. The sliding door was slowly closed until it was touching the subject’s other arm. The door was opened before the subject was instructed to open his or her eyes.
2.23 Passability Experiment
Subjects stood 1.5 meters behind the sliding doorway with their eyes closed while the aperture was set to approximately 200% of the subject’s actual body width. The subject was then instructed to open his or her eyes and answer the question, “Could you walk through this doorway without squeezing or rotating your torso?” The procedure and question were repeated with an increasingly narrow doorway: the doorway was closed by about 10 cm per repetition until it was about 130% of the subject’s actual width. Then it was closed by 1–2 cm until the subject answered, “No.” At this point, the doorway was closed all the way and opened in units of around 10 cm until it was about 80% of the subject’s actual width, then opened in increments of 1–2 cm until the subject answered, “Yes.” The experimenter attempted to use different starting apertures and increments every time, so that the subject could not just count repetitions to arrive at the same answer every time. This procedure was repeated until there were three transitions from “yes” to “no” and three transitions from “no” to “yes.” The average of these six numbers was computed as the subject’s mean transition, and the ratio of each subject’s mean transition to his or her actual width was recorded as that subject’s passability estimate.
2.24 Imagery Experiment
After completing the doorway passability estimates experiment, subjects participated in a two-part motor imagery experiment: (A) Doorway Width manipulation and (B) Distance manipulation as a control. In Part A, subjects timed themselves imagining walking through the doorway while it was open to different widths. The experimenter emphasized that the subjects should imagine the movement as realistically as possible, including rotating the body, if necessary, to pass through the doorway. Before beginning, subjects were informed that after completing the imaginary walking task, they would be walking in the same conditions they imagined, and they should imagine it exactly as they would actually do it. To begin, subjects stood behind the 1.5 m line and closed their eyes while the doorway was adjusted to 130% of their actual width. Then they were invited to open their eyes and begin when ready. For each trial, subjects pressed the ‘start’ button on a hand-held stopwatch, imagined walking through the doorway to a line 1 m beyond it, and pressed the button again to stop the stopwatch. Then, without looking at the stopwatch, they handed it to an experimenter, who recorded the time and reset the stopwatch. This procedure was repeated with the doorway set to different percentages of the subject’s actual width, beginning at 130%, decreasing by 10% to 80%, then repeating 80% and increasing by 10% to 130%. In the second part of this experiment, the doorway was set to 130% again, and the same set of doorway widths were repeated, only instead of imagining walking, subjects actually walked through the doorway. Again, they timed themselves and then handed the stopwatch to an experimenter.
Part B was like Part A, except that instead of varying the width of the doorway, the experimenter varied the distance walked. The doorway was left at a constant 130% of the subject’s actual width, and the subject stood behind lines placed at three distances from the doorway: 1.5 m, 3 m, and 6 m. Subjects imagined walking through the doorway from each of these distances in increasing and then decreasing order, then actually walked the distances in the same order, timing themselves in both conditions as before.
For both Part A and Part B, we computed the difference (mismatch) in duration between actual walking and imagined walking for each subject in each condition (Personnier, Kubicki, Laroche, & Papaxanthis, 2010).
2.25 Statistics
Before analyzing our data, we tested it for normality. Data that passed the test for normality were subjected to ANOVA, and data that failed the test for normality were subjected to non-parametric tests, the Mann-Whitney U or Wilcoxon rank sum (Wilcoxon) tests. Passability estimates failed the test for normality. Therefore, to evaluate differences in passability estimates between groups in the Passability experiment, we compared scores in each of the three groups to scores in the two other groups, using independent sample, two-tailed Wilcoxon tests; we also report confidence intervals (CI).
Data from the Imagery experiment passed the test for normality. Therefore, to compare actual and imagined walking durations in the Imagery experiment, we used ANOVA with a linear mixed model approach that included subject as a random factor. Fixed factors were group, trial type (actual and imagined), and condition (width in Part A and distance in Part B). The dependent measure was duration. We began by conducting two separate 3-way ANOVAs for Part A and Part B. Significant interactions were followed up with post hoc tests.
We also tested for associations between clinical variables (disease duration, side of onset, and UPDRS) and experimental variables (passability estimates and mismatch between imagined and executed walking durations). Spearman’s correlation was used to test association with disease duration and UPDRS, and Wilcoxon tests were used for the side of onset. Alpha was set at .05 for all tests.
3. Results
3.1 Subject Demographics
Subject demographics are presented in Tables 1 and 2. Subjects in all groups were well matched in age and body width. Among the subjects with PD, those with and without FoG had similar disease durations, and sides of disease onset were equally balanced, but the subjects with FoG had more severe Hoehn & Yahr stages and UPDRS scores than the subjects without FoG.
