Abstract
BACKGROUND
Prostate cancer (PCa) frequently metastasizes to the bone and induces osteoblastic lesions. We previously demonstrated through over-expression of the Wnt inhibitor dickkopf-1 (DKK-1) that Wnts contribute to the osteoblastic component of PCa osseous lesions in vivo.
METHODS
To test the clinical significance of DKK-1 expression during PCa progression, tissue microarrays were stained for DKK-1 protein by immunohistochemistry.
RESULTS
DKK-1 expression index (EI) was found to increase in PIN and primary lesions compared to non-neoplastic tissue (106±10 vs. 19±6, respectively, where the EI is the product of the percent expression and staining intensity). DKK-1 expression was also found to be higher in all PCa metastatic lesions (56±21 EI) compared to non-neoplastic tissues but was significantly decreased vs. primary PCa lesions (p<0.008). The decline in DKK-1 correlated with a shift of β-catenin staining from the nucleus to the cytoplasm suggesting possible mechanism for the observed decrease in DKK-1 levels during PCa progression. Within metastatic lesions, DKK-1 expression was least abundant in PCa bone metastases relative to all soft tissue PCa metastatic lesions except lymph node metastases. High DKK-1 expression within PCa metastases was further associated with shorter over-all patient survival.
CONCLUSIONS
Taken together, these data demonstrate that elevated DKK-1 expression is an early event in PCa and that as PCa progresses DKK-1 expression declines, particularly in advanced bone metastases. The decline of DKK-1 in bone metastases can unmask Wnts’ osteoblastic activity. These data support a model in which DKK-1 is a molecular switch that transitions the phenotype of PCa osseous lesions from osteolytic to osteoblastic.
Keywords: prostate cancer, DKK-1, Wnt, metastasis, bone
Introduction
Prostate cancer (PCa) remains a significant health problem in men within the United States. Metastases of the axial skeleton are a frequent complication and occur in over 80% of all men who die of PCa (1). PCa bone metastases are characterized by regions of new bone formation (osteogenesis) with underlying areas of bone resorption (osteolysis). A number of factors have been implicated in PCa osteogenesis in vitro (2) and in vivo including bone morphogenetic protein-6 (3) and Wnt (4).
The Wnt proteins are a large family of soluble glycoproteins that are essential for normal bone development (reviewed in (5,6). The activity of Wnt proteins is controlled by secreted antagonists including secreted FZD-related proteins (sFRP), Wnt inhibitory factor-1 (WIF-1), Cerberus, Sclerostin, and Dickkopfs (DKK) (7). The spatial and temporal expression of Wnt inhibitors is critically important for proper skeletalgenesis. Deletion of a single allele of DKK-1 leads to increased bone mass (8) where as DKK-1 null mice develop fusion and duplication of digits (9). Conversely, the osteoblast specific expression of DKK-1 in the mouse led to severe osteopenia further supporting that disruption of canonical Wnt signaling results in significant limb defects in the developing embryo (10).
Proper Wnt signaling is also necessary for maintenance of bone mass in adult animals. Specifically, mice deficient in sFRP1 were shown to have increased trabecular bone accrual without defects in cortical bone (11). This demonstration that changes in Wnt antagonists can affect adult bone suggests a mechanism that can be co-opted by tumor cells to influence bone remodeling. Tian et al observed that DKK-1 was detected in the bone marrow plasma of patients with multiple myeloma (MM) (12). Elevated DKK1 levels were associated with the presence of focal bone lesions suggesting that DKK-1 participated in the development of osteolytic disease (12). Subsequently, DKK-1 expression was found to be evaluated in patient specimens of MM, breast cancer, and Barrett’s metaplasia suggesting that DKK1 is a potential prognostic and diagnostic marker in these diseases (13–16).
We have shown that reducing Wnt activity in PCa by increasing DKK-1 expression affects the phenotype of bone metastases in a murine model (4). Specifically, the enforced expression of DKK-1 into C4-2B PCa cells (a PCa cell line that produces mixed osteoblastic and osteolytic lesions in bone) transformed these cells into a highly osteolytic tumor in vivo. As PCa bone metastases contain areas of both osteolytic and osteoblastic activity, these data suggest that modulation of DKK-1 expression could play a role in the development of PCa bone metastasis. Accordingly, we preformed a retrospective analysis of DKK-1 protein expression in human PCa tissue samples to determine whether DKK-1 was expressed or altered during PCa progression. We report that DKK-1 protein is found early in PCa development and decreases in advanced bone metastases supporting a role for Wnts in PCa-induced osteogenesis. In addition, elevated DKK-1 protein levels within PCa metastases were found to be associated with shorter over-all patient survival. These data support a model in which DKK-1 is a potential therapeutic target and a molecular switch between osteolytic and osteoblastic disease.
