Abstract
Organ deposition of autoantibodies against the noncollagenous-1 domain of the α3 chain of type IV collagen leads to severe kidney and lung injury in anti-glomerular basement membrane disease. The origin and regulation of these highly pathogenic autoantibodies remains unknown. Anti-α3(IV) collagen B lymphocytes are predicted to mature in vivo ignorant of target antigen because α3(IV) collagen expression is highly tissue restricted and pathogenic epitopes are cryptic. However, a recent analysis of an anti-α3(IV)NC1 collagen autoantibody transgenic mouse model revealed that developing B cells are rapidly silenced by deletion and editing in the bone marrow. To dissect the role of collagen as central tolerogen in this model, we determined B cell fate in autoantibody transgenic mice genetically lacking α3(IV) collagen. We found that absence of the tissue target autoantigen has little impact on the fate of anti-α3(IV)NC1 B cells. This implies a more complex regulatory mechanism for preventing anti-glomerular basement membrane disease than has been previously considered, including the possibility that a second antigen present in bone marrow engages and tolerizes anti-α3(IV)NC1 collagen B cells.
Keywords: anti-glomerular basement membrane, alpha3(IV) collagen, B cell, tolerance, Goodpasture
1. Introduction
Autoantibodies directed against epitopes of various collagen isoforms are associated with debilitating damage in kidney, joints, skin, and lung [1–6]. In anti-glomerular basement membrane (anti-GBM) nephritis and Goodpasture’s disease, pathogenic autoantibodies destroy kidney and lung by targeting the globular C-terminal noncollagenous-1 (NC1) domain of the α3 chain of type IV collagen, hereafter α3(IV)NC1 (reviewed in [7]). The organ-specificity reflects the highly tissue-restricted expression of the α3(IV) collagen chain [8].
Several features of anti-GBM disease underscore the role of B cell dysregulation in disease origin and pathogenesis. Detection of circulating autoantibodies and linear Ig deposits in tissue specimens is key to diagnosis, and serum autoantibody levels guide treatment decisions, including duration of plasmapheresis and timing of transplantation. Direct pathogenicity of human anti-GBM autoantibodies was demonstrated by induction in primates of severe renal failure, proliferative glomerulonephritis, and linear GBM Ig deposits after passive transfer of patients’ renal eluate Ig [9]. Epitopes recognized by the pathogenic Ig are conformational and cryptic, buried within the cross-linked native NC1 hexamer [10–14]. This suggests both that renal epitopes must be exposed by environmental or other stressors to facilitate Ig binding and that relevant epitopes are not normally accessible to the immune system to engage and regulate developing anti-α3(IV)NC1 B lymphocytes. However, certain disease features are difficult to reconcile with this paradigm: Anti-GBM nephritis and Goodpasture’s disease are rare, and anti-α3(IV)NC1 Ig are not detected in the sera of most healthy individuals using conventional assays. Moreover, renal inflammation and injury from diverse and common causes, such as pyelonephritis, do not induce anti-α3(IV)NC1 autoantibodies despite generating a vigorous inflammatory and immunogenic microenvironment.
We previously explored this paradox using an autoantibody transgenic model, constructed such that transgenic (IgMa/kappa) B cell receptors bind epitopes of α3(IV)NC1 collagen recognized by serum pathogenic IgG from patients with anti-GBM disease but not by serum IgG from healthy individuals [15]. Initial experiments tested the hypothesis that naïve anti-α3(IV) collagen B lymphocytes are immunologically ignorant because they do not see hidden antigen during development in vivo. This scenario also predicts that the cells are available in the periphery for activation by environmental stimulants. However, analysis of the Ig transgenic mice instead revealed B cell depletion and light chain editing, suggesting that anti-α3(IV) collagen B cells are regulated centrally. Subsequent generation of Ig transgenic mice incapable of Ig receptor editing due to genetic elimination of the recombinase activating gene, Rag, revealed a default phenotype of profound central tolerance: mature B cells were absent from spleen and bone marrow [15]. An identical phenotype was observed in Rag-deficient Ig transgenic progeny derived from independent founders.
These results indicate the presence of a tolerizing cognate self-antigen in bone marrow, as well as unsuspected complexity either in distribution and exposure of α3(IV) collagen B cell epitopes or in the nature of self-antigen-lymphocyte interactions in anti-α3(IV)NC1 Ig-mediated autoimmune diseases. Although α3(IV) collagen is not known to be expressed in bone marrow [16], serum is reported to contain soluble α3(IV)NC1 monomers generated by metalloproteinase cleavage [17]. To test the possibility that intact α3(IV) collagen or circulating NC1 monomers mediate central tolerance, we bred our anti-α3(IV)NC1 IgMa/kappa transgenes into mice with genetic α3(IV) collagen deficiency [18], both in the absence and presence of the Rag gene. The results reported here indicate that tolerance persists despite elimination of the known Ig tissue target antigen.