Table 2.
Clinical features of PD subjects.
| PD + FoG | PD − FoG | C.I. (chi2) | p-value | |
|---|---|---|---|---|
| Disease duration | 9.9± 7.8 | 6.2 ± 3.6 | −1.3:8.7 | .13 |
| Side of Onset (R/L/N) | 5/5/1 | 5/5/3 | (0) | 1 |
| Hoehn & Yahr Stage | 3.0 ± 0.8 | 2.1 ± 0.5 | 0.3:1.4 | .003 |
| UPDRS | 44.9 ± 15.1 | 32.2 ± 7.6 | 2.9:22.6 | .01 |
| FOGQ Score | 10.7 ± 4.1 | N/A |
Values are mean ± SD, except where indicated. UPDRS = Unified Parkinson’s Disease Rating Scale (motor symptoms); FOGQ = Freezing of Gait Questionnaire. C.I. = Confidence Interval. N/A = not administered.
3.2 Passability Experiment
Figure 2 shows passability estimates. Healthy subjects judged that they could easily pass through a doorway that was 97% of their body width, whereas PD subjects (both with and without FoG) judged that they needed 109% of their body width in order to easily pass. Wilcoxon tests indicated that control subjects’ estimates were narrower than estimates of PD subjects with FoG (CI = 5.3: 21.8, p = .01), and PD subjects without FoG (CI = 0.9: 19.8, p = .03). However, PD subjects with and without FoG did not differ in their estimates (CI = −12.5: 8.1, p=.41). Passability estimates were not related to side of disease onset, UPDRS scores, or disease duration.
Figure 2. Passability Estimates.
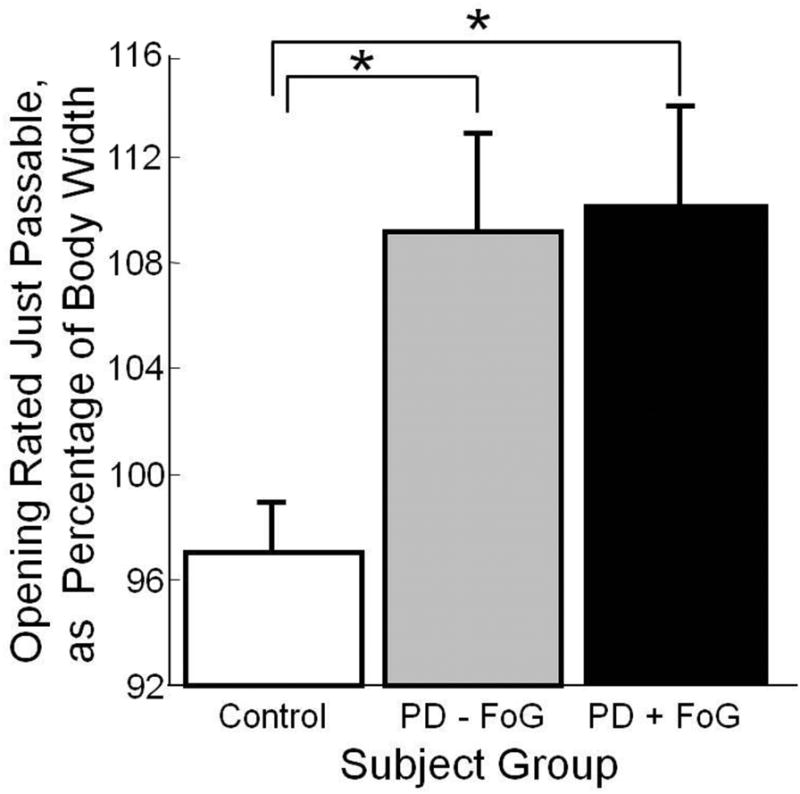
Doorway opening rated just passable, as percentage of body width. Error bars represent standard error of the mean. One subject with PD and FoG was not included in this protocol, due to experimenter error. Significant differences (p < .05) are indicated by *.
3.3 Motor Imagery Experiment
3.31 Part A. Width Manipulation
All subjects were asked during debriefing if they froze during imagined walking, and all subjects reported that they did not. However, inspection of the videotapes of subjects walking through the door reveals that many of the PD subjects with FoG experienced one or more brief freezing episodes (hesitations, festination, or double-stepping) when approaching or turning their bodies to pass through the narrowest doorways. The data collection method was not sophisticated enough to determine whether these episodes could account for the large (over 2s) discrepancy between actual and imagined walking times. Of note, two PD subjects with FoG experienced severe freezing episodes (>30s with no forward step) when attempting to walk through the narrowest two doorways, and one other subject’s walking times were more than twice as slow as the next-slowest subject. Because inclusion of these subjects’ data would have introduced very large variability into the walking durations, they are not included in the figures or analysis. Mean imagined walking durations of the subjects who froze were 4.9s and 5.0s. Although these durations were longer than those of most of the other subjects, they do not reflect the freezing of gait lasting over 30 seconds that was experienced by these subjects.
