Abstract
Objective
To estimate the global burden of chronic pulmonary aspergillosis (CPA) after pulmonary tuberculosis (PTB), specifically in cases with pulmonary cavitation.
Methods
PTB rates were obtained from the World Health Organization and a scoping review of the literature was conducted to identify studies on residual pulmonary cavitation after PTB and estimate the global incidence of CPA after PTB. Having established that from 21% (United States of America) to 35% (Taiwan, China) of PTB patients developed pulmonary cavities and that about 22% of these patients developed CPA, the authors applied annual attrition rates of 10%, 15% and 25% to estimate the period prevalence range for CPA over five years. Analysis was based on a deterministic model.
Findings
In 2007, 7.7 million cases of PTB occurred globally, and of them, an estimated 372 000 developed CPA: from 11 400 in Europe to 145 372 in South-East Asia. The global five-year period prevalence was 1 174 000, 852 000 and 1 372 000 cases at 15%, 25% and 10% annual attrition rates, respectively. The prevalence rate ranged from < 1 case per 100 000 population in large western European countries and the United States of America to 42.9 per 100 000 in both the Democratic Republic of the Congo and Nigeria. China and India had intermediate five-year period prevalence rates of 16.2 and 23.1 per 100 000, respectively.
Conclusion
The global burden of CPA as a sequel to PTB is substantial and warrants further investigation. CPA could account for some cases of smear-negative PTB. Since CPA responds to long-term antifungal therapy, improved case detection should be urgently undertaken.
Résumé
Objectif
Estimer la charge globale de l’aspergillose pulmonaire chronique (APC) après la tuberculose pulmonaire (TBP), en particulier dans les cas présentant une cavitation pulmonaire.
Méthodes
Les taux de TBP ont été fournis par l’Organisation mondiale de la Santé, et un examen de la portée de la documentation a été réalisé afin d’identifier les études sur la cavitation pulmonaire résiduelle après la TBP et d’estimer l’incidence globale de l’APC après la TBP. Après avoir établi que 21% (États-Unis d’Amérique) à 35% (Taiwan, Chine) des patients atteints de TBP ont développé des cavités pulmonaires et qu’approximativement 22% de ces patients ont développé l’APC, les auteurs ont appliqué des taux d’attrition annuelle de 10%, 15% et 25% afin d’évaluer l’étendue de la prévalence de période de l’APC sur cinq ans. L’analyse reposait sur un modèle déterministe.
Résultats
En 2007, on a enregistré à l’échelle mondiale 7,7 millions de patients atteints de TBP, et on estime que 372 000 d’entre eux ont ensuite développé l’APC: de 11 400 en Europe à 145 372 en Asie du Sud-est. La prévalence de période de cinq ans globale était de 1 174 000, 852 000 et 1 372 000 cas à des taux d’attrition annuelle de 15%, 25% et 10%, respectivement. Le taux de prévalence était compris entre moins de 1 cas pour 100 000 habitants dans les grands pays d’Europe de l’Ouest et les États-Unis d’Amérique à 42,9 cas pour 100 000 habitants au Nigéria et en République démocratique du Congo. La Chine et l’Inde présentaient des taux de prévalence de période de cinq ans intermédiaires de 16,2 et 23,1, respectivement, pour 100 000 habitants.
Conclusion
La charge globale de l’APC en tant que séquelle de la TBP est considérable et justifie des examens ultérieurs. L’APC pourrait expliquer certains cas de TBP à frottis négatifs. Comme l’ACP répond à la thérapie antifongique à long terme, une meilleure détection des cas devrait immédiatement être entreprise.
Resumen
Objetivo
Calcular la carga global de la aspergilosis pulmonar crónica (APC) después de la tuberculosis pulmonar (TBP), específicamente, en casos con cavitación pulmonar.
Métodos
Se obtuvieron las tasas de TBP de la Organización Mundial de la Salud y se realizó una revisión de evaluación de la bibliografía con el fin de identificar los estudios sobre cavitación pulmonar residual después de la TBP y calcular la incidencia global de la APC después de la TBP. Habiendo establecido que del 21% (Estados Unidos de América) al 35% (Taiwán, China) de los pacientes con TBP desarrollaron cavidades pulmonares y que alrededor del 22% de dichos pacientes desarrollaron APC, los autores aplicaron tasas de abandono anuales del 10%, 15% y 25% para calcular el rango de prevalencia para la APC en un periodo de cinco años. Los análisis se basaron en un modelo determinista.
Hallazgos
En 2007, se produjeron 7,7 millones de casos de TBP en todo el mundo, de los cuales se calcula que unos 372 000 desarrollaron APC: desde 11 400 en Europa hasta 145 372 en Asia Sudoriental. La prevalencia global en un periodo de cinco años fue de 1 174 000, 852 000 y 1 372 000 casos con tasas de abandono anuales del 15%, 25% y 10%, respectivamente. La tasa de prevalencia osciló de < 1 caso por cada 100 000 habitantes en los grandes países de Europa Occidental y los Estados Unidos de América a 42,9 por cada 100 000 habitantes en tanto Nigeria como la República Democrática del Congo. China e India presentaron tasas intermedias de prevalencia en un periodo de cinco años de 16,2 y 23,1 por cada 100 000 habitantes, respectivamente.
Conclusión
La carga global de la APC como consecuencia de la TBP es sustancial, algo que justifica que se continúe investigando. La APC podría ser la responsable de algunos casos de TBP con frotis negativo. Dado que la APC responde al tratamiento antifúngico a largo plazo, es necesario mejorar con urgencia la detección de los casos.
ملخص
الغرض قياس العبء العالمي لداء الرشاشيات الرئوي المزمن في أعقاب السل الرئوي، ولاسيما في حالات التكهف الرئوي.
الطريقة جمعت معدلات السل الرئوي من منظمة الصحة العالمية، وأجريت مراجعة متأنية للنشريات لتحديد الدراسات المتصلة بالتكهف الرئوي المتبقي بعد الإصابة بالسل الرئوي، وقياس معدل الانتشار العالمي لداء الرشاشيات الرئوي المزمن بعد الإصابة بالسل الرئوي. والذي تبين أنه كان يتراوح بين 21% (في الولايات المتحدة الأمريكية) إلى 35% (في تايوان، الصين) بين المرضى المصابين بالسل الرئوي وتطورت حالتهم بظهور كهوف رئوية، وأن حوالي 22% من هؤلاء المرضى قد تطورت حالتهم بإصابتهم بداء الرشاشيات الرئوي المزمن، وطبق مؤلفو البحث معدلات الانسحال السنوية بنسبة 10%، 15%، 25% لقياس مجال فترة الانتشار لداء الرشاشيات الرئوي المزمن طوال خمس سنوات. واستند التحليل على النموذج الحتمي.