2. Materials and Methods
2.1 Animals
Generation of transgenic mice carrying both the H and L chain constructs of the prototypic anti-α3(IV)NC1 monoclonal antibody and deficient in the Rag1 enzyme was previously described [15], with a detailed description of the prototypic antibody and H and L chain variable region gene sequences, showing minimal mutation, found in [19] and GenBank accession numbers DQ889716.1 and DQ889719.1. Antibody transgenic and collagen mutant lines were genotyped by PCR using specific forward and reverse primers:
| Heavy chain | 5′-CTTCAGTGAAGATGTCCTGTAA – 3′ |
| 5′-AGACTGTGAGAGTGGTGCCT – 3′ | |
| Light chain | 5′-CCAACAGAAACCAGGACAGC -3′ |
| 5′-CGTTTTATTTCCAGCTTGGTC – 3′ | |
| α3(IV) collagen | 5′ - AAC ACC AGC TCT GAT GCC AAT G – 3′ |
| 5′-AAT GAA AGA AAA ACC TTT CCA GAG – 3′ | |
| Knockout α3(IV)collagen | 5′ - ACG ACC TTT GTT AAA CTA GAA GAA GTC – 3′ |
| 5′-TGC TAA AGC GCA TGC TCC AGA CTG C -3′ |
The experiments described here were carried out on mice of either gender, age 9 to 24 weeks, hemizygous for the introduced Ig chain transgenes and reared under conventional specific pathogen-free conditions. C57BL/6J (hereafter B6) breeders, BALB/c (IgMa allotype) controls, and mice carrying a targeted mutation in Rag1 on the B6 background (RagKO) were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice carrying a targeted disruption of the α3 chain of type IV collagen on the B6 background [18, 20] were graciously provided by Dr. Jeffrey Miner, Washington University, St. Louis, MO. Absence of α3(IV) collagen protein in this model has been confirmed, as assessed by the absence of anti-collagen α3(IV) antibody binding to kidney sections [18] and the absence of circulating α3(IV)NC1 monomers [17]. Whereas α3(IV)-deficient mice develop a progressive renal disease marked by abnormal GBM morphology and proteinuria similar to human Alport syndrome, kidney disease is attenuated and later in onset in the B6 background [20]. Tg+ mice were generated on a B6SJLF1/J background and were subsequently crossed onto the B6 background 2–3 generations for the Tg+ Col-KO studies, and an additional 2–3 generations for Tg+ Col-KO RagKO studies. The care and use of all experimental animals was in accordance with institutional guidelines and all studies and procedures were approved by the Animal Care and Use Committees of Duke University and the Durham Veterans Affairs Medical Center.
2.2 Cell staining
Single-cell suspensions containing one million RBC-depleted splenocytes or bone marrow cells were rinsed three times in 1X PBS plus 2% fetal bovine serum, incubated for 30 minutes with the appropriate fluorescently-labeled antibodies as described below, rinsed two additional times, and fixed in 1% paraformaldehyde prior to analysis by flow cytometry. Fluorescent stains include: anti-CD45R/B220-PE and –PerCP, anti-IgMb-FTC, and anti-IgMa-PE (Becton Dickinson-Pharmingen, San Jose, CA, USA), and anti-IgM-FTC, anti-IgD-FTC, anti-lambda-FTC, and anti-kappa-PE (Southern Biotech, Birmingham, Alabama, USA). Cells were analyzed on a FACScan analyzer (Becton Dickinson), and results analyzed using FlowJo (Treestar, Ashland, OR, USA). Lymphocytes were gated based on FSC and SSC properties.
2.3 Antibody isotype, allotype, and binding specificity
Ig concentrations and isotype- and allotype-specific binding in serum were determined by ELISA. 96-well Immulon II HB plates (Thermo Scientific, Waltham, MA) were coated overnight with goat-anti-mouse IgM (Southern Biotech). Plate coat was removed, and plates were blocked using 3% bovine serum albumin (BSA, Sigma, St. Louis, MO, USA). Diluted sera (range 1:20-1:10000) were assessed in duplicate against a standard curve generated by a commercial mouse IgMa/kappa antibody (BD Pharmingen, for IgM and IgMa ELISAs), or by a dilution series from a previously characterized murine serum (for IgMb ELISAs.) Following three rinses, bound antibody was detected via incubation with either alkaline-phosphatase conjugated anti-IgM (Southern Biotech, 1:1000 dilution in PBS), or with biotinylated anti-IgMa or anti-IgMb antibodies (BD Pharmingen, 1:500 dilution in PBS). For IgMa and IgMb detection, plates were rinsed then incubated with Streptavidin-AP (1:500 dilution in PBS, Southern Biotech). Colorimetric detection was provided by phosphatase substrate (Sigma), and assays were monitored at 405 nm for up to 2 hours. Interpolation into the standard curve was performed by SoftMax software.
