Abstract
Peutz–Jeghers syndrome (PJS) is a hereditary disorder caused by LKB1 gene mutations, and is associated with considerable morbidity and decreased life expectancy. This study was conducted to assess the attitude of PJS patients towards family planning, prenatal diagnosis (PND) and pregnancy termination, and pre-implantation genetic diagnosis (PGD). In a cross-sectional study, 61 adult PJS patients were asked to complete a questionnaire concerning genetic testing, family planning, PND and PGD. The questionnaire was completed by 52 patients (85% response rate, 44% males) with a median age of 44 (range 18–74) years. A total of 37 (71%) respondents had undergone genetic testing. In all, 24 respondents (46%, 75% males) had children. A total of 15 (29%) respondents reported that their diagnosis of PJS had influenced their decisions regarding family planning, including 10 patients (19%, 9/10 females) who did not want to have children because of their disease. Termination of pregnancy after PND in case of a foetus with PJS was considered ‘acceptable' for 15% of the respondents, whereas 52% considered PGD acceptable. In conclusion, the diagnosis of PJS influences the decisions regarding family planning in one third of PJS patients, especially in women. Most patients have a negative attitude towards pregnancy termination after PND, while PGD in case of PJS is judged more acceptable. These results emphasise the importance of discussing aspects regarding family planning with PJS patients, including PND and PGD.
Keywords: Peutz–Jeghers syndrome, family planning, genetic testing, pre-implantation genetic diagnosis, prenatal diagnosis
Introduction
Peutz–Jeghers syndrome (PJS) is a rare, autosomal dominant inherited disorder caused by germline mutations in the LKB1 gene.1, 2 The syndrome is clinically characterised by gastrointestinal hamartomas and mucocutaneous pigmentation.3, 4 Hamartomatous polyps can develop already in the first decade of life and may cause various complications, including anaemia, bleeding and acute intestinal obstruction.5, 6 Furthermore, PJS is associated with an increased cancer risk in adult life. Lifetime cumulative cancer risks as high as 93% have been described.7, 8 These clinical aspects of the disease affect the psychological condition and quality of life of PJS patients. They suffer from mild depression and experience a poorer mental quality of life, more limitations in daily functioning due to emotional problems and a poorer general health perception compared with the general population.9, 10
Diagnostic mutation analysis is available for patients clinically suspected of PJS. Performing genetic testing might influence family planning of patients. If a pathogenic mutation is confirmed, antenatal genetic testing of offspring is available through prenatal diagnosis (PND) (ie, chorionic villus sampling and amniocentesis), which may result in the wish to terminate the pregnancy in case of an affected foetus. In addition, pre-implantation genetic diagnosis (PGD) has become available. PGD involves in vitro fertilisation (IVF). One or two cells of a 3-day old embryo created in vitro are analysed for the genetic defect, and only embryos with an unaffected genotype are selected for transfer to the uterus.11 Although PND and PGD are available for hereditary cancer syndromes in most European countries, the application of these techniques remains controversial in the social, ethical and political domain.12
Data concerning family planning of patients with PJS are lacking. Therefore, the aim of this study was to investigate the desire to have children in PJS patients, and their attitudes towards PND with the implication of pregnancy termination and towards PGD.
Patients and Methods
Patients
A total of 61 PJS patients from 39 families from two Dutch academic hospitals were invited to complete a questionnaire on genetic testing, family planning, PND and PGD. The study was approved by the Institutional Review Boards of both participating hospitals. Patients were eligible if they were aged 18 years or older and fulfilled the diagnostic criteria for PJS recommended by the World Health Organisation (see Supplementary Information online).13 The questionnaire, an information folder, a consent form and a reply paid envelope were sent to all potential participants by mail. After 6 and 12 weeks a reminder was sent to non-respondents.
Measures
The questionnaire was earlier described in detail by van Lier et al.10 Briefly, it comprised a range of demographic variables including age, gender and parenthood. As psychological determinants, concerns regarding cancer were assessed with the cancer worry scale (CWS),14 and illness perceptions were evaluated by the Illness Perception Questionnaire-Revised (IPQ-R).15 Clinical variables including history of cancer and family history of PJS were derived from medical records.