النتائج في عام 2007، وقعت عالمياً 7.7 مليون حالة من السل الرئوي، منها 372000 حالة تطورت إلى داء الرشاشايات الرئوي المزمن: بدءاً من 11400 حالة في أوروبا إلى 145372 حالة في جنوب شرق أسيا. وكانت معدل الانتشار العالمي لفترة خمس سنوات 1174000، و 852000، و 1372000 حالة عند معدلات انسحال سنوية قدرها 15%، و25%، و10% بالترتيب. وترواح معدل الانتشار بين أقل من حالة لكل 100000 شخص من السكان في بلدان غرب أوروبا الكبرى وفي الولايات المتحدة الأمريكية، إلى 42.9 حالة لكل 100000 في شمال نيجيريا وجمهورية الكونغو الديمقراطية. وكان للصين والهند معدلات انتشار متوسطة لفترة خمس سنوات بلغت 16.2، و 23.1 لكل 100000 بالترتيب.
الاستنتاج يشكل داء الرشاشيات الرئوي المزمن كنتيجة للسل الرئوي عبئاً عالمياً ثقيلاً، ويتطلب المزيد من التقصي. ويمكن أن يكون داء الرشاشيات الرئوي المزمن مسؤولاً عن بعض الحالات السلبية اللطاخة للسل الرئوي. وحيث أن داء الرشاشيات الرئوي المزمن يستجيب للعلاج بمضادات الفطريات، فيجب اتخاذ ما يلزم على وجه السرعة لتحسين الكشف عن الحالات المصابة به.
摘要
目的
旨在估计肺结核(PTB)特别是肺空洞形成之后慢性肺曲霉病(CPA)的全球负担。
方法
肺结核发病率从世界卫生组织获得,同时对相关文献进行大范围回顾,从而确定有关肺结核之后残余肺空洞方面的研究,进而估计肺结核之后慢性肺曲霉病的全球发病率。经查实,21%(美国)到35%(中国台湾)的肺结核病人产生肺空洞,并且其中约22%的病人患有慢性肺曲霉病,本文作者运用10%,15%和25%的年度损耗率来估计五年期内慢性肺曲霉病的期间患病率范围。分析基于确定性模型进行。
结果
2007年,全球有770万例肺结核,其中估计有372,000例患有慢性肺曲霉病,各地发病数介于欧洲的11,400例和东南亚的145,372例之间。以15%,25%和10%的年度损耗率计算,全球五年期患病数分别为1,174,000例,852,000例和1,372,000例。患病率处于每10万人小于1例(西欧大国和美国)至每10万人42.9例(尼日利亚和刚果民主共和国)之间。中国和印度五年期患病率居中,分别为每10万人16.2例和23.1例。
结论
慢性肺曲霉病作为肺结核的一种后续发展带来相当大的全球负担,需要进一步调查。慢性肺曲霉病可导致部分涂阴肺结核病例。由于长期抗真菌治疗对慢性肺曲霉病有很好的疗效,应尽快加强病例检测。
Резюме
Цель
Оценить глобальное бремя хронического пульмонального аспергиллеза (ХПА), развившегося после излечения туберкулеза легких (ТБЛ), в частности, у больных с легочной кавитацией.
Методы
Показатели ТБЛ были получены от Всемирной организации здравоохранения. Был также выполнен оценочный обзор литературы с тем, чтобы выявить исследования, посвященные остаточной легочной кавитации после излечения ТБЛ, и провести глобальную оценку заболеваемости ХПА после излеченного ТБЛ. Установив, что от 21 (США) до 35% (Тайвань, Китай) пациентов с ТБЛ имели полости в легких, и что примерно у 22% этих больных развился ХПА, авторы применили годовые коэффициенты оттока, равные 10, 15 и 25%, чтобы оценить диапазон распространенности ХПА за пятилетний период. Анализ основывался на детерминистской модели.
Результаты
В 2007 году в мире произошло 7,7 млн случаев заболевания ТБЛ; из них, по оценкам, у 372 тыс. пациентов развился ХПА; разброс региональных показателей составлял от 11 400 случаев в Европе до 145 372 в Юго-Восточной Азии. Глобальная распространенность заболевания за пятилетний период составила 1 174, 852 и 1 372 тыс. случаев при годовом коэффициенте оттока в 15, 25 и 10%, соответственно. Коэффициент распространенности варьировался менее чем от одного случая на 100 тыс. чел. населения в крупных странах Западной Европы и в США до 42,9 случая на 100 тыс. чел. населения в Нигерии и Демократической Республике Конго. В Китае и Индии промежуточный показатель распространенности за пятилетний период составлял 16,2 и 23,1 случаев на 100 тыс. чел. населения, соответственно.
Вывод
Глобальное бремя ХПА, как осложнения ТБЛ, значительно и нуждается в углубленном исследовании. В некоторых случаях ХПА мог быть причиной заболевания ТБЛ с отрицательным мазком. Поскольку ХПА поддается лечению в случае длительного применения антигрибковой терапии, срочно необходимо повысить выявляемость случаев этого заболевания.
Introduction
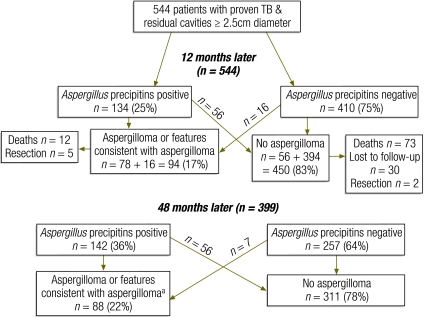
With more than 36 million people cured of tuberculosis between 1995 and 20081 and 9 million new cases diagnosed worldwide each year,2 the health of those affected over the long term warrants attention. Treated pulmonary tuberculosis (PTB) can lead to complications, including progressive loss of lung function,3 persistent pulmonary symptoms3 and chronic pulmonary aspergillosis (CPA).4–6 Of the long-term sequelae of PTB, CPA is perhaps the most subtle, yet the most severe.4–11 In the 1960s the Research Committee of the British Thoracic and Tuberculosis Association estimated the prevalence of CPA in patients who had a residual cavity of at least 2.5 cm on the chest radiograph following treatment for PTB.9,12 It assessed more than 500 patients from 55 chest clinics twice – once about 12 months after the sputum became negative for acid fast bacilli,12 and again three years later.9 Remarkably, 25% of the patients had detectable Aspergillus precipitins in blood and both precipitins and radiological features of an aspergilloma were detectable in 14% at 12 months and in 22% at 3–4 years. PTB and CPA present with similar symptoms. This, combined with inadequate facilities for testing for immunoglobulin G (IgG) antibodies (precipitins) against A. fumigatus in many places, probably results in the underdiagnosis of CPA both at initial presentation13 and following treatment for PTB. For example, in early case series of people with respiratory illness and negative acid fast bacillus (AFB) sputum smears in sub-Saharan Africa, A. fumigatus was among the pathogens identified.14 CPA is an important differential diagnosis of what appears to be smear-negative tuberculosis.