Figure 3 shows actual and imagined walking durations for the three subject groups in the doorway width manipulation trials. The 3-way ANOVA indicated no main effects of group, doorway width, or trial type. However, there was a significant interaction between group and width, F(1, 376) = 22.7, p < .0001, and a significant 3-way interaction, F(1,376) = 4.2, p=.04. Post-hoc analysis revealed that imagined walking durations were not different between groups, but that in actual walking, the groups were differently affected by doorway width. PD subjects with FoG were more slowed by the narrow doorway than control subjects, F(1,105) = 21.4, p<.0001, or PD subjects without FoG, F(1,122) = 26.3, p<.0001. Furthermore, PD subjects with FoG (but not other subjects) imagined that they could walk more quickly than they actually could, as revealed by a significant finding in a one-way ANOVA testing doorway width, F(1,54) = 20.7, p<.0001.
Figure 3. Durations of Actual and Imagined Walking as a Function of Door Width.
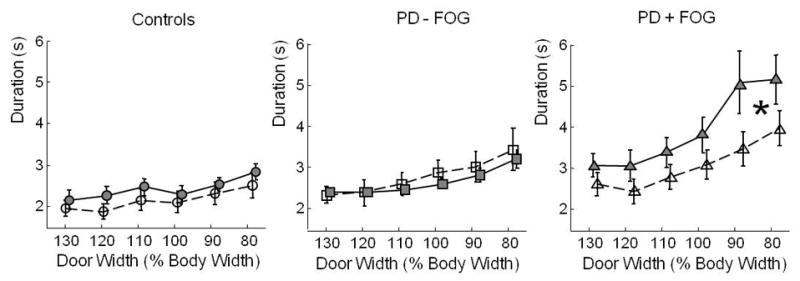
Duration in seconds of actual walking (filled shapes and solid lines) and imagined walking (hollow shapes and dashed lines) in the different conditions of the Imagery Experiment, Part A. Door width was varied as a percentage of body width, and distance from the start line to the doorway was fixed at 1.5 m. Circles: healthy control subjects; squares: PD subjects without FoG; triangles: PD subjects with FoG. The locus of the significant 3-way interaction is indicated by a *.
Overall, motor imagery was 26% faster than actual walking in PD subjects with FoG, 10% faster than actual walking in healthy control subjects, and 4% slower than actual walking in PD subjects without FoG.
The mismatch (difference) between actual and imagined walking durations in PD subjects with FoG was not related to their disease duration, side of onset, or motor UPDRS score.
3.32 Part B. Distance Manipulation
Figure 4 shows actual and imagined walking durations for all subject groups in the distance manipulation trials. One subject with PD and FoG did not participate in this task, due to fatigue. ANOVA indicated an overall effect of distance, F(1,174) = 18.1, p<.0001. There were no differences between groups and no differences between actual and imagined walking for either group.
Figure 4. Durations of Actual and Imagined Walking as a Function of Distance Covered.
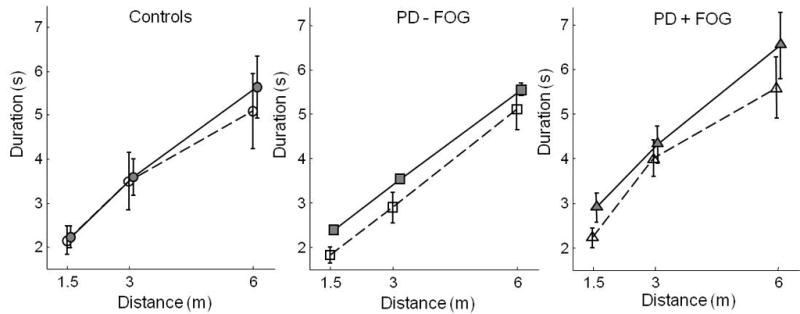
Duration in seconds of actual walking (filled shapes and solid lines) and imagined walking (hollow shapes and dashed lines) in the different conditions of the Imagery Experiment, Part B. Distance from the start line to the doorway was varied, and door width was fixed at 130% of body width. Error bars represent standard error of the mean. Circles: healthy control subjects; squares: PD subjects without FoG; triangles: PD subjects with FoG.