To assay for collagen binding activity, Immulon II plates were coated overnight at 4°C with bovine α3(IV)NC1 collagen (Wieslab) diluted in 6M guanidine-HCl (Sigma). Plates were blocked, rinsed, and detected as above with minimal changes. Sera were diluted at 1:20 and 1:100 for assay. Control Ig included anti-α3(IV)NC1 IgG mAb (Wieslab) and transfectant IgM. Results were recorded as the mean sample OD after subtraction of mean OD on diluent-coated plates. Ag binding by transgene-encoded Ig was confirmed using biotin-labeled anti-allotype (IgMa)-specific second-step reagents (BD Biosciences) detected with Streptavidin- alkaline phosphatase (Southern Biotechnology Associates) as above.
2.4 Immunohistochemistry
Kidneys from experimental subjects were frozen in Tissue-Tek O.C.T. (Sakura Finetek USA, Torrance, CA, USA) and sectioned. Following acetone fixation, sections were rinsed in PBS, blocked in a solution of 3% BSA and 3% normal goat serum (Gibco Invitrogen, Carlsbad, CA, USA) in PBS, then incubated with a 1:300 dilution of MAB3 anti-α3(IV) collagen antibody (Wieslab, Sweden) in 5% milk powder/PBS solution overnight at 4C in a humid chamber. Slides were then rinsed in PBS followed by incubation with goat anti-mouse IgG-FITC (Southern Biotech) at a 1:100 dilution in blocking buffer for 2 hours. After a final PBS rinse, slides were mounted in Fluoromount G (Southern Biotech) containing DAPI nuclear stain (Sigma) for analysis.
2.5 Statistical analyses
All data are shown as mean values +/− standard deviation. Comparisons between two groups were analyzed with the Wilcoxon test. Analyses were performed with JMP software (SAS Institute, Cary, NC). A value of p < 0.05 was considered to be significant.
3. Results
3.1 In vivo absence of α3(IV) collagen does not rescue anti-α3(IV)NC1 collagen B cells
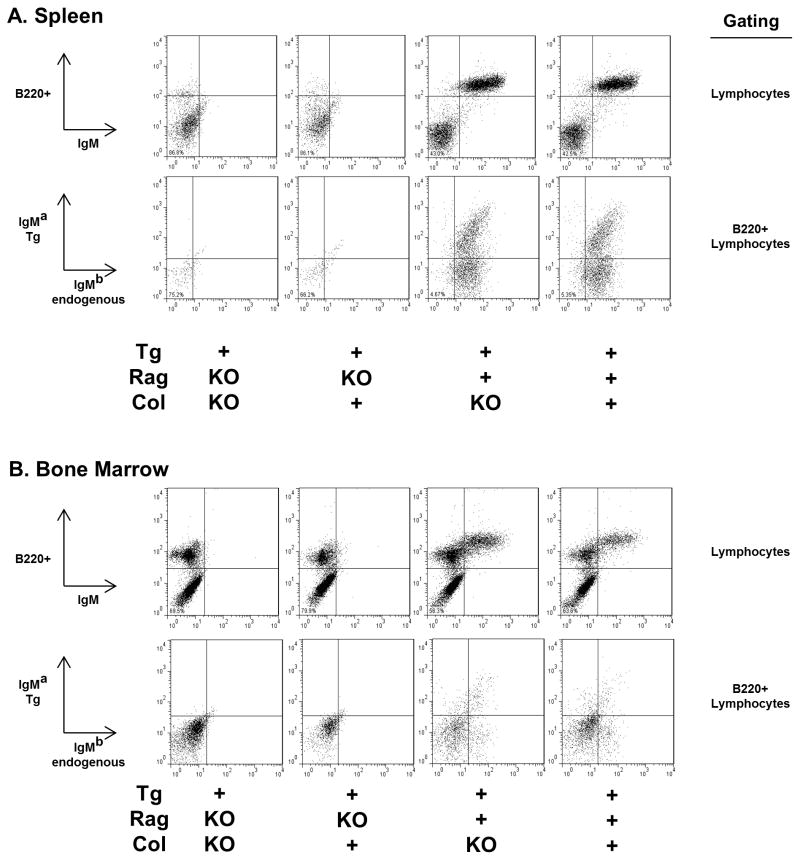
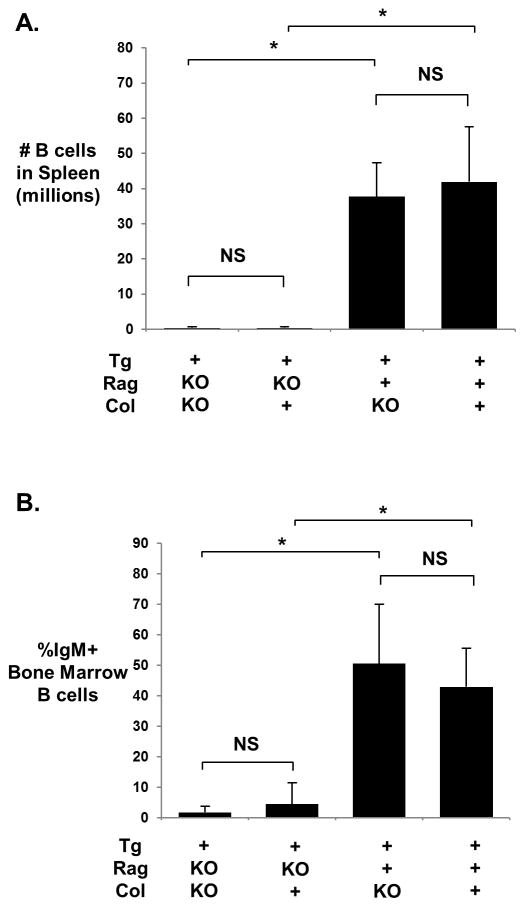
Immune phenotyping of transgenic (Tg+) collagen α3(IV)-deficient (Col-KO) mice indicates that the absence of target antigen, including its circulating fragments, does not rescue autoreactive B cells from central deletion. In Tg+ Rag-deficient (Tg+RagKO) mice, IgM+ B cells are absent in both spleen and bone marrow of Col-KO as well as collagen-sufficient (Col+) groups (Figs. 1 and 2). This phenotype is identical to that of the non-Tg RagKO mice, which by default have no B cells [15]. Consistent with this finding, serum Tg+ IgMa is undetectable or present only in minute quantities (range 0 – 0.359 μg/ml) in both groups of mice. As expected, endogenous IgMb is not detected in any mouse with homozygous Rag deficiency, whereas IgMb cell surface and serum expression is abundant in all Rag-sufficient (Rag+) mice (Fig. 1 and data not shown). Absence of α3(IV) collagen expression in homozygous α3(IV) collagen-deficient mice was confirmed in a subset of mice using immunofluorescent staining of frozen kidney sections (not shown).
Figure 1.