CPA occurs in various forms: simple aspergilloma, chronic cavitary pulmonary aspergillosis and chronic fibrosing pulmonary aspergillosis, both with and without an aspergilloma.4 Unlike invasive aspergillosis, CPA occurs in immunocompetent patients. Morbidity is considerable and is marked by both systemic and respiratory symptoms and haemoptysis.7,8Weight loss, profound fatigue, severe shortness of breath and life-threatening haemoptysis are common. Progressive pulmonary fibrosis and loss of lung function, also common, could partly account for the unexplained loss of lung function in these patients. Even when treated, CPA has a case fatality rate of 20–33% in the short-term and of 50% over a span of 5 years.5,8
The country-specific PTB statistics and mortality rates published by the World Health Organization (WHO)15 make it possible to estimate the burden of chronic sequelae after treatment for PTB. Our objective was to use these published clinical and population data as inputs to model estimates of the likely burden of CPA related to PTB worldwide.
Methods
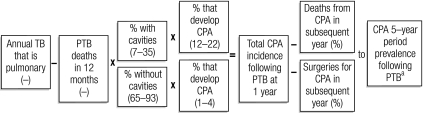
We developed a deterministic scenario model using Excel (Microsoft, Bellevue, United States of America). Fig. 1 shows our approach to estimating the adult burden of CPA in the largest countries of every WHO region. We started with WHO estimates of the number of new cases of PTB and of deaths from PTB15 and assumed that the mortality figures quoted by the WHO were for the point 12 months after the diagnosis of PTB.
Fig. 1.
Factors used to estimate the annual incidence and five-year period prevalence of chronic pulmonary aspergillosis (CPA)
PTB, pulmonary tuberculosis, TB, tuberculosis.
a Attrition applied annually over 5 years.
We searched the literature with the following questions in mind: (i) What is the frequency of pulmonary cavitation after completion of the treatment for PTB? (ii) How common is CPA following PTB? (iii) Are there any radiological risk factors (such as cavitation) for CPA? (iv) What is the range of the 12-month survival for PTB (to estimate the numbers at risk of developing CPA development)? and (v) What is the range of the 12-month survival for CPA (to estimate attrition and convert incidence to period prevalence)? We initially adopted a systematic search strategy but quickly realized that the literature was limited and that scoping reviews for all five questions were more appropriate.16
To identify the primary literature on cavitation after PTB, we searched several electronic bibliographic databases using the search terms “tuberculosis” and all of (separately) “follow up”, “radiology”, “cavitation”, “cavity”, “CT”, “radiograph”, “radiographic”, “outcome”, “fungal ball”, “aspergilloma”, “aspergillosis” and “haemoptysis” (using both British and American English spellings). All literature on aspergilloma was retrieved back to 1936. Our primary source was Medline, but we also searched the Aspergillus web site (including the historical paper archive [pre-1964]). We did not search meeting abstracts, doctoral theses or other grey literature sources, but we hand searched our extensive files of pre-1990 papers on all forms of aspergillosis. Every paper retrieved with information on cavitation following PTB was scrutinized and additional referenced papers were also retrieved. The term “aspergilloma” entered the medical literature in the 1940s, but the phrase “chronic pulmonary aspergillosis” was not formally used until 2003. Thus, searches with the terms “aspergillosis” and “aspergilloma” yielded very different numbers of papers. We initially identified over 400 papers and scanned their abstracts. We read over 100 papers to determine the availability of data on the treatment of pulmonary cavitation. Five sources contained relevant quantitative information on cavitation; the rest contained only qualitative data. To minimize selection bias, we established as inclusion criteria a cohort study or case series design and a minimum of 20 study subjects. We also contacted the original authors of the primary combination studies of PTB conducted by the Medical Research Council of the United Kingdom of Great Britain and Northern Ireland to establish if the original end-of-treatment chest radiographs or readings were available for review. Unfortunately, they were not.
We identified five papers that provided data on the proportion of cases with pulmonary cavities following anti-tuberculous therapy. The studies yielded different figures: they ranged from 21% in the United States17 to 35% in Taiwan, China18 and > 50% in South Africa in some studies,19 while in others the figures were 21–23% in South Africa20 and the United States17 and 30% in Brazil21 (Table 1). We decided on an intermediate figure of 22% and applied it to countries across the globe except for the 22 members of the European Community. For Europe we took into account our own (unpublished) observations from the United Kingdom, which showed a post-treatment cavitation rate of less than 10%, and selected an intermediate figure of 12% that will require prospective validation. We also performed sensitivity analyses using figures of 10% and 30% for countries outside the European Community.
Table 1. Summary of published studies evaluating cavitation on chest radiograph or computerized tomography (CT) scan after anti-tuberculous treatment.
| Reference | Design | Population | Relevant measure(s) | Frequencies |
|---|---|---|---|---|
| Sonnenberg (2000)20 | Prognostic cohort for TB outcomes (exclusion of MDR cases and those not cured of TB), stratified by HIV status | Gold miners in South Africa | Chest X-ray at 3 & 6 months after cure | Residual cavitation at cure - 69/326 (21%) |
| [HIV strong first factor for TB recurrence among those without cavitation] | ||||
| Bombarda (2003)21 | Repeat measures (unclear if losses to follow-up) during active disease and then after treatment conclusion | Referral hospital PTB patients | Repeated conventional CT scans | Thick-walled cavities post treatment 1/20 (5%) and thin-walled cavities post treatment 5/20 (25%); together, 30% |
| ? selected population | ||||
| De Vallière (2004)19 | Post-treatment cross-sectional survey (reporting on 33/42 patients who completed treatment – no comment on selection) | Patients registered in MDR-TB programme in Limpopo province, South Africa | Chest X-ray based on two observers | % with cavities – observer 2, 17/33 (52%) to observer 1, 23/33 (70%) |
| Hamilton (2008)17 | Prognostic cohort of post-treatment (6 months), 170 exclusions from 1 004 subjects primarily for missing test results | Multi-centre North American TB trials Consortium RCT | Chest X-ray, consensus criteria, reading kappa 0.54 for cavity (80% raw agreement) | EOT cavity, 23.3% (n = 834) in EOT chest X-ray analysis vs 19.1% among those excluded from main analysis (n = 170) |
| Lee (2008)18 | Pre- and post-treatment repeat scans (excluded 31/83, primarily loss to follow-up, 23) | Taiwan, China, general hospital n = 52 | High resolution chest CT scan | Post-treatment, 18/52 (35%) vs pre-treatment, 38/52 (73%) |
EOT, end-of-treatment; HIV, human immunodeficiency virus; MDR, multi-drug resistant; PTB, pulmonary tuberculosis; RCT, randomized controlled trial; TB, tuberculosis.