The difference between actual and imagined walking durations in Part B was not related to disease duration or UPDRS scores. However, it was related to side of disease onset (CI = .03–.45, p = 0.007). Subjects with left side onset had faster motor imagery, relative to their walking speed, than subjects with right side onset.
4. Discussion
4.1 Main Findings
In two experiments with the same subjects, we tested two hypotheses about the FoG that many subjects with PD experience when passing through doorways. Results suggest that FoG is not associated with a problem judging passability, but that it is associated with a discrepancy between motor imagery and motor execution when passing through narrow doors.
4.11 Passability Experiment
Because PD is associated with visuospatial deficits (Levin, et al., 1991) and with proprioceptive deficits (Konczak, et al., 2009), it has been suggested that FoG may be aggravated by failure to correctly perceive environmental constraints such as doorway width, in reference to one’s own body size (Almeida and Lebold, 2010). We tested this hypothesis by asking subjects to judge the passability of doorways based on visual observation of different apertures. We found that although subjects with PD selected just-passable doorways that were wider, relative to their bodies, than those chosen by control subjects, there was no difference between PD subjects with and without FoG. Thus, while visuospatial or body schema deficits may contribute to the underlying state that allows FoG to occur, these deficits are unlikely to be primarily responsible for FoG.
4.12 Imagery Experiment
Because PD is associated with deficits in motor imagery (Braun, Beurskens, Kleynen, Schols, & Wade, 2011; Cunnington, et al., 1997; Helmich, et al., 2007), and because of the intermittent nature of FoG, we proposed that FoG may be associated with a mismatch between motor imagery and motor execution when walking in difficult conditions. We tested this hypothesis by asking subjects to time themselves while performing imagined and actual walking through doorways of different apertures and, to control for general problems with imagery, from different distances. We found that, unlike control subjects and PD subjects without FoG, PD subjects with FoG had a poor match between their imagined and actual walking speeds; in particular, they slowed more than other subjects when walking through narrow doorways, but their motor imagery durations did not reflect this difference. The discrepancy was not accounted for by disease duration or severity.
4.2 Relation to Previous Findings
4.21 Perception of Passability
The finding of a difference in perception of passability between subjects with and without PD is consistent with recent brain imaging results. Snijders et al (2010) found that when contrasting motor imagery to visual imagery, subjects with PD showed a smaller difference in activation than healthy control subjects in the right superior parietal lobe. This cortical region has been associated with the extraction of spatial information necessary for planning visually-guided action (Culham & Valyear, 2006; Goodale & Milner, 1992). If PD subjects have trouble extracting the necessary spatial information for planning visually guided action, allowing more space to pass through a doorway (allowing a “buffer”) would be a logical response.
In contrast to our study, Cowie et al recently reported an absence of difference in perceptions of passability between subjects with and without PD (Cowie, et al., 2010). We attribute this discrepancy to the fact that in the Cowie study, subjects performed passability estimates after having walked through doorways of different widths, so they could rely on memory rather than motor imagery to complete the task. The finding by Cowie et al. that PD subjects reported passability estimates that were 80% of their body widths, similar to those reported by our control subjects, suggests that the practice walking (during which subjects were allowed to turn their bodies if necessary) was a major factor in the null results of that study. Another difference between the two studies is that in the Cowie et al. study, the doorway was a simple wooden frame, whereas in the present study it was a solid object that blocked off the hallway; this might have been a more realistic representation of a doorway to the subjects. A third difference is that subjects in the previous study were positioned 5 m from the doorway, whereas subjects in the present study were positioned 1.5 m from the doorway (following Lee et al, 2001). Again, this could have increased the salience of the doorway to the subjects.
The absence of an effect of the more parkinsonian side on passability estimates in the present study differs from previous results in which PD subjects with left-side onset were found to have poorer estimates of passability than PD subjects with right-side onset (Lee, et al., 2001). We note two differences between the present study of passability estimates and the previous study. In the Lee et al. study, subjects were instructed to imagine that they were standing on a trolley that passed through the door, rather than actively walking through as in the present study. Thus, the previous task may have drawn more on visual imagery than on motor imagery. Also, stimuli in the Lee et al. study were a visual image projected onto a screen, rather than an actual doorway. This could have made the task seem more abstract, again engaging less of the motor system than the present study. In the present study, differences depending on side of disease onset were found only in the motor imagery condition that included longer walking distances, which occurred after actual walking through the doorways. Noting that this was also a condition in which we did not find a difference between subjects with and without FoG, we speculate that subjects might have used a different strategy for this task, relying on memory and estimation rather than imagining themselves in the doorway. Thus, side of onset might have a greater effect on memory and estimation than on motor imagery.