Representative dot plots for lymphocytes in spleen (A) and bone marrow (B) of mice bearing the anti-α3(IV)NC1 collagen IgMa,kappa antibody transgenes (Tg+). Log fluorescence data for stained unstimulated cells gated on lymphocytes on the basis of forward and side scatter (all plots), with further gating on B220+ populations where indicated. B220 is a B cell marker. IgM-a allotype identifies the transgene heavy chain, IgM-b allotype identifies endogenous heavy chains.
Figure 2.
Mean ± standard deviation for spleen B cell count (A) and % IgM+ Bone Marrow B cells (B). N=4–5 for all groups using data from 4 experimental replications. Significant differences were determined by pairwise comparison using the Wilcoxon test and are indicated by asterisks; *, p<0.05; NS, p>0.05.
3.2 Editing is prominent in Rag-sufficient Col-KO transgenic mice
Immunophenotyping also reveals no difference in B cell numbers, B cell phenotype, serum IgM levels, and transgene expression between Tg+ Col-KO and Tg+ Col+ mice in which the Rag enzyme is active (Tg+Rag+, Fig. 1 and Table 1). Analysis of bone marrow reveals a similar frequency of surface IgM+ B220+ B cells in Tg+ Col-KO and Tg+ Col+ mice (Table 1). Roughly one-third of spleen B cells express the transgene IgMa allotype in both groups, and both groups have detectable but low levels of serum transgene IgMa and transgene-encoded anti-α3(IV)NC1 reactivity. There are no differences in circulating total IgM concentration, and the majority of splenic B cells and predominance of serum IgM express the endogenous IgMb allotype (Table 1), indicating more extensive rearrangement at the endogenous Ig heavy chain alleles than was observed in early generation backcross mice [15]. The majority of splenic B cells in both groups also coexpress endogenous surface IgD, similar to expression levels in non-Tg Rag+ mice (not shown.) Approximately 7% of Tg+ mouse B cells express endogenous lambda light chains (Table 1), indicating editing also at this light chain locus.
Table 1.
B cell and serum antibody profiles in Tg+ Rag-sufficient mice in presence (Col+) and absence (Col-KO) of α3(IV) collagen. B cell data are from spleen except for BM (bone marrow). IgMa identifies the Tg+ heavy chain; host endogenous Ig is IgMb. The percent IgMa+, IgMb+, and other calculations are based on total B cells as determined by B220+ staining. Because a subset of B cells express both transgene IgMa and endogenous IgMb, the total percent IgMa+ plus IgMb+ staining is greater than 100%. Serum anti-collagen IgMa is OD405 for binding of 1:20 serum dilution to α3(IV)NC1 minus diluent binding, with positive control MAB3 mean OD405 2.0 ± 1.4 and non-Tg mice mean OD405 0.002 ± 0.004. Anti-collagen binding was not detected at 1:100 serum dilution in either group. There were no significant differences between Tg+ Col+ and Tg+ Col-KO as determined by the Wilcoxon test. All data are mean ± standard deviation. N=7–12 mice per group, mean age 3.4 months, for B cell phenotype data and 4–6 mice per group for serum studies.