In addition, respondents were asked whether or not they had undergone genetic testing and, if they had, what the result had been. Self-reported data regarding genetic testing were confirmed by medical records where possible. Questions were posed about the current desire to have (more) children, and if the diagnosis of PJS had influenced the desire to have (more) children. Furthermore, after a short introductory text about PND and PGD, respondents were asked whether or not they considered termination of pregnancy after PND or the use of PGD acceptable; (1) in general, and (2) in case of PJS (see Supplementary Information online). Response categories were ‘yes', ‘no' or ‘unsure'.16
Statistical analysis
Data were analysed using the SPSS 17.0 statistical software for Windows (IBM, Somers, NY, USA). Descriptive statistics were used to characterise the study sample. Continuous variables were reported by means (and standard deviation) and medians (and range). Univariate analyses (χ2, Fisher's exact test, independent t-test and Mann–Whitney U-test) were used to evaluate which sociodemographic, clinical and psychological variables were related to attitudes towards genetic testing, PND and PGD. A two-sided P-value <0.05 was considered statistically significant. Multivariate logistic regression analyses using backward selection with a P-value of 0.1 for removal of the variable was carried out to determine associations between possible confounders (sociodemographic, personal and family medical history and psychosocial determinants) and three outcome measures: genetic testing (‘yes' or ‘no'), termination of pregnancy after PND acceptable in case of PJS (‘yes' or ‘no/unsure') and PGD acceptable in case of PJS (‘yes' or ‘no/unsure').
Results
Baseline characteristics
The questionnaire was completed by 52 PJS patients (response rate 85%) from 34 families. Median age of respondents was 44 (18–74) years and 23 (44%) were male. Baseline characteristics of the respondents and non-respondents are shown in Table 1.
Table 1. Baseline characteristics of respondents and non-respondents.
| Respondents | Non-respondents | |
|---|---|---|
| N (%) | N (%) | |
| 52 | 9 | |
| Median age (range)a | 44 (18–74) | 34 (18–67) |
| ≤45 yrs (child-bearing age) | 29 (55.8) | 5 (55.6) |
| >45 yrs | 23 (44.2) | 4 (44.4) |
| Gendera | ||
| Male | 23 (44.2) | 6 (66.7) |
| Female | 29 (55.8) | 3 (33.3) |
| Partner | ||
| Yes | 36 (69.2) | Unknown |
| No | 16 (30.8) | Unknown |
| Children | ||
| Yes | 24 (46.2) | 5 (55.6) |
| No | 28 (53.8) | 4 (44.4) |
| Educational level | ||
| Low | 29 (55.8) | Unknown |
| High | 23 (44.2) | Unknown |
| Genetic testing performed | ||
| Yes | 37 (71.2) | 9 (100) |
| No | 15 (28.8) | 0 (0) |
| Family history | ||
| Familial PJS | 33 (63) | 5 (55.6) |
| Sporadic PJS/family unknown | 19 (37) | 4 (44.4) |
Abbreviation: PJS, Peutz–Jeghers syndrome.
Age (P=0.86) and gender distribution (P=0.29) did not differ between respondents and non-respondents.
There were no significant differences in age (P=0.056) or cancer incidence between male and female respondents (P=0.144). However, women in our cohort scored significantly higher than men on the CWS (6.41 vs 5.13, P=0.038), and on the IPQ-R subscale emotional representations (16.21 vs 12.87, P=0.019). Scores on the other six IPQ-R subscales did not differ significantly between male and female respondents.
Genetic testing
Of the 52 patients who completed the questionnaire, 37 patients had undergone genetic testing, of which 33 (89%) were actually carrier of a pathogenic LKB1 mutation. Multivariate logistic regression analysis showed female gender (P=0.035) and parenthood (P=0.016) as positive predictors for genetic test uptake (Supplementary Table 1).
Parenthood and influence of PJS on family planning
In all, 24 respondents (46% median age 50 years) had children. Female PJS patients less often had children than male patients (25 vs 75%, P<0.001).
Of the 52 respondents, 15 (29%, median age 44 years) reported that the diagnosis of PJS had influenced their desire to have children (ie, less or no children). Ten of these 15 respondents (19% median age 45 years) stated that they had decided to have no children because of PJS, including nine females and one male, the latter who had adopted a child. Cancer incidence was higher in these 10 patients (56 vs 44%, P=0.011), and they scored higher on the CWS (8.0 vs 5.2, P=0.039) compared with the other respondents. In all, 23 of the respondents (44%, median age 45 years) indicated that PJS had not influenced their desire to have children.