4.23 Internal Representation of Locomotor Speed
The finding of overall slowing of imagined and actual gait as a function of both distance and door width is consistent with previous results from motor imagery studies (Decety & Jeannerod, 1995) and with results from many studies showing a speed-accuracy trade-off for rapid, target-directed movement (Fitts, 1954). The present findings are also consistent with recent reports that subjects with PD reduce forward velocity, decrease step length, and increase step length variability when passing through a doorway; these changes were more extreme when the doorway was narrow than when it was wide, and more extreme in subjects with FoG than in subjects without FoG (Almeida & Lebold, 2010; Cowie, et al., 2010). One explanation previously put forth to explain this slowing is that FoG is associated with a perceptual deficit (Almeida & Lebold, 2010). The present results suggest that, instead, there is an underlying concern or perceptual difference with respect to passing through narrow spaces that affects all subjects with PD, and that subjects with FoG respond differently to that concern than subjects without FoG.
This study reports, for the first time, a substantial discrepancy between actual and imagined locomotor times by PD subjects who have FoG. This discrepancy was particularly large in the conditions that significantly slowed gait and caused freezing, the narrowest doorways, Previous studies using mental chronometry have emphasized the similarity in motor imagery between control subjects and subjects with mild or moderate PD (Dominey, Decety, Broussolle, Chazot, & Jeannerod, 1995; Heremans, et al., 2011). However, a recent study of PD subjects with and without FoG who were matched for severity showed a nonsignificant trend for motor imagery of walking to be of shorter duration in PD subjects with FoG than in PD subjects without FoG (Snijders, et al., 2011). The authors did not report actual walking times, but presumably the PD subjects with FoG did not walk faster than PD subjects without FoG. Thus, those data appear consistent with our suggestion that motor imagery may sometimes be dissociated from motor execution in PD subjects with FoG. Another recent study found that for subjects with advanced PD, mental rehearsal of locomotor tasks did not evoke greater improvement in walking than relaxation training did (Braun, et al., 2011). Given that FoG is increasingly common as PD severity increases, this result also supports the idea of a breakdown in the link between imagery and execution in advanced PD and associated FoG.
4.3 Implications for Understanding FoG
In this study, the finding that FoG was associated with an underestimation of duration of actual walking through a doorway suggests two broad categories of interpretation: abnormal locomotor execution or abnormal internal representation of locomotion. One could postulate that the absence of a significant motor imagery duration difference between groups in the present study indicates that motor representations are not more impaired in PD subjects with FoG than in PD subjects without FoG, but that FoG arises downstream from these representations, in the execution stage. This explanation is consistent with the theory that FoG is associated with problems in the locomotor centers in the brainstem – specifically, in the pedunculopontine nucleus or mesencephalic locomotor region (Lewis & Barker, 2009; Takakusaki, Tomita, & Yano, 2008).
Alternatively, one could postulate that the inability of PD subjects with FOG to accurately imagine their walking times through narrow doorways indicates a problem with representing their own ability to perform a movement – especially a complex motor act requiring coordination of multiple elements with accurate timing. Walking through a narrow doorway is a complex form of locomotion, requiring the coordination of forward walking with rotating the shoulders (Warren & Whang, 1987). The failure to accurately represent one’s own locomotor ability could indicate a failure of subjects with FoG to take into account their own slowness of movement (bradykinesia) and difficulty with torso rotation (axial rigidity). Although anosognosia (unawareness of impairment) is not often reported in PD, evidence suggests that a subgroup of people with severe PD are less aware of some of their motor symptoms than others (Seltzer, Vasterling, Mathias, & Brennan, 2001). Perhaps this subgroup is more likely to develop FoG.
If the motor representation that is generated does not allow sufficient time for each element to be carried out, the motor system could attempt to execute simultaneously motor acts that should be executed sequentially, such as attempting to step with a foot before shifting weight off it, and this could lead to FoG. This explanation is consistent with the observation that FoG typically occurs in situations requiring execution of complex motor sequences, such as when working in one’s own kitchen, where one takes only a few steps in any direction at a given time, generally with the intention of performing another action immediately upon arriving at the new destination. Thus, failure to account for one’s motor deficits when generating a representation for a complex motor act could lead one to expect to move more quickly than the motor execution system can support, resulting in FoG.