| Tg+ Col+ | Tg+ Col-KO | |
|---|---|---|
| B cell count millions | 25.9 ± 7.1 | 25.8 ± 10.8 |
| %IgMa+ of B cells | 32.1 ± 3.6 | 32.5 ± 6.7 |
| %IgMb+ of B cells | 91.2 ± 4.1 | 91.5 ± 5.6 |
| %lambda+ of B cells | 7.4 ± 1.3 | 6.6 ± 1.3 |
| BM %IgM+ of B220+ | 51.7 ± 5.0 | 53.5 ± 8.8 |
| Serum IgM μg/mL | 122.8 ± 18.4 | 124.0 ± 36.6 |
| Serum Tg IgMa μg/mL | 5.6 ± 3.4 | 4.5 ± 4.0 |
| Serum Tg anti-collagen (OD405) | 0.116 ± 0.061 | 0.105 ± 0.42 |
4. Discussion
These data indicate that presence of the tissue target protein, α3(IV) collagen, is not essential for the in vivo regulation of anti-α3(IV)NC1 B cells in this Ig transgenic model. These findings further suggest the existence of a distinct bone-marrow localized antigen that is capable of binding to the transgenic anti-α3(IV)NC1 Ig receptors and inducing tolerance in these B cells. The striking bone marrow phenotype in the Tg Col-KO RagKO mice, in which virtually no Tg+ cells are detected, is consistent with the findings of other investigators in the setting of a known potent central tolerogen concurrent with the absence of Rag enzyme activity [21]. We conclude that in a healthy host, newly formed dual-specific anti-α3(IV)NC1 collagen B cells engage this second antigen in the bone marrow immediately upon expression of surface Ig. Intracellular signals emanating from interaction with this as yet unidentified tolerogen arrest B cell development, leading to cell deletion unless a Rag-dependent process alters the receptor, thus shielding the host from deadly anti-GBM disease.
The indistinguishable immune phenotype in Rag-sufficient Tg+ Col-KO versus Tg+ Col+ mice confirms that receptor editing is also triggered by the unknown tolerogen. Thus either central regulatory mechanism, editing or cell deletion, may be disrupted to promote disease in genetically susceptible individuals. Notably, if tolerance fails, the second self-antigen, or structurally related environmental antigens, become potential potent immune activators.
The existence of a tolerizing antigen distinct from α3(IV)NC1 collagen provides a step toward resolving the underlying paradox wherein the relative rarity of anti-GBM disease and of anti-α3(IV)NC1 serological reactivity suggest a high degree of B cell regulation that is discordant with the cryptic and tissue-restricted nature of the pathogenic Ig epitopes. Our findings suggest a more complex paradigm for B cell regulation that is dependent on anti-α3(IV)NC1 Ig binding to multiple self-antigens, including one that regulates B cell fate and a distinct antigen targeted by soluble pathogenic Ig. A regulatory network based on such self dual specificity is fully plausible. The literature is replete with examples of presumably selective enzymes, receptors, and antibodies that bind structurally dissimilar substrates, ligands, or antigens, respectively, with equal or greater affinity than the natural or originally identified substrate [22–24]. A molecular basis for antibody dual self-specificity was recently elegantly revealed in a therapeutic monoclonal IgG capable of targeting the structurally distinct antigens human epidermal growth factor receptor-2 and vascular endothelial growth factor [25].
The identity of the second antigen that acts as central tolerogen in our Ig transgenic model is as yet unknown, and its identity is difficult to predict a priori. Unlike T cell epitopes that may be predicted from primary structure and protein sequence databases [26], Goodpasture antibodies, like many Ig and B cell receptors, recognize conformational epitopes. It is also possible that the second antigen is structurally unique compared to the α3(IV)NC1 epitope, such that binding requires plasticity in the antibody binding site, analogous to that described by Bostrom and colleagues [25]. The pool of endogenous molecules with access to the bone marrow is large and structurally diverse. Candidate central tolerogens include epitopes on circulating cells, resident bone marrow cells, bone marrow matrix, and serum analytes, of which there are an estimated one thousand. Candidate cell surface tolerogens also include glycoproteins and glycolipids, though it is notable that the murine anti-α3(IV)NC1 antibodies identified no targets on a mammalian glycan array (data not shown). Screening strategies using a broad range of unselected native structures or ligand prediction modeling may be required to delineate the responsible ligand.
Complex central regulation of anti-GBM B cells may also explain the rarity of anti-GBM nephritis despite the presence of T cell help. Although α3(IV) collagen is expressed in thymus [27], antigen-reactive CD4+ and CD8+ T cells have been isolated from healthy individuals as well as patients [28–30]. Anti-α3(IV)NC1 T cells are likely crucial for induction of pathogenic anti-GBM IgG responses and contribute directly to disease pathogenesis, as shown in some animal models [31–33]. Independent regulation of the highly autoreactive B cells as demonstrated here would thus prevent inadvertent activation of ignorant B cells by bystander pathways, during immune responses to environmental pathogens [33], or by intramolecular spread after immunization with nephritogenic T cell epitopes [32].