We examined the papers found through our searches for data on the frequency of an association between PTB and either aspergilloma or chronic pulmonary aspergillosis, as well as on the radiological characteristics of these entities. We excluded case reports, qualitative studies and papers describing an association with invasive aspergillosis. Papers reporting the frequency of aspergilloma or fungal balls without serological confirmation were included but those describing cavitation without a fungal ball were included only if CPA was confirmed serologically or through direct histological exam or culture of the lesions. Finally, only two papers from the same Medical Research Council study in the United Kingdom linked radiological findings with the subsequent development of aspergilloma (and included serologic testing for Aspergillus) and, therefore, chronic pulmonary aspergillosis (Fig. 2).9,12 The figures shown in Fig. 2 were checked and confirmed by all authors. We found no papers describing the rate of CPA after PTB without reference to pulmonary cavitation or in patients without cavitation.
Fig. 2.
Relative frequency of chronic pulmonary aspergillosis after standard anti-tuberculous treatment
TB, tuberculosis
a Including resection (n = 7) showing an aspergilloma.
Source: Anonymous9 and Research Committee of the British Tuberculosis Association.12
On a country by country basis, we multiplied the number of survivors 12 months following initiation of therapy for PTB by the designated percentage of patients with pulmonary cavities. We then estimated the number of patients with cavities who were likely to develop CPA (22% according to the studies from the United Kingdom,9,12 Fig. 2). We found little published data on CPA among patients without visible cavities on a chest radiograph following PTB, so we chose 2% as the best estimate, with upper and lower bounds of 1% and 4% for sensitivity analyses. This allowed us to estimate the number of cases with CPA 1 year following completion of treatment of PTB.
The purpose of the fourth review was to establish the range of survival rates 12 months after diagnosis of PTB. A full systematic review could have been conducted to address this question, but since the rates of survival were used to identify patients at risk for CPA for modelling purposes only, we chose to perform a scoping review to establish the survival range. We assumed that all cases of PTB had been correctly diagnosed in these studies. Five studies were identified and 12-month mortality following PTB ranged from 5% to 26%.
We also conducted a scoping review to estimate12-month survival after the development of CPA and found only three published studies that allowed us to make such an estimate (85%) and convert annual incident cases into five-year period prevalence. Patients undergoing resection surgery for aspergilloma are so few compared with the number who die from CPA that we did not estimate their proportion.
Long-term survival from PTB varies widely and is affected by co-infection with the human immunodeficiency virus (HIV), age, treatment adherence and the presence of multidrug-resistant or extensively drug-resistant PTB (MDR-PTB and XDR-PTB, respectively). Annual mortality following PTB has been shown to vary from < 2% in Denmark22 and 9% in Guinea-Bissau23 to 15% in Uzbekistan.24 (Table 2). Annual mortality from MDR-PTB may be no higher than mortality from PTB responsive to medication (41% over 5 years),26 but it is higher in HIV-positive individuals (26%) before they are treated with antiretroviral therapy.25,27 Thus, to estimate the five-year period prevalence of CPA as a complication of PTB, we applied 10%, 15% and 25% annual attrition rates to deduct deaths annually over the 5-year period.
Table 2. Papers (chronological) on 12-month survival among pulmonary tuberculosis (PTB) patients after treatment.
| Reference | Design | Population | Mortality/Survival |
|---|---|---|---|
| Lillebaek (1999)22 | Retrospective prognostic cohort – not inception, as differential times since first diagnosis and clinical presentations. Four-year follow-up | Danish population (all 350 cases with information notified in country), mix of older Danes and immigrants across ages. PTB and EPTB | Overall 45/350 (12.9%) died during treatment often up to one year and overall 51/350 (14.6%). Culture positive PTB died during treatment (17/210 or 8.1%). Overall 19.7% mortality and 19/350 not available for post Rx FU. Maximum post-treatment mortality = 2% |
| Connolly (1999)25 | Prognostic cohort, two-year follow up, 78/403 (19%) left the area | Hlabisa health district of KwaZulu-Natal, South Africa primarily rural, follow-up of 403 cured TB patients (53% HIV-infected) | 58/403 (14%) died, mortality was four times higher among HIV-infected patients (17.8 and 4.4 deaths per 100 PYO for HIV-infected and uninfected patients, respectively; P < 0.0001). Probability of survival at 24 months was estimated at 59% and 81%, respectively |
| Winquist (2000)23 | Prognostic cohort - 206 bacteriologically verified PTB patients, 168 were followed up for 3 years, 149 discharged to ambulatory treatment | Initially attending hospital clinic Guinea-Bissau, then discharged to ambulatory treatment | At 36 months, of 130 patients discharged to follow ambulatory treatment and alive > 9 months after diagnosis, 23 (17.7%) died over the next 27 months |
| Cox (2006)24 | Retrospective, mixed duration and 40% previously treated, prognostic cohort, follow-up, median of 22 months from diagnosis, valid follow-up data obtained for 197 (92%) patients | Karakalpakstan, Uzbekistan, 213 patients who were sputum-smear-positive for TB, included in drug resistance survey and diagnosed consecutively in 2001–2002 from four districts (68% of eligible) | Mortality - 48 (24%) of the 197 patients dead at the time of follow-up average of 15% (95% confidence interval, CI: 11% to 19%) dying per year after diagnosis (6% of 73 pan-susceptible cases and 43% of 55 MDR-PTB cases also died per year). 11 (41%) of the 27 patients defined as treatment failures had died; 37/170 (22%) of patients successfully treated had died |
| Shean (2008)26 | Retrospective prognostic cohort study of 491 treated | Chest hospital patients, Western Cape, South Africa. All MDR-TB patients starting treatment during 1992–2002. 491 (66%) of 747 MDR-TB patients received treatment with two or more second-line drugs | Of 491 treated, 239 (49%) were cured or completed treatment; 68 (14%) died. Of 410 patients who had not transferred out or died during treatment for MDR-PTB, 281 (69%) had 2-year data available: 185 (66%) were cured or completed treatment; 32 (11%) were retreated for PTB and 64 (23%) died. Analysis of five-year outcomes of 154/233 (66%) patients who were treated in cohorts 1992–1998 and were known to be alive at the end of treatment revealed that 77 (50%) were alive, 14 (9%) had been re-treated for drug-susceptible PTB and 63 (41%) had died |
EPTB, extrapulmonary tuberculosis; HIV, human immunodeficiency virus; MDR, multi-drug resistant; PYO, person–years of observation; TB, tuberculosis.
All papers retrieved that contained quantitative data were reviewed by all authors to ensure that the samples surveyed in each paper were not highly selected and hence biased. The proportions of patients with pulmonary cavities and the annual post-treatment mortality rates were extracted independently by two authors (DWD and DCC) and discrepancies were resolved by discussion. The list of excluded papers is available from the authors on request.