Materials and Methods
Case selection and tissue microarrays
A PCa progression tissue microarray (TMA) was constructed from cases of clinically localized PCa obtained from a radical prostatectomy series at the University of Michigan. PCa metastases TMAs were developed from samples obtained through the Rapid Autopsy Program within the Michigan Prostate SPORE Tissue Core (17). The progression TMA 100 consisted of 309 evaluable cores taken from 99 total patients; 92 cores of non-neoplastic prostate (from 39 cases), 23 cores of BPH (from 8 cases), 19 cores of PIN (from 12 cases), 142 cores of localized PCa (from 50 cases), and 33 cores of metastatic, hormone-refractory PCa (from 11 cases) (18). Two PCa autopsy arrays 79A and 79B were constructed from soft tissue and bone metastases taken from 30 available autopsies. TMA 79A consists of 303 evaluable cores of primary PCa and soft tissue metastases of the liver, lung, lymph node, adrenal, bladder, dura, and seminal vesicles. The TMA 79B consisted of 129 evaluable cores included 72 cores (from 17 cases) of bone metastases in addition to primary PCa and soft tissue metastases. All tissue procurement and analysis in this study was approved by the Institutional Review Board. Histological processing of all clinical samples was performed in the University of Michigan Hospital’s accredited Pathology Department using a standardized procedure to assure uniform sample preparation for each TMA.
Immunohistochemistry and evaluation
TMA slides were deparaffinized, rehydrated to water, and antigen retrieved by pretreatment with Citrate Buffer, pH6.0 for 10 minutes with microwaving. After Peroxidase blocking, the slide were incubated with 1:400 dilution of goat anti DKK-1 pAb (ab22827, Abcam Inc, Cambridge, MA) on a DAKO AutoStainer using the LSAB+ detection kit and counterstain with Hematoxylin. Staining intensity was scored by a genitor-urinary pathologist as negative [1], weak [2], moderate [3], or strong [4] based on the amount of stain detected as previously described (19). The percent of positive stained cells was determined by counting 100 cells in 2 random fields. DKK-1 protein levels in each sample were measured based on its Expression Index (EI), which is a product of DKK-1 staining intensity and the percentage of positive staining as described previously (20). Identical TMAs were also stained for β-catenin expression using a mouse anti β-catenin mAb (clone 14, BD-Transduction, San Diego, CA) as above.
To confirm the specificity of both the DKK-1 and β-catenin antibodies, control IHC studies were preformed. Serial sections from paraffin embedded tumor tissue produced by DKK-1+/β-catenin− PC-3 shRNA control transfected PCa cells or DKK-1−/β-catenin+ PC-3 DKK-1shRNA transfected PCa cells were stained for DKK-1 and β-catenin using the above described methodology. Primary antibody was also excluded on identical sections to evaluate non-specific binding of secondary antibody.
Statistical analysis
A box plot was used to chart DKK-1 expression in the progression array. The Expression Index (EI), which is the product of the staining intensity and percent expression, was analyzed to determine differences in expression between the primary tumor and metastases samples using a linear regression mixed model to account for the correlation of the samples within each patient. Product-limit methods were used to determine the median survival times in patients with low and high expressions of DKK-1. The Kaplan-Meier estimates were plotted for each group and the log-rank p-value was reported. SAS 9.1 (SAS Institute, Cary, NC) was used to perform all statistical analyses. Tests were performed with alpha=0.05.
Results
DKK-1 protein levels decrease with PCa progression
To conduct a retrospective analysis of DKK-1 protein expression in human PCa patient specimens, tissue microarrays (TMA) were prepared by the University of Michigan PCa SPORE tissue core. Two types of TMAs were evaluated: A progression TMA that contained a selection of non-neoplastic prostate, BPH, PIN lesions, primary lesions, and metastases and two autopsy arrays that were composed of soft tissue and skeletal metastases. The advantage of a TMA for biomarker analysis is that the TMA provides a platform for the simultaneous evaluation of multiple patients, tumor grades and stages on a single slide thus reducing the variability inherent in multiple single sample analyses. This coupled with uniform sample preparation gives one a high degree of confidence that differences in DKK-1 staining are reflective of the disease stage. These TMAs were used to evaluate DKK-1 protein levels during progression and to explore the impact of organ site on DKK-1 protein expression.