We propose a working model as follows: Under healthy conditions, developing and potentially deadly anti-α3(IV)NC1 B cells engage a second self-antigen in bone marrow, by which they are tolerized by deletion or editing. Anti-α3(IV)NC1 B cells may also periodically arise de novo in the periphery by somatic mutation or loss of editor chains; these cells are presumably also regulated, either by the same ubiquitous self-antigen or by other mechanisms such as Treg, lack of T cell help, or follicular exclusion. Peripheral tolerance may also be crucial to control B cells that incidentally escape the bone marrow due to subthreshold interaction with the central tolerogen. If occasional anti-α3(IV)NC1 B cells become activated, disease is averted because tissue target epitopes are hidden and local inflammation is absent. To develop disease, “multiple hit” susceptibility would be necessary: a combination of genetic susceptibility and superimposed environmental factors (e.g., infection, or chemical or toxin exposure, as suspected of triggering Goodpasture’s disease in some patients) [34], disrupts regulatory pathways, promotes anti-α3(IV)NC1 B and T cell activation, and facilitates epitope exposure and local inflammation.
The proposed scenario should provide effective regulation of the anti-GBM humoral response if it encompasses major pathogenic epitopes. It is notable that several α(IV)NC1 collagen epitopes are targeted by Goodpasture sera IgG [35–37], and it is unclear if the phenomenon revealed by our transgenic B cell receptor is generalizable to multiple GBM epitopes. Nonetheless, the majority of antibodies from most patients react with one or two epitopes on the α3(IV)NC1 domain [11,12,36]. Moreover, the findings of Chen and colleagues indicate that the amino terminal third of α3(IV)NC1 that contains an immunodominant epitope as defined by Ig binding [11,12,36] can also induce autoantibodies and disease, indicating this epitope’s involvement in proximal regulation as well as in tissue deposition [38]. Disease relevance of our transgenic BCR is suggested by the finding that multiple different Goodpasture patients’ sera inhibit α3(IV)NC1 binding by the transgene prototype antibody and by transgenic serum Ig [15]. Identification of the endogenous tolerogen and elucidation of its structural relationship to α3(IV)NC1 and the structural plasticity of the transgenic BCR should provide additional insight into in vivo regulatory networks.
5. Conclusion
These data support a novel model for regulation of anti-α3(IV)NC1 collagen B cells that cause anti-GBM disease. We propose that anti-α3(IV)NC1 collagen B cells are regulated by a bone marrow tolerogen distinct from α3(IV) collagen. This model reconciles the clinical observation of rare serological reactivity despite a hidden antigen. Identification of the tolerizing second antigen will provide key insight into disease origins, regulation, and pathogenesis and potential new therapeutic targets.
Research Highlights.
Pathogenic anti-α3(IV)NC1 collagen B cells are centrally regulated.
Regulation of anti-α3(IV)NC1 collagen B cells is independent of α3(IV) collagen.
These findings implicate a second, cross-reactive antigen present in bone marrow.
Acknowledgments
This work was supported by Award Number R01DK47424 from the National Institute Of Diabetes And Digestive And Kidney Diseases (MHF), the DVAMC Research Service, and a grant from the American Society of Nephrology. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Diabetes And Digestive And Kidney Diseases or the National Institutes of Health. Glycan array and analyses were provided by the Consortium for Functional Glycomics Grant funded by NIGMS number GM62116. We thank the Duke University Comprehensive Cancer Center Flow Cytometry Shared Resource and the Duke University Light Microscopy Core Facility. We thank Ying Zhang for assistance with phenotyping and Melissa Weston and Ting Yu for technical assistance. We thank Dr. Jeffrey Miner, Washington University, St. Louis for graciously providing α3(IV) collagen knockout mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Amy G. Clark, Email: amy.clark@duke.edu.
Mary H. Foster, Email: mhfoster@notes.duke.edu.