Results
According to WHO, in 2007 an estimated 7.7 million cases of PTB occurred worldwide, and 5.96 million (77.1%) of them survived to at least 12 months after diagnosis. Nearly 50% of these cases occurred in China and India, whose combined population in 2005 was 2.4 billion (Table 3).28 We estimate that annually at least 372 385 patients in the world develop CPA following PTB, but incidence estimates vary substantially by country and WHO region. The number of new cases of CPA associated with PTB was estimated at 11 420 in the European Region, 20 615 in the Eastern Mediterranean Region and 12 610 in the Region of the Americas (Table 4). However, minimum estimates were 98 551 for the African Region, 83 815 for the Western Pacific Region and 145 372 for the South-East Asia Region. Individual country estimates for the 24 most populous countries are provided in Table 3.
Table 3. Relative frequency of pulmonary tuberculosis (PTB) and chronic pulmonary aspergillosis (CPA) for countries with populations exceeding 50 million, 2005.
| Country | Populationa (2005) | Annual PTB cases alive at 1 year | Estimated annual CPA cases after PTB | 5-year estimated CPA prevalenceb | 5-year estimated CPA prevalence ratec |
|---|---|---|---|---|---|
| Global total | 6 512 276 000 | 5 899 619 | 372 385 | 1 173 881 | 18.0 |
| China | 1 312 253 000 | 1 052 925 | 67 387 | 212 427 | 16.2 |
| India | 1 130 618 000 | 1 297 047 | 83 011 | 261 679 | 23.1 |
| United States | 302 741 000 | 8 907 | 588 | 1 853 | 0.6 |
| Indonesia | 219 210 000 | 420 853 | 26 935 | 84 907 | 38.7 |
| Brazil | 186 075 000 | 70 789 | 5 663 | 17 852 | 9.6 |
| Pakistan | 165 816 000 | 204 955 | 13 117 | 41 350 | 24.9 |
| Bangladesh | 153 122 000 | 243 361 | 15 575 | 49 098 | 32.1 |
| Russian Federation | 143 470 000 | 116 234 | 7 439 | 23 450 | 16.3 |
| Nigeria | 140 879 000 | 299 297 | 19 155 | 60 383 | 42.9 |
| Japan | 127 449 000 | 17 724 | 1 134 | 3 576 | 2.8 |
| Mexico | 105 330 000 | 15 326 | 981 | 3 092 | 2.9 |
| Philippines | 85 496 000 | 216 228 | 13 839 | 43 624 | 51.0 |
| Viet Nam | 84 074 000 | 97 497 | 3 412 | 10 757 | 12.8 |
| Germany | 82 409 000 | 3 339 | 100 | 316 | 0.4 |
| Egypt | 77 154 000 | 9 266 | 593 | 1 869 | 2.4 |
| Ethiopia | 74 661 000 | 124 710 | 7 981 | 25 160 | 33.7 |
| Turkey | 71 169 000 | 11 042 | 707 | 2 228 | 3.1 |
| Islamic Republic of Iran | 70 765 000 | 9 278 | 594 | 1 872 | 2.6 |
| Thailand | 65 946 000 | 64 566 | 4 132 | 13 026 | 19.8 |
| France | 61 013 000 | 5 517 | 166 | 522 | 0.9 |
| United Kingdom | 60 261 000 | 4 189 | 118 | 370 | 0.6 |
| Democratic Republic of the Congo | 59 077 000 | 125 538 | 8 034 | 25 327 | 42.9 |
| Italy | 58 645 000 | 2 807 | 84 | 265 | 0.5 |
Table 4. Estimated global burden of chronic pulmonary aspergillosis (CPA) after pulmonary tuberculosis (TB), by World Health Organization (WHO) region, for different rates of annual attrition (10–25%) and CPA frequency estimatesa.
| CPA frequency |
Global |
Europe |
Africa |
Eastern Mediterranean |
Americas |
Western Pacific |
South-East Asia |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TB cavity | No TB cavity | 10% | 15% | 25% | 10% | 15% | 25% | 10% | 15% | 25% | 10% | 15% | 25% | 10% | 15% | 25% | 10% | 15% | 25% | 10% | 15% | 25% |
| 30% | 4% | 2 088 604 | 1 786 412 | 1 296 646 | 68 111 | 58 256 | 42 284 | 551 639 | 471 825 | 342 469 | 115 395 | 98 699 | 71 639 | 70 584 | 60 371 | 43 820 | 469 154 | 401 274 | 291 260 | 813 721 | 695 987 | 505 174 |
| 22% | 4% | 1 713 570 | 1 465 640 | 1 063 817 | 58 927 | 56 401 | 36 583 | 451 754 | 386 392 | 280 458 | 94 500 | 80 828 | 58 668 | 57 803 | 49 440 | 35 885 | 384 204 | 328 615 | 238 522 | 666 381 | 569 965 | 413 702 |
| 22% | 2% | 1 372 457 | 1 173 881 | 852 048 | 42 091 | 36 001 | 26 131 | 363 219 | 310 667 | 225 494 | 75 980 | 64 987 | 47 170 | 46 475 | 39 751 | 28 852 | 308 908 | 264 213 | 191 776 | 535 783 | 458 263 | 332 625 |
| 22% | 1% | 1 201 900 | 1 028 002 | 746 163 | 33 673 | 28 801 | 20 905 | 318 952 | 272 804 | 198 012 | 66 720 | 57 067 | 41 421 | 40 811 | 34 906 | 25 336 | 271 260 | 232 012 | 168 403 | 470 485 | 402 412 | 292 086 |
| 10% | 1% | 639 349 | 546 844 | 396 920 | 19 898 | 17 019 | 12 353 | 169 124 | 144 654 | 104 995 | 35 378 | 30 260 | 21 964 | 21 640 | 18 509 | 13 434 | 143 835 | 123 024 | 89 296 | 249 474 | 213 379 | 154 878 |
a Estimated frequency was 10–30% in patients with cavities and 1–4% in patients without cavities after completion of anti-tuberculous therapy.
Our best estimate of the global five-year period prevalence of CPA following PTB was 1 173 881 patients, with a range from 852 048 at 25% annual attrition to 1 372 457 at 10% annual attrition (Table 4). Sensitivity analyses using rates of cavitation after PTB of 10% and 30% and rates of CPA in people without cavities of 1% and 4% altered the estimates from a low global five-year period prevalence of 546 844 to a high of 1 786 421 when a 15% attrition rate was applied.
The five-year period prevalence of CPA indicated a predicted prevalence rate of 18 per 100 000 population (Table 3). The prevalence rate of CPA varies widely, however. Among the 23 largest countries in the world it ranges from as low as 0.4 per 100 000 in Germany to 42.9 per 100 000 in both Nigeria and the Democratic Republic of the Congo. China and India have intermediate predicted prevalence rates of 16.2 and 23.1 per 100 000, respectively. In the largest developed countries, the predicted prevalence rate is invariably below 1 per 100 000.