4.4 Implications for Motor Imagery as a Tool for Studying FoG
Researchers wanting to investigate brain areas associated with locomotion are confronted with a substantial problem: most neuroimaging techniques require subjects to lie supine and still; thus, they do not permit actual locomotion. The abundant evidence supporting the similarity of neural activation between actual and imagined upper-limb movement (Grezes & Decety, 2001; Porro, et al., 1996; Rizzolatti & Luppino, 2001; Roth, et al., 1996; Stephan, et al., 1995) has been used to justify studying the brain during motor imagery of locomotion as a way to study neural substrates of posture and gait (Bakker, et al., 2008; Jahn, et al., 2008; Jahn, et al., 2004; Malouin, Richards, Jackson, Dumas, & Doyon, 2003; Miyai, et al., 2001; Sacco, et al., 2006). This method has also been used to investigate brain areas associated with FoG (Snijders, et al., 2011). However, the results of the present study suggest that, when carrying out a complex motor act such as walking through a doorway, there is a greater discrepancy between imagined and executed movement in subjects with FoG than in either healthy subjects or PD subjects without FoG. Although existing evidence suggests that motor imagery can shed light on internal representations associated with motor execution in healthy subjects, the brain activity associated with motor imagery may not be an accurate reflection of the brain activity associated with motor execution in subjects with FoG, especially for complex motor acts. Thus, while the results of studies using neuroimaging of subjects with freezing of gait performing motor imagery of locomotion are promising, they need corroboration from other results based on observing brain activity during actual movement.
4.5 Limitations
Because we were most interested in the width manipulation when we designed the Imagery Experiment, all subjects completed both the motor imagery and the actual walking at the shortest distance with different apertures (Part A) before they performed motor imagery of walking different distances with a constant aperture (Part B). It is possible that, even though we provided no external feedback, subjects learned from practicing the walking tasks. Therefore, the finding of no difference in imagining distance-related walking times across groups in Part B must be interpreted with caution. However, the results from Part A, in which we saw a group difference when walking through narrow apertures, were unconfounded by practice.
Another limitation of this study is the difference in disease severity (but not disease duration) between the two groups of PD subjects. Because the likelihood of experiencing FoG increases with the severity of the disease, it is difficult to match severity between PD subjects with and without FoG. The discrepancy between actual and imagined walking durations for narrow doorways demonstrated here did not correlate with disease duration or UPDRS score. This suggests that the experiment was capturing an aspect of FoG, not merely disease severity.
5. Conclusion
We found that, although changes in visuospatial processing distinguished PD subjects from age-matched healthy control subjects, they did not distinguish between PD subjects with and without FoG. In addition, there was no general motor imagery deficit associated with FoG. However, FoG was specifically associated with a tendency to imagine passing through a narrow doorway more quickly than one could execute such a passage. The observed discrepancy between imagined and actual walking times may point to a specific problem that contributes to the occurrence of FoG. One possible explanation for the discrepancy is that motor representation is fine and only execution is impaired. If this is the case, there is no causal link between the mismatch and FoG itself. Another possibility is that the mismatch indicates a problem representing complex motor acts that require fully functional basal ganglia. If this is the case, the mismatch may actually be a clue to how FoG is caused. Further studies are needed to distinguish these possibilities. In either case, caution is warranted when using motor imagery to study the neural substrates of FoG.
Whatever the underlying cause of the mismatch between motor imagery and execution, its association with FoG suggests that rehabilitation strategies should include a focus on improving that match. We therefore suggest that therapies that include both representation and execution of a movement, with attention to the difference between them (Farley & Koshland, 2005; Morris, Iansek, & Kirkwood, 2009), should be investigated with respect to FoG.
HIGHLIGHTS.
PD subjects estimate more space needed to pass through a doorway than control subjects estimate.
Subjects with FoG imagine walking more quickly through narrow spaces than they actually walk.
The execution-imagery discrepancy may point to a specific problem that contributes to FoG.