References
- 1.Gammon WR, Briggaman RA. Epidermolysis bullosa acquisita and bullous systemic lupus erythematosus. Diseases of autoimmunity to type VII collagen. Dermatologic Clinics. 1993;11:535–547. [PubMed] [Google Scholar]
- 2.Ghohestani R, Hudson B, Claudy A, Uitto J. The α5 chain of type IV collagen is the target of IgG autoantibodies in a novel autoimmune disease with subepidermal blisters and renal insufficiency. J Biol Chem. 2000;275:16002–16006. doi: 10.1074/jbc.275.21.16002. [DOI] [PubMed] [Google Scholar]
- 3.Haque MA, Mizobuchi T, Yasufuku K, Fujisawa T, Brutkiewicz RR, Zheng Y, et al. Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol. 2002;169:1542–1549. doi: 10.4049/jimmunol.169.3.1542. [DOI] [PubMed] [Google Scholar]
- 4.Luross JA, Williams NA. The genetic and immunopathological processes underlying collagen-induced arthritis. Immunology. 2001;103:407–416. doi: 10.1046/j.1365-2567.2001.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu L, Robinson N, Miller SD, Chan LS. Characterization of BALB/c mice B lymphocyte autoimmune responses to skin basement membrane component type XVII collagen, the target antigen of autoimmune skin disease bullous pemphigoid. Immunol Lett. 2001;77:105–111. doi: 10.1016/s0165-2478(01)00212-7. [DOI] [PubMed] [Google Scholar]
- 6.Morgan K. What do anti-collagen antibodies mean? Ann Rheum Dis. 1990;49:62–65. doi: 10.1136/ard.49.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med. 2003;348:2543–2556. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- 8.Hudson BG, Reeders ST, Tryggvason K. Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem. 1993;268:26033–26036. [PubMed] [Google Scholar]
- 9.Lerner RA, Glassock RJ, Dixon FJ. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med. 1967;126:989–1004. doi: 10.1084/jem.126.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan JJ, Mason PJ, Pusey CD, Turner N. Recombinant alpha-chains of type IV collagen demonstrate that the amino terminal of the Goodpasture autoantigen is crucial for antibody recognition. Clin Exp Immunol. 1998;113:17–27. doi: 10.1046/j.1365-2249.1998.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellmark T, Segelmark M, Unger C, Burkhardt H, Saus J, Wieslander J. Identification of a clinically relevant immunodominant region of collagen IV in Goodpasture disease. Kidney Int. 1999;55:936–944. doi: 10.1046/j.1523-1755.1999.055003936.x. [DOI] [PubMed] [Google Scholar]
- 12.Netzer KO, Leinonen A, Boutaud A, Borza DB, Todd P, Gunwar S, et al. The Goodpasture Autoantigen. Mapping the major conformational epitope(s) of α3(IV) collagen to residues 17–31 and 127–141 of the NC1 domain. J Biol Chem. 1999;274:11267–11274. doi: 10.1074/jbc.274.16.11267. [DOI] [PubMed] [Google Scholar]
- 13.Borza D, Bondar O, Todd P, Sundaramoorthy M, Sado Y, Ninomiya Y, et al. Quaternary organization of the Goodpasture autoantigen, the α 3(IV) collagen chain. Sequestration of two cryptic autoepitopes by intrapromoter interactions with the α4 and α5 NC1 domains. J Biol Chem. 2002;277:40075–40083. doi: 10.1074/jbc.M207769200. [DOI] [PubMed] [Google Scholar]
- 14.Vanacore RM, Ham AJ, Cartailler JP, Sundaramoorthy M, Todd P, Pedchenko V, et al. A role for collagen IV cross-links in conferring immune privilege to the Goodpasture autoantigen: structural basis for the crypticity of B cell epitopes. J Biol Chem. 2008;283:22737–22748. doi: 10.1074/jbc.M803451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Su SC, Hecox DB, Brady GF, Mackin KM, Clark AG, et al. Central tolerance regulates B cells reactive with Goodpasture antigen α3(IV)NC1 collagen. J Immunol. 2008;181:6092–6100. doi: 10.4049/jimmunol.181.9.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wieczorek G, Steinhoff C, Schulz R, Scheller M, Vingron M, Ropers HH, et al. Gene expression profile of mouse bone marrow stromal cells determined by cDNA microarray analysis. Cell Tissue Res. 2003;311:227–237. doi: 10.1007/s00441-002-0671-3. [DOI] [PubMed] [Google Scholar]
- 17.Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, et al. Physiological levels of tumstatin, a fragment of collagen IV α3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via αV β3 integrin. Cancer Cell. 2003;3:589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miner JH, Sanes JR. Molecular and functional defects in kidneys of mice lacking collagen α3(IV): implications for Alport syndrome. J Cell Biol. 1996;135:1403–1413. doi: 10.1083/jcb.135.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sackey FN, Congdon KL, Brady GF, Hopfer H, Zhang Y, Mackin KM, et al. Shared variable domain elements among anti-collagen antibodies reactive with Goodpasture epitopes. In: Vogel FL, Zimmermann LF, editors. Autoimmunity: Role, Regulation and Disorder. Hauppauge, NY: Nova Science Publishers, Inc; 2008. [Google Scholar]