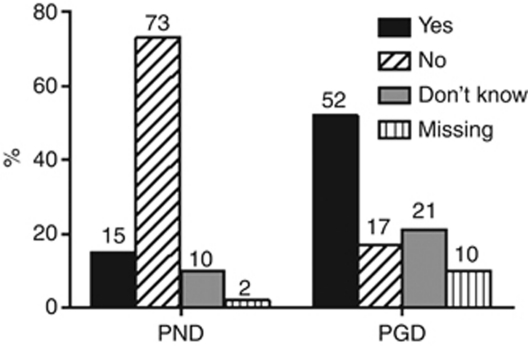
In general, the majority of respondents considered termination of pregnancy after PND and the use of PGD as ‘acceptable' (62% and 61%, respectively). The attitude of respondents regarding these two techniques in relation to PJS is shown in Figure 1. Fifteen percent of patients considered pregnancy termination after PND acceptable, while 52% accepted the use of PGD in case of PJS. Results of univariate and multivariate analyses are shown in Tables 2 and 3. No significant associations were found for the attitude towards pregnancy termination after PND or towards PGD.
Figure 1.
Attitude of PJS patients towards termination of pregnancy after PND and PGD in case of PJS. PND: acceptance of termination of pregnancy after PND in case of PJS. PGD: acceptance of the use of PGD in case of PJS.
Table 2. Determinants of the attitude towards termination of pregnancy in case of a foetus with PJS (N=51).
| Univariate analysis | Multivariate logistic regression analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Gender; male/female | 2.609 (0.472; 14.406) | 0.271 | — | |
| Age | 0.936 (0.877; 0.998) | 0.042 | — | |
| Aware of mutation status; yes/noa | 0.680 (0.149; 3.099) | 0.618 | ||
| Children; yes/no | 0.124 (0.014; 1.098) | 0.061 | — | |
| PJS familial; yes/no | 0.655 (0.133; 3.218) | 0.602 | ||
| Malignancy; yes/no | 0.625 (0.067; 5.822) | 0.680 | ||
| CWS score | 1.165 (0.881; 1.540) | 0.283 | ||
Abbreviations: 95% CI, 95% confidence interval; CWS, cancer worry scale; OR, odds ratio; PJS, Peutz–Jeghers syndrome.
In all, 29 respondents were aware of their mutation status; 27 LKB1 mutation positive and 2 LKB1 mutation negative.
Table 3. Determinants of the attitude towards pre-implantation genetic diagnosis in case of PJS (N=47).
| Univariate analysis | Multivariate logistic regression analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Gender; male/female | 1.455 (0.454;4.664) | 0.529 | — | |
| Age | 1.021 (0.978;1.067) | 0.341 | — | |
| Aware of mutation status; yes/noa | 1.700 (0.525;5.500) | 0.376 | ||
| Children; yes/no | 1.135 (0.356;3.621) | 0.831 | ||
| PJS familial; yes/no | 0.343 (0.078;1.500) | 0.155 | ||
| Malignancy; yes/no | 0.375 (0.078;1.803) | 0.221 | ||
| CWS score | 1.187 (0.884;1.593) | 0.254 | ||
Abbreviations: 95% CI, 95% confidence interval; CWS, cancer worry scale; OR, odds ratio; PJS, Peutz–Jeghers syndrome.
In all, 29 respondents were aware of their mutation status; 27 LKB1 mutation positive and 2 LKB1 mutation negative.