Acknowledgments
We are grateful to the subjects who participated in this study, to Triana Nagel for help scheduling and testing subjects, and to Charlie Russell for building the equipment. This study was funded by a grant from NIH (R37AG006457).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida QJ, Lebold CA. Freezing of gait in Parkinson’s disease: a perceptual cause for a motor impairment? Journal of Neurology, Neurosurgery, and Psychiatry. 2010;81(5):513–518. doi: 10.1136/jnnp.2008.160580. [DOI] [PubMed] [Google Scholar]
- Bakker M, De Lange F, Helmich R, Scheeringa R, Bloem B, Toni I. Cerebral correlates of motor imagery of normal and precision gait. Neuroimage. 2008;41(3):998–1010. doi: 10.1016/j.neuroimage.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Movement Disorders. 2004;19(8):871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- Braun S, Beurskens A, Kleynen M, Schols J, Wade D. Rehabilitation with mental practice has similar effects on mobility as rehabilitation with relaxation in people with Parkinson’s disease: a multicentre randomised trial. Journal of Physiotherapy. 2011;57(1):27–34. doi: 10.1016/S1836-9553(11)70004-2. [DOI] [PubMed] [Google Scholar]
- Cowie D, Limousin P, Peters A, Day BL. Insights into the neural control of locomotion from walking through doorways in Parkinson’s disease. Neuropsychologia. 2010;48(9):2750–2757. doi: 10.1016/j.neuropsychologia.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Culham JC, Valyear KF. Human parietal cortex in action. Current Opinion in Neurobiology. 2006;16(2):205–212. doi: 10.1016/j.conb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Iansek R, Johnson KA, Bradshaw JL. Movement-related potentials in Parkinson’s disease. Motor imagery and movement preparation. Brain. 1997;120(Pt 8):1339–1353. doi: 10.1093/brain/120.8.1339. [DOI] [PubMed] [Google Scholar]
- Decety J. The neurophysiological basis of motor imagery. Behavioural Brain Research. 1996;77(1–2):45–52. doi: 10.1016/0166-4328(95)00225-1. [DOI] [PubMed] [Google Scholar]
- Decety J, Jeannerod M. Mentally simulated movements in virtual reality: does Fitts’s law hold in motor imagery? Behav Brain Res. 1995;72(1–2):127–134. doi: 10.1016/0166-4328(96)00141-6. [DOI] [PubMed] [Google Scholar]
- Dominey P, Decety J, Broussolle E, Chazot G, Jeannerod M. Motor imagery of a lateralized sequential task is asymmetrically slowed in hemi-Parkinson’s patients. Neuropsychologia. 1995;33(6):727–741. doi: 10.1016/0028-3932(95)00008-q. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R. Unified Parkinson’s Disease Rating Scale. Florham Park: NJ: Macmillan Health Care Information; 1987. [Google Scholar]
- Farley BG, Koshland GF. Training BIG to move faster: the application of the speed-amplitude relation as a rehabilitation strategy for people with Parkinson’s disease. Experimental Brain Research. 2005;167(3):462–467. doi: 10.1007/s00221-005-0179-7. [DOI] [PubMed] [Google Scholar]
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. Journal of Experimental Psychology. 1954;47(6):381–391. [PubMed] [Google Scholar]
- Giladi N, McMahon D, Przedborski S, Flaster E, Guillory S, Kostic V, et al. Motor blocks in Parkinson’s disease. Neurology. 1992;42(2):333–339. doi: 10.1212/wnl.42.2.333. [DOI] [PubMed] [Google Scholar]
- Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism and Related Disorders. 2000;6(3):165–170. doi: 10.1016/s1353-8020(99)00062-0. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends in Neuroscience. 1992;15(1):20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Grezes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta-analysis. Human Brain Mapping. 2001;12(1):1–19. doi: 10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmich RC, de Lange FP, Bloem BR, Toni I. Cerebral compensation during motor imagery in Parkinson’s disease. Neuropsychologia. 2007;45(10):2201–2215. doi: 10.1016/j.neuropsychologia.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Movement Disorders. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- Heremans E, Feys P, Nieuwboer A, Vercruysse S, Vandenberghe W, Sharma N, et al. Motor imagery ability in patients with early-and mid-stage Parkinson disease. Neurorehabilitation and Neural Repair. 2011;25(2):168–177. doi: 10.1177/1545968310370750. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Ivanenko Y, Dominici N, Daprati E, Nico D, Cappellini G, Lacquaniti F. Locomotor body scheme. Human Movement Science. 2011;30:341–351. doi: 10.1016/j.humov.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Lou JS, Kraakevik JA, Horak FB. The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without parkinson’s disease. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain. 1995;118(Pt 4):913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Jahn K, Deutschlander A, Stephan T, Kalla R, Wiesmann M, Strupp M, et al. Imaging human supraspinal locomotor centers in brainstem and cerebellum. Neuroimage. 2008;39(2):786–792. doi: 10.1016/j.neuroimage.2007.09.047. [DOI] [PubMed] [Google Scholar]