- 20.Andrews KL, Mudd JL, Li C, Miner JH. Quantitative trait loci influence renal disease progression in a mouse model of Alport syndrome. Am J Pathol. 2002;160:721–730. doi: 10.1016/S0002-9440(10)64892-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spanopoulou E, Roman CAJ, Corcoran LM, Schlissel MS, Silver DP, Nemazee D, et al. Functional immunoglobulin transgenes guide ordered B-cell differentiation in Rag-1-deficient mice. Genes Dev. 1994;8:1030–1042. doi: 10.1101/gad.8.9.1030. [DOI] [PubMed] [Google Scholar]
- 22.Kramer A, Keitel T, Winkler K, Stocklein W, Hohne W, Schneider-Mergener J. Molecular basis for the binding promiscuity of an anti-p24 (HIV-1) monoclonal antibody. Cell. 1997;91:799–809. doi: 10.1016/s0092-8674(00)80468-7. [DOI] [PubMed] [Google Scholar]
- 23.DeLano WL, Ultsch MH, de Vos AM, Wells JA. Convergent solutions to binding at a protein-protein interface. Science. 2000;287:1279–1283. doi: 10.1126/science.287.5456.1279. [DOI] [PubMed] [Google Scholar]
- 24.James LC, Roversi P, Tawfik DS. Antibody multispecificity mediated by conformational diversity. Science. 2003;299:1362–1367. doi: 10.1126/science.1079731. [DOI] [PubMed] [Google Scholar]
- 25.Bostrom J, Yu SF, Kan D, Appleton BA, Lee CV, Billeci K, et al. Variants of the antibody herceptin that interact with HER2 and VEGF at the antigen binding site. Science. 2009;323:1610–1614. doi: 10.1126/science.1165480. [DOI] [PubMed] [Google Scholar]
- 26.Stefferl A, Schubart A, Storch M, Amini A, Mather I, Lassmann H, et al. Butyrophilin, a milk protein, modulates the encephalitogenic T cell response to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis. J Immunol. 2000;165:2859–2865. doi: 10.4049/jimmunol.165.5.2859. [DOI] [PubMed] [Google Scholar]
- 27.Wong D, Phelps RG, Turner AN. The Goodpasture antigen is expressed in the human thymus. Kidney Int. 2001;60:1777–1783. doi: 10.1046/j.1523-1755.2001.00014.x. [DOI] [PubMed] [Google Scholar]
- 28.Derry CJ, Ross CN, Lombardi G, Mason PD, Rees AJ, Lechler RI, et al. Analysis of T-cell responses to the autoantigen in Goodpasture’s Disease. Clin Exp Immunol. 1995;100:262–268. doi: 10.1111/j.1365-2249.1995.tb03663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merkel F, Kalluri R, Marx M, Enders U, Stevanovic S, Giegerich G, et al. Autoreactive T-cells in Goodpasture’s syndrome recognize the N-terminal NCI domain on α3 type 1V collagen. Kidney Int. 1996;49:1127–1133. doi: 10.1038/ki.1996.163. [DOI] [PubMed] [Google Scholar]
- 30.Zou J, Hannier S, Cairns LS, Barker RN, Rees AJ, Turner AN, et al. Healthy individuals have Goodpasture autoantigen-reactive T cells. J Am Soc Nephrol. 2008;19:396–404. doi: 10.1681/ASN.2007050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolton WK, Tucker FL, Sturgill BC. New avian model of experimental glomerulonephritis consistent with mediation by cellular immunity. J Clin Invest. 1984;73:1263–1276. doi: 10.1172/JCI111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolton WK, Chen L, Hellmark T, Wieslander J, Fox JW. Epitope spreading and autoimmune glomerulonephritis in rats induced by a T cell epitope of Goodpasture’s antigen. J Am Soc Nephrol. 2005;16:2657–2666. doi: 10.1681/ASN.2004100823. [DOI] [PubMed] [Google Scholar]
- 33.Arends J, Wu J, Borillo J, Troung L, Zhou C, Vigneswaran N, et al. T cell epitope mimicry in antiglomerular basement membrane disease. J Immunol. 2006;176:1252–1258. doi: 10.4049/jimmunol.176.2.1252. [DOI] [PubMed] [Google Scholar]
- 34.Shah MK, Hugghins SY. Characteristics and outcomes of patients with Goodpasture’s syndrome. South Med J. 2002;95:1411–1418. [PubMed] [Google Scholar]
- 35.Hellmark T, Johansson C, Wieslander J. Characterization of anti-GBM antibodies involved in Goodpasture’s syndrome. Kidney Int. 1994;46:823–829. doi: 10.1038/ki.1994.338. [DOI] [PubMed] [Google Scholar]
- 36.Borza DB, Netzer KO, Leinonen A, Todd P, Cervera J, Saus J, et al. The Goodpasture autoantigen. Identification of multiple cryptic epitopes on the NC1 domain of the alpha3(IV) collagen chain. J Biol Chem. 2000;275:6030–6037. doi: 10.1074/jbc.275.8.6030. [DOI] [PubMed] [Google Scholar]
- 37.Pedchenko V, Bondar O, Fogo A, Vanacore R, Voziyan P, Kitching AR, et al. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med. 2010;363:343–354. doi: 10.1056/NEJMoa0910500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L, Hellmark T, Wieslander J, Bolton WK. Immunodominant epitopes of alpha3(IV)NC1 induce autoimmune glomerulonephritis in rats. Kidney Int. 2003;64:2108–2120. doi: 10.1046/j.1523-1755.2003.00332.x. [DOI] [PubMed] [Google Scholar]