Discussion
This is the first survey among PJS patients that evaluated their decisions regarding family planning, and their attitude towards PND with possible pregnancy termination, and towards PGD. In all, 24 respondents (46, 75% males) had children. Interestingly, there was a notable gender difference in our study population with respect to parenthood. Female patients less often had children than men with PJS. Furthermore, 90% of patients (9/10) who explicitly indicated that they did not want to have children because of PJS were female. The reason for this difference is not clear. As PJS is associated with an increased risk for the development of gynaecological tumours,8, 17 disabilities (eg, hysterectomy or oophorectomy) might have prevented female patients from having children. However, this was the case in only two females from our cohort (at the age of 36 and 39 years). In addition, there were no significant differences in age or cancer incidence between male and female respondents. One could postulate that psychosocial explanations for this difference exist. Women in our cohort did have more cancer worries than men, and had a higher emotional response to PJS. These findings could imply that women are more emotionally affected by their disease, which can render to a higher sense of responsibility towards their offspring.18
All respondents, irrespective of parenthood, were asked about their attitude towards termination of pregnancy after PND. More patients accepted the use of PGD in case of PJS than pregnancy termination after PND, suggesting a preference for PGD. This preference has been observed before in couples with different genetic disorders, including cancer susceptibility syndromes as hereditary breast and ovarian cancer and familial adenomatous polyposis syndrome.19, 20, 21, 22, 23 In a recent study among couples with a broad spectrum of genetic disorders, 74% of couples preferred PGD over PND for diagnostic testing in a future pregnancy.24 The preference for PGD can partly be explained by the fact that PGD offers patients the possibility to have an unaffected genetically related child while termination of a pregnancy can be avoided. Furthermore, early reassurance is seen as an important advantage.19 Though, many individuals with a hereditary condition for which PGD has been permitted, are unfamiliar with the technique or even unaware of its existence.24 In practise, PGD is physically and psychologically burdensome.25 Our questionnaire did not explore the knowledge of respondents about PND and PGD. Although both techniques were shortly described, the information might have been too limited. Furthermore, positive attitudes towards PND and PGD do not necessarily translate into actual use.26
This study is hampered by some limitations. First of all, the cross-sectional study design makes evaluation of causal interactions impossible. Instead, we can only demonstrate statistical associations between determinants and the attitude towards genetic testing and reproductive decision-making. Second, only affected individuals were asked to fill in the questionnaire, not their partners, yet it is likely that partners of PJS patients have an important role in the reproductive decision making and family planning. Third, the actual use of PND and subsequent pregnancy termination and PGD amongst PJS patients is not known, and questions regarding religion were not included in our questionnaire, while religion can be of influence on the attitude towards both PND as well as PGD. Finally, in spite of the response rate of over 85%, our conclusions are drawn from a small sample size. However, as PJS is a rare disorder it is difficult to assess a larger group. We managed to approach nearly all known Dutch PJS patients, thereby creating a heterogeneous cohort of patients enrolled in similar surveillance programs and with similar access to medical care. To our knowledge this is the first report concerning reproductive decision-making and the attitude towards antenatal diagnostics amongst PJS patients.
In conclusion, this study demonstrates that the diagnosis of PJS influences decisions regarding family planning in approximately one third of PJS patients, especially in women. The majority of patients undergo genetic testing, and many PJS patients have a positive attitude towards PGD as an option to prevent transmission of PJS to their offspring. In contrast, the attitude of respondents was predominantly negative towards pregnancy termination after PND in case of a foetus affected with the syndrome. Our results emphasise not only the importance of accurate genetic counselling for these patients; it also indicates that medical specialists dealing with patients suffering from hereditary cancer syndromes, including PJS, should discuss aspects regarding family planning, such as PND and PGD.
Acknowledgments