- Jahn K, Deutschlander A, Stephan T, Strupp M, Wiesmann M, Brandt T. Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. Neuroimage. 2004;22(4):1722–1731. doi: 10.1016/j.neuroimage.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Mental imagery in the motor context. Neuropsychologia. 1995;33(11):1419–1432. doi: 10.1016/0028-3932(95)00073-c. [DOI] [PubMed] [Google Scholar]
- Kohl RM, Roenker DL. Bilateral transfer as a function of mental imagery. Journal of Motor Behavior. 1980 doi: 10.1080/00222895.1980.10735220. [DOI] [PubMed] [Google Scholar]
- Konczak J, Corcos DM, Horak F, Poizner H, Shapiro M, Tuite P, et al. Proprioception and motor control in Parkinson’s disease. Journal of Motor Behavior. 2009;41(6):543–552. doi: 10.3200/35-09-002. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Ghilardi MF, Ghez C. Independent learning of internal models for kinematic and dynamic control of reaching. Nature Neuroscience. 1999;2(11):1026–1031. doi: 10.1038/14826. [DOI] [PubMed] [Google Scholar]
- Lee AC, Harris JP, Atkinson EA, Fowler MS. Disruption of estimation of body-scaled aperture width in Hemiparkinson’s disease. Neuropsychologia. 2001;39(10):1097–1104. doi: 10.1016/s0028-3932(01)00032-x. [DOI] [PubMed] [Google Scholar]
- Levin B, Llabre M, Reisman S, Weiner W, Sanchez-Ramos J, Singer C, et al. Visuospatial impairment in Parkinson’s disease. Neurology. 1991;41(3):365–369. doi: 10.1212/wnl.41.3.365. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Barker RA. A pathophysiological model of freezing of gait in Parkinson’s disease. Parkinsonism and Related Disorders. 2009;15(5):333–338. doi: 10.1016/j.parkreldis.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J. Brain activations during motor imagery of locomotor-related tasks: a PET study. Human Brain Mapping. 2003;19(1):47–62. doi: 10.1002/hbm.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, et al. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage. 2001;14(5):1186–1192. doi: 10.1006/nimg.2001.0905. [DOI] [PubMed] [Google Scholar]
- Morris ME, Iansek R, Kirkwood B. A randomized controlled trial of movement strategies compared with exercise for people with Parkinson’s disease. Movement Disorders. 2009;24(1):64–71. doi: 10.1002/mds.22295. [DOI] [PubMed] [Google Scholar]
- Personnier P, Kubicki A, Laroche D, Papaxanthis C. Temporal features of imagined locomotion in normal aging. Neuroscience Letters. 2010;476(3):146–149. doi: 10.1016/j.neulet.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Porro CA, Francescato MP, Cettolo V, Diamond ME, Baraldi P, Zuiani C, et al. Primary motor and sensory cortex activation during motor performance and motor imagery: a functional magnetic resonance imaging study. Journal of Neuroscience. 1996;16(23):7688–7698. doi: 10.1523/JNEUROSCI.16-23-07688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31(6):889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Roland PE, Larsen B, Lassen N, Skinhøj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. Journal of Neurophysiology. 1980;43(1):118. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- Roth M, Decety J, Raybaudi M, Massarelli R, Delon-Martin C, Segebarth C, et al. Possible involvement of primary motor cortex in mentally simulated movement: a functional magnetic resonance imaging study. Neuroreport. 1996;7(7):1280. doi: 10.1097/00001756-199605170-00012. [DOI] [PubMed] [Google Scholar]
- Sacco K, Cauda F, Cerliani L, Mate D, Duca S, Geminiani GC. Motor imagery of walking following training in locomotor attention. The effect of “the tango lesson”. Neuroimage. 2006;32(3):1441–1449. doi: 10.1016/j.neuroimage.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Vasterling JJ, Mathias CW, Brennan A. Clinical and neuropsychological correlates of impaired awareness of deficits in Alzheimer disease and Parkinson disease: a comparative study. Cognitive and Behavioral Neurology. 2001;14(2):122–129. [PubMed] [Google Scholar]
- Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, et al. Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait. Brain. 2011;134(1):59–72. doi: 10.1093/brain/awq324. [DOI] [PubMed] [Google Scholar]
- Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, et al. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. Journal of Neurophysiology. 1995;73(1):373–386. doi: 10.1152/jn.1995.73.1.373. [DOI] [PubMed] [Google Scholar]
- Stevens JA. Interference effects demonstrate distinct roles for visual and motor imagery during the mental representation of human action. Cognition. 2005;95(3):329–350. doi: 10.1016/j.cognition.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Tomita N, Yano M. Substrates for normal gait and pathophysiology of gait disturbances with respect to the basal ganglia dysfunction. Journal of Neurology. 2008;255(Suppl 4):19–29. doi: 10.1007/s00415-008-4004-7. [DOI] [PubMed] [Google Scholar]
- Warren WH, Jr, Whang S. Visual guidance of walking through apertures: body-scaled information for affordances. Journal of Experimental Psychology: Human Perception and Performance. 1987;13(3):371–383. doi: 10.1037//0096-1523.13.3.371. [DOI] [PubMed] [Google Scholar]