Discussion
According to our model, which resembles many models used by WHO to estimate the burden of other diseases, around 1.2 million people in the world have CPA as a sequel to PTB. Most CPA cases occur in WHO’s South-East Asia, Western Pacific and African regions, where PTB has the highest prevalence. In many series PTB is an underlying condition among CPA cases, but this varies widely. Only 17% of referred CPA patients in Manchester, England,29 had underlying PTB, compared with 93% in Seoul, Republic of Korea.6 This variation reflects differences in clinician awareness, in diagnostic approaches in patients with persistent pulmonary shadowing, and in the relative frequency of underlying pulmonary diseases in each locality. The progressive loss of pulmonary function and/or the presence of symptoms after PTB could be caused by CPA, but this possibility has never been studied. In cases in which CPA is diagnosed, symptoms such as fatigue, cough, shortness of breath, weight loss and haemoptysis are best managed with antifungal therapy. Identifying CPA early in patients with residual pulmonary shadows from PTB is only possible by means of microbiological testing (principally for Aspergillus IgG antibodies). If tests are not conducted, patients are often diagnosed as having “smear-negative pulmonary tuberculosis”, “progressive upper lobe fibrosis” or “recurrent pulmonary tuberculosis”, all of which result in inappropriate therapy or none at all. In areas with a high prevalence of tuberculosis, criteria for the diagnosis of CPA and PTB are so similar that distinguishing between the two entities is not possible, without serological testing for Aspergillus precipitins, even if sputum culture is positive for A. fumigatus.14
Accuracy of pulmonary tuberculosis case estimates
We based our estimates of CPA prevalence following PTB on WHO tuberculosis rates.15 The data are robust in some countries but not others. Under-reporting is common, especially in countries such as China. Therefore, we have probably underestimated the burden of CPA. In addition, both incident cases and cure rates are changing relatively rapidly, thanks to the Stop TB Partnership. Case fatality rates dropped from 8% to 4% between 1995 and 2008.1 A recent estimate of mortality in patients with HIV and TB co-infection yielded a rate of 5% for countries in Africa with a low prevalence of HIV infection, but closer to 20% in those with a high prevalence,25 consistent with our estimates. Increased survival is likely to lead to greater numbers of people at risk of sequelae, including CPA. Estimating post-treatment survival was challenging, mostly because accurately estimating prognostic denominators was difficult, as others have found.27
CPA case ascertainment
To estimate CPA burden we have used radiographic findings primarily. Our own data suggests that about 25% of patients with CPA have an aspergilloma.30 The original United Kingdom survey on PTB was conducted with chest radiographs, yet computerized tomography (CT) scanning of the thorax is much more sensitive, especially in the apex of the lungs, which is the site of most PTB and CPA. The cavitation rates of 30%18 and 35%21 after PTB are based on CT, whereas estimates based on chest radiographs are generally 21–23%.17,20 An even higher rate of residual cavitation (> 50%) was demonstrated in a population with MDR-PTB.19 We arbitrarily applied to all of Europe the cavitation rate in the United Kingdom instead of conducting a prospective assessment, a clear study limitation. Plain chest X-rays have reasonable sensitivity (70%) for the detection of pulmonary cavities when anti-tuberculous treatment is initiated and while it is being administered, but it drops by the end of treatment (49%)17 and few centres undertake CT scans at that time. Although we have accounted for this variation in our upper and lower estimates, additional work is necessary to validate these frequencies in different populations, especially in North America and Europe. We recognize that a robust estimate of CPA based on rates of cavitation after PTB needs to be fully validated at the local level, especially in countries where data are old or do not exist, such as the United Kingdom.
All patients who have had a pulmonary insult are probably at some risk of developing CPA. The relative risk of CPA following PTB in patients with smaller cavities or with none has not been estimated. A cavity is thought to be important in pathogenesis because the insult to the lung in that area probably undermines local host defences, allowing Aspergillus conidia to germinate. In other groups of patients who develop CPA, notably those with sarcoidosis and emphysematous bullae, pulmonary cavities predate the development of CPA. In others, including patients with survived lung cancer or who have allergic bronchopulmonary aspergillosis, cavities are not present before the development of CPA. We have estimated that the risk of CPA among PTB patients without discernible cavities is about 2% (range: 1–4%), but this may not be an accurate estimate across all populations. Prospective studies are needed to substantiate these rates.
In addition to pulmonary cavitations on chest X-ray, with or without an aspergilloma, either the presence of hyphae in a pulmonary cavity (preferably with a positive culture) or the presence of Aspergillus IgG antibodies must be definitively demonstrated for the diagnosis of CPA to be made. Serologic tests for A. fumigatus IgG antibodies have a sensitivity of about 90% for CPA.31,32 Rare cases of CPA caused by A niger, A. nidulans and A. flavus instead of the more common pathogen, A. fumigatus, have been documented8,33–35 and may be a source of false negative serology results. Thus, case detection is likely to be incomplete. Detectable Aspergillus desoxyribonucleic acid (DNA) in respiratory samples36 could suggest CPA, prior to A. fumigatus IgG antibodies being requested.
Accounting for CPA-related mortality
CPA can be cured in 1% to 17% of patients who undergo surgery, usually within a year of diagnosis.9,11 Mortality from surgery is extremely low for simple aspergilloma37–40 but much higher for complex disease.4,11 Even with antifungal treatment, CPA develops gradually and leads to progressive loss of lung function. The case fatality rate after admission to hospital ranges from 10% to 30%.6,8,10 We have therefore introduced an annual attrition rate of 15% by default, with a range from 10% to 25%, when converting annual incident cases to five-year period prevalence. In our experience, survival is determined primarily by the combined effect of the severity of the underlying pulmonary disease and the extent and pace of lung destruction.
Other risk factors
Many risk factors for CPA probably exist and they include some genetic defects. Deficiency of surfactant A2 and toll-like receptor 4 has been shown to alter innate immune function.41,42 In CPA patients, cytokine production profiles typically show a Th2 cytokine profile41 and gamma interferon production may be absent or poor.43 Other risk factors such as these, whose frequency probably varies in different ethnic groups, could affect both the incidence and progression of CPA.
Future directions
CPA is a sequel of PTB more commonly than is generally appreciated. It can account for progressive lung destruction and the persistence of symptoms after successful anti-tuberculous treatment and can mimic smear-negative PTB. Antifungal therapy is very beneficial in CPA patients, as it reduces both morbidity and mortality.6–8,30,43–46 Little data exist on the development of CPA after PTB. Prospective clinical and epidemiological studies using the best diagnostic tools available are needed to ascertain its frequency in different places and among different ethnic groups. Recognition of CPA and treatment with generic itraconazole have the potential to reduce morbidity and mortality from CPA worldwide at a modest cost.