We would like to thank all participating PJS patients.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- Jenne DE, Reimann H, Nezu J, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- Peutz JLA. Over een zeer merkwaardige, gecombineerde familiaire polyposis van de slijmliezen van den tractus intestinalis met die van de neuskeelholte en gepaard met eigenaardige pigmentaties van huid en slijmvliezen. Ned Maandschr v Geneesk. 1921;10:134–146. [Google Scholar]
- McGarrity TJ, Amos C. Peutz-Jeghers syndrome: clinicopathology and molecular alterations. Cell Mol Life Sci. 2006;63:2135–2144. doi: 10.1007/s00018-006-6080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsunomiya J, Gocho H, Miyanaga T, Hamaguchi E, Kashimure A. Peutz-Jeghers syndrome: its natural course and management. Johns Hopkins Med J. 1975;136:71–82. [PubMed] [Google Scholar]
- Hearle N, Schumacher V, Menko FH, et al. STK11 status and intussusception risk in Peutz-Jeghers syndrome. J Med Genet. 2006;43:e41. doi: 10.1136/jmg.2005.040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- van Lier MG, Wagner A, Mathus-Vliegen EM, Kuipers EJ, Steyerberg EW, van Leerdam ME. High cancer risk in Peutz-Jeghers syndrome: a systematic review and surveillance recommendations. Am J Gastroenterol. 2010;105:7. doi: 10.1038/ajg.2009.725. [DOI] [PubMed] [Google Scholar]
- Woo A, Sadana A, Mauger DT, Baker MJ, Berk T, McGarrity TJ. Psychosocial impact of Peutz-Jeghers syndrome. Fam.Cancer. 2009;8:59–65. doi: 10.1007/s10689-008-9202-z. [DOI] [PubMed] [Google Scholar]
- van Lier MG, Mathus-Vliegen EM, van Leerdam ME, et al. Quality of life and psychological distress in patients with Peutz-Jeghers syndrome. Clin Genet. 2010;78:219–226. doi: 10.1111/j.1399-0004.2010.01469.x. [DOI] [PubMed] [Google Scholar]
- Sermon K, Van Steirteghem A, Liebaers I. Preimplantation genetic diagnosis. Lancet. 2004;363:1633–1641. doi: 10.1016/S0140-6736(04)16209-0. [DOI] [PubMed] [Google Scholar]
- Lammens C, Bleiker E, Aaronson N, et al. Attitude towards pre-implantation genetic diagnosis for hereditary cancer. Fam Cancer. 2009;8:457–464. doi: 10.1007/s10689-009-9265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SR, Aaltonen LA. World Health Organization Classification of Tumours. Pathology and Genetics. Tumours of the Digestive System. IARC Press: Lyon; 2001. [Google Scholar]
- Lerman C, Trock B, Rimer BK, Jepson C, Brody D, Boyce A. Psychological side effects of breast cancer screening. Health Psychol. 1991;10:259–267. doi: 10.1037//0278-6133.10.4.259. [DOI] [PubMed] [Google Scholar]
- Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The revised illness perception questionnaire (IPQ-R) Psychol Health. 2002;17:1–16. [Google Scholar]
- Douma KF, Aaronson NK, Vasen HF, Verhoef S, Gundy CM, Bleiker EM. Attitudes toward genetic testing in childhood and reproductive decision-making for familial adenomatous polyposis. Eur J Hum Genet. 2010;18:186–193. doi: 10.1038/ejhg.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res. 2006;12:3209–3215. doi: 10.1158/1078-0432.CCR-06-0083. [DOI] [PubMed] [Google Scholar]
- d'Agincourt-Canning L, Baird P. Genetic testing for hereditary cancers: the impact of gender on interest, uptake and ethical considerations. Crit Rev Oncol Hematol. 2006;58:114–123. doi: 10.1016/j.critrevonc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Snowdon C, Green JM. Preimplantation diagnosis and other reproductive options: attitudes of male and female carriers of recessive disorders. Hum Reprod. 1997;12:341–350. doi: 10.1093/humrep/12.2.341. [DOI] [PubMed] [Google Scholar]
- Chamayou S, Guglielmino A, Giambona A, et al. Attitude of potential users in Sicily towards preimplantation genetic diagnosis for beta-thalassaemia and aneuploidies. Hum Reprod. 1998;13:1936–1944. doi: 10.1093/humrep/13.7.1936. [DOI] [PubMed] [Google Scholar]
- Lavery SA, Aurell R, Turner C, et al. Preimplantation genetic diagnosis: patients' experiences and attitudes. Hum Reprod. 2002;17:2464–2467. doi: 10.1093/humrep/17.9.2464. [DOI] [PubMed] [Google Scholar]
- Kastrinos F, Stoffel EM, Balmana J, Syngal S. Attitudes toward prenatal genetic testing in patients with familial adenomatous polyposis. Am J Gastroenterol. 2007;102:1284–1290. doi: 10.1111/j.1572-0241.2007.01168.x. [DOI] [PubMed] [Google Scholar]
- Menon U, Harper J, Sharma A, et al. Views of BRCA gene mutation carriers on preimplantation genetic diagnosis as a reproductive option for hereditary breast and ovarian cancer. Hum Reprod. 2007;22:1573–1577. doi: 10.1093/humrep/dem055. [DOI] [PubMed] [Google Scholar]
- Musters AM, Twisk M, Leschot NJ, et al. Perspectives of couples with high risk of transmitting genetic disorders. Fertil Steril. 2010;94:1239–1243. doi: 10.1016/j.fertnstert.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Van Voorhis BJ. Clinical practice. In vitro fertilization. N Engl J Med. 2007;356:379–386. doi: 10.1056/NEJMcp065743. [DOI] [PubMed] [Google Scholar]
- de Die-Smulders CE, Land JA, Dreesen JC, Coonen E, Evers JL, Geraedts JP. Results from 10 years of preimplantation-genetic diagnostics in The Netherlands. Ned Tijdschr Geneeskd. 2004;148:2491–2496. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.