Acknowledgements
We are indebted to Joanne Gill and the library staff for sourcing the papers and to multiple colleagues who answered questions about the frequency of disease in their countries, including Ashok Shah (Vallabhbhai Patel Chest Institute, University of Delhi), Won-Jung Koh (Sungkyunkwan University School of Medicine, Seoul) and Peter Omerod (Blackburn, England). Helen Carruthers drew Fig. 2.
Funding:
Funding was provided by the University Hospital of South Manchester, Manchester, England.
Competing interests:
David W Denning holds founder shares in F2G Ltd, a University of Manchester spin-out company, and has received grant support from F2G as well as the Fungal Research Trust, the Wellcome Trust, the Moulton Trust, The Medical Research Council, The Chronic Granulomatous Disease Research Trust, the National Institute of Allergy and Infectious Diseases, National Institute of Health Research and the European Union, AstraZeneca and Basilea. He has been as an advisor/consultant over the last 5 years to F2G, Lab21, Basilea, Vicuron (now Pfizer), Pfizer, Schering Plough (now Merck), Nektar, Daiichi, Astellas, Gilead and York Pharma. He has been paid for talks on behalf of Schering, Astellas, Novartis, Merck, Dainippon and Pfizer. Alex Pleuvry is a Director and shareholder in Oncalex, an independent consultancy, with no specific financial interest in respiratory or fungal disorders. Donald C Cole is a tenured professor with consultancies on environmental health to public health units but none on respiratory or fungal disorders or their treatment.
References
- 1.Lönnroth K, Castro KG, Chakaya JM, Chauhan LS, Floyd K, Glaziou P, et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet. 2010;375:1814–29. doi: 10.1016/S0140-6736(10)60483-7. [DOI] [PubMed] [Google Scholar]
- 2.Global tuberculosis control: epidemiology, strategy, financing: WHO report 2009. Geneva: World Health Organization; 2009. [Google Scholar]
- 3.Ross J, Ehrlich RI, Hnizdo E, White N, Churchyard GJ. Excess lung function decline in gold miners following pulmonary tuberculosis. Thorax. 2010;65:1010–5. doi: 10.1136/thx.2009.129999. [DOI] [PubMed] [Google Scholar]
- 4.Denning DW. Chronic aspergillosis. In: Latge JP, Steinbach WJ, editors. Aspergillus fumigatus and aspergillosis Washington: ASM Press; 2009. [Google Scholar]
- 5.Jewkes J, Kay PH, Paneth M, Citron KM. Pulmonary aspergilloma: analysis of cavitating invasive pulmonary aspergillosis in immunocompromised patients. Ann Thorac Surg. 1983;53:621. [Google Scholar]
- 6.Nam HS, Jeon K, Um SW, Suh GY, Chung MP, Kim H, et al. Clinical characteristics and treatment outcomes of chronic necrotizing pulmonary aspergillosis: a review of 43 cases. Int J Infect Dis. 2010;14:e479–82. doi: 10.1016/j.ijid.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Denning DW, Riniotis K, Dobrashian R, Sambatakou H. Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin Infect Dis. 2003;37(Suppl 3):S265–80. doi: 10.1086/376526. [DOI] [PubMed] [Google Scholar]
- 8.Camuset J, Nunes H, Dombret MC, Bergeron A, Henno P, Philippe B, et al. Treatment of chronic pulmonary aspergillosis by voriconazole in nonimmunocompromised patients. Chest. 2007;131:1435–41. doi: 10.1378/chest.06-2441. [DOI] [PubMed] [Google Scholar]
- 9.Aspergilloma and residual tuberculous cavities–the results of a resurvey. Tubercle. 1970;51:227–45. [PubMed] [Google Scholar]
- 10.Tomlinson JR, Sahn SA. Aspergilloma in sarcoid and tuberculosis. Chest. 1987;92:505–8. doi: 10.1378/chest.92.3.505. [DOI] [PubMed] [Google Scholar]
- 11.Massard G, Roeslin N, Wihlm JM, Dumont P, Witz JP, Morand G.Surgical treatment of pulmonary and bronchial aspergilloma. Ann Chir 199347141French [PubMed] [Google Scholar]
- 12.Research Committee of the British Tuberculosis Association Aspergillus in persistent lung cavities after tuberculosis. A report from the Research Committee of the British Tuberculosis Association. Tubercle. 1968;49:1–11. doi: 10.1016/S0041-3879(68)80002-9. [DOI] [PubMed] [Google Scholar]
- 13.International standards for tuberculosis care (ISTC) The Hague: Tuberculosis Coalition for Technical Assistance; 2006.
- 14.Harries AD, Maher D, Nunn P. An approach to the problems of diagnosing and treating adult smear-negative pulmonary tuberculosis in high-HIV-prevalence settings in sub-Saharan Africa. Bull World Health Organ. 1998;76:651–62. [PMC free article] [PubMed] [Google Scholar]
- 15.Global tuberculosis database [Internet]. Incidence, mortality and percentage pulmonary tuberculosis. Geneva: World Health Organization; Available from: http://apps.who.int/globalatlas/ [accessed 3 August 2011].
- 16.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 17.Hamilton CD, Stout JE, Goodman PC, Mosher A, Menzies R, Schluger NW, et al. Tuberculosis Trials Consortium The value of end-of-treatment chest radiograph in predicting pulmonary tuberculosis relapse. Int J Tuberc Lung Dis. 2008;12:1059–64. [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JJ, Chong PY, Lin CB, Hsu AH, Lee CC. High resolution chest CT in patients with pulmonary tuberculosis: characteristic findings before and after antituberculous therapy. Eur J Radiol. 2008;67:100–4. doi: 10.1016/j.ejrad.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 19.de Vallière S, Barker RD. Residual lung damage after completion of treatment for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2004;8:767–71. [PubMed] [Google Scholar]
- 20.Sonnenberg P, Murray J, Glynn JR, Thomas RG, Godfrey-Faussett P, Shearer S. Risk factors for pulmonary disease due to culture-positive M. tuberculosis or nontuberculous mycobacteria in South African gold miners. Eur Respir J. 2000;15:291–6. doi: 10.1034/j.1399-3003.2000.15b12.x. [DOI] [PubMed] [Google Scholar]
- 21.Bombarda S, Figueiredo CM, Seiscento M, Terra Filho M. Pulmonary tuberculosis: tomographic evaluation in the active and post-treatment phases. Sao Paulo Med J. 2003;121:198–202. doi: 10.1590/S1516-31802003000500004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lillebaek T, Poulsen S, Kok-Jensen A. Tuberculosis treatment in Denmark: treatment outcome for all Danish patients in 1992. Int J Tuberc Lung Dis. 1999;3:603–12. [PubMed] [Google Scholar]
- 23.Winqvist N, Nauclér A, Gomes V, Djamanca I, Koivula T, Jensen H, et al. Three-year follow-up of patients with pulmonary tuberculosis in Guinea-Bissau, West Africa. Int J Tuberc Lung Dis. 2000;4:845–52. [PubMed] [Google Scholar]
- 24.Cox H, Kebede Y, Allamuratova S, Ismailov G, Davletmuratova Z, Byrnes G, et al. Tuberculosis recurrence and mortality after successful treatment: impact of drug resistance. PLoS Med. 2006;3:e384. doi: 10.1371/journal.pmed.0030384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connolly C, Reid A, Davies G, Sturm W, McAdam KP, Wilkinson D. Relapse and mortality among HIV-infected and uninfected patients with tuberculosis successfully treated with twice weekly directly observed therapy in rural South Africa. AIDS. 1999;13:1543–7. doi: 10.1097/00002030-199908200-00015. [DOI] [PubMed] [Google Scholar]
- 26.Shean KP, Willcox PA, Siwendu SN, Laserson KF, Gross L, Kammerer S, et al. Treatment outcome and follow-up of multidrug-resistant tuberculosis patients, West Coast/Winelands, South Africa, 1992–2002. Int J Tuberc Lung Dis. 2008;12:1182–9. [PubMed] [Google Scholar]
- 27.Au-Yeung C, Kanters S, Ding E, Glaziou P, Anema A, Cooper CL, et al. Tuberculosis mortality in HIV-infected individuals: a cross-national systematic assessment. Clin Epidemiol. 2011;3:21–9. doi: 10.2147/CLEP.S15574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat. World population prospects: the 2008 revision. Available from: http://esa.un.org/unpp [accessed 3 August 2011].
- 29.Smith N, Denning DW. Underlying pulmonary disease frequency in patients with chronic pulmonary aspergillosis. Eur Respir J. 2011;37:865–72. doi: 10.1183/09031936.00054810. [DOI] [PubMed] [Google Scholar]
- 30.Felton TW, Baxter C, Moore CB, Roberts SA, Hope WW, Denning DW. Efficacy and safety of posaconazole for chronic pulmonary aspergillosis. Clin Infect Dis. 2010;51:1383–91. doi: 10.1086/657306. [DOI] [PubMed] [Google Scholar]
- 31.Kappe R, Schulze-Berge A, Sonntag HG. Evaluation of eight antibody tests and one antigen test for the diagnosis of invasive aspergillosis. Mycoses. 1996;39:13–23. doi: 10.1111/j.1439-0507.1996.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 32.Coleman RM, Kaufman L. Use of the immunodiffusion test in the serodiagnosis of aspergillosis. Appl Microbiol. 1972;23:301–8. doi: 10.1128/am.23.2.301-308.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longbottom JL, Pepys J, Clive FT. Diagnostic precipitin test in Aspergillus pulmonary mycetoma. Lancet. 1964;1:588–9. doi: 10.1016/S0140-6736(64)91335-2. [DOI] [PubMed] [Google Scholar]
- 34.Severo LC, Geyer GR, Porto Nda S, Wagner MB, Londero AT. Pulmonary Aspergillus niger intracavitary colonization. Report of 23 cases and a review of the literature. Rev Iberoam Micol. 1997;14:104–10. [PubMed] [Google Scholar]
- 35.Pasqualotto AC, Denning DW. An aspergilloma caused by Aspergillus flavus. Med Mycol. 2008;46:275–8. doi: 10.1080/13693780701624639. [DOI] [PubMed] [Google Scholar]
- 36.Denning DW, Park S, Lass-Florl C, Fraczek MG, Kirwan M, Gore R, et al. High-frequency triazole resistance found In nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis. 2011;52:1123–9. doi: 10.1093/cid/cir179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regnard JF, Icard P, Nicolosi M, Spagiarri L, Magdeleinat P, Jauffret B, et al. Aspergilloma: a series of 89 surgical cases. Ann Thorac Surg. 2000;69:898–903. doi: 10.1016/S0003-4975(99)01334-X. [DOI] [PubMed] [Google Scholar]
- 38.Kim YT, Kang MC, Sung SW, Kim JH. Good long-term outcomes after surgical treatment of simple and complex pulmonary aspergilloma. Ann Thorac Surg. 2005;79:294–8. doi: 10.1016/j.athoracsur.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 39.Pratap H, Dewan RK, Singh L, Gill S, Vaddadi S. Surgical treatment of pulmonary aspergilloma: a series of 72 cases. Indian J Chest Dis Allied Sci. 2007;49:23–7. [PubMed] [Google Scholar]
- 40.Brik A, Salem AM, Kamal AR, Abdel-Sadek M, Essa M, El Sharawy M, et al. Surgical outcome of pulmonary aspergilloma. Eur J Cardiothorac Surg. 2008;34:882–5. doi: 10.1016/j.ejcts.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 41.Vaid M, Kaur S, Sambatakou H, Madan T, Denning DW, Sarma PU. Distinct alleles of mannose-binding lectin (MBL) and surfactant proteins A (SP-A) in patients with chronic cavitary pulmonary aspergillosis and allergic bronchopulmonary aspergillosis. Clin Chem Lab Med. 2007;45:183–6. doi: 10.1515/CCLM.2007.033. [DOI] [PubMed] [Google Scholar]
- 42.Carvalho A, Pasqualotto AC, Pitzurra L, Romani L, Denning DW, Rodrigues F. Polymorphisms in toll-like receptor genes and susceptibility to pulmonary aspergillosis. J Infect Dis. 2008;197:618–21. doi: 10.1086/526500. [DOI] [PubMed] [Google Scholar]
- 43.Kelleher P, Goodsall A, Mulgirigama A, Kunst H, Henderson DC, Wilson R, et al. Interferon-γ therapy in two patients with progressive chronic pulmonary aspergillosis. Eur Respir J. 2006;27:1307–10. doi: 10.1183/09031936.06.00021705. [DOI] [PubMed] [Google Scholar]
- 44.Jain LR, Denning DW. The efficacy and tolerability of voriconazole in the treatment of chronic cavitary pulmonary aspergillosis. J Infect. 2006;52:e133–7. doi: 10.1016/j.jinf.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 45.Sambatakou H, Dupont B, Lode H, Denning DW. Voriconazole treatment for subacute invasive and chronic pulmonary aspergillosis. Am J Med. 2006;119:527.e17–24. doi: 10.1016/j.amjmed.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 46.Kohno S, Izumikawa K, Ogawa K, Kurashima A, Okimoto N, Amitani R, et al. Japan Chronic Pulmonary Aspergillosis Study Group (JCPASG) Intravenous micafungin versus voriconazole for chronic pulmonary aspergillosis: a multicenter trial in Japan. J Infect. 2010;61:410–8. doi: 10.1016/j.jinf.2010.08.005. [DOI] [PubMed] [Google Scholar]