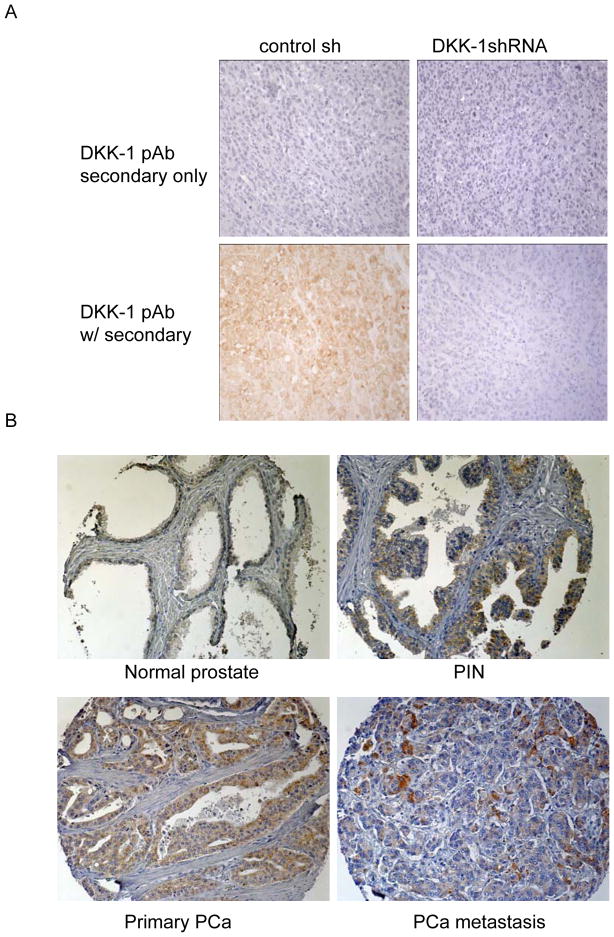
To confirm the specificity of the DKK-1 antibody, serial sections from paraffin embedded tumor tissue produced by DKK-1+ PC-3 shRNA control transfected PCa cells or DKK-1− PC-3 DKK-1shRNA transfected PCa cells were stained for DKK-1 protein. Primary antibody was also excluded on identical sections to evaluate non-specific binding of secondary antibody. In these control studies, secondary antibody alone did not stain the human PCa tumor tissue (figure 1A, top row). DKK-1 was detected in only those tumors formed by PC-3 shRNA control transfected cells (figure 1A, bottom row) thus confirming the antibody selectively detected human DKK-1 protein.
Figure 1. DKK-1 decreases in PCa clinical samples with progression.
A. Specificity of DKK-1 antibody. Serial sections from paraffin embedded tumor tissue produced by DKK-1+ PC-3 shRNA control transfected PCa cells or DKK-1− PC-3 DKK-1shRNA transfected PCa cells were incubated using the protocol described in the Materials and Methods section with secondary only or primary antibody to DKK-1. B. A progression TMA that contains a selection of non-neoplastic prostate, BPH, PIN, primary tumors (mostly Gleason score 7–8) and soft tissue metastases was stained for DKK-1 using routine immunohistochemistry. Shown are tissue cores that represent the median of DKK-1 Expression Index of normal prostate, PIN, primary PCa and PCa metastases presented in Figure 2A (200x). Brown color indicates DKK-1 staining.
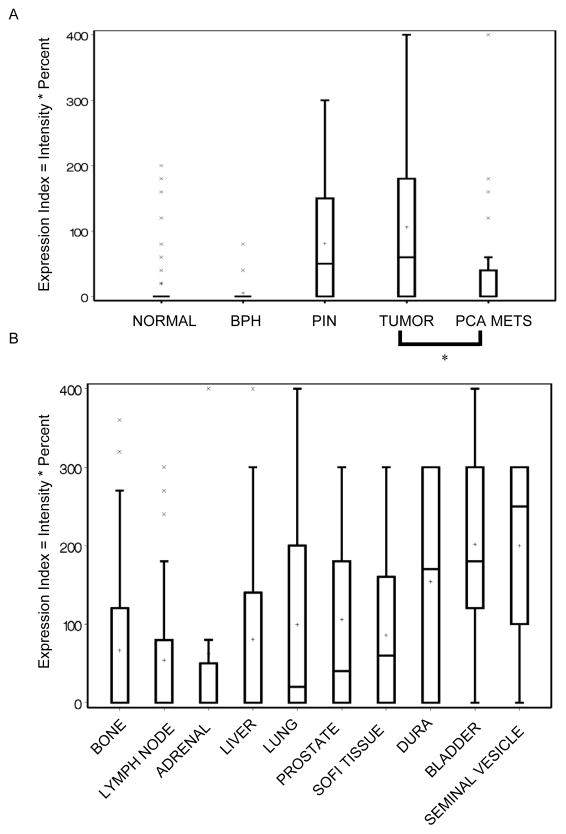
The progression TMA contained a total of 309 evaluable cores representing 99 total PCa patients. The metastases on the progression array consisted of PCa lymph node and liver metastases only. Analysis of DKK-1 staining demonstrated that the protein was restricted to epithelial cells in all samples (Figure 1B). Qualitative evaluation of DKK-1 staining showed that DKK-1 was found at low levels in normal prostate tissue but increased significantly in both PIN and primary PCa lesions (Figure 1B). In PCa metastases, DKK-1 staining appeared greater than normal prostate but at significantly lower levels compared to primary PCa tumors (Figure 1B). Total changes in DKK-1 protein staining were evaluated as previously described using an Expression Index (EI), which is the product of the staining intensity and percent expression (20). DKK-1 EI increased 500% in primary lesions compared to normal prostate and BPH (Figure 2A). However, DKK-1 EI decreased 47% in metastatic lesions compared to primary tumors (mean±SEM, median; normal prostate: 19.3±5.6, 0; BPH: 5.2±3.8, 0; primary PCa: 106.0±10.4, 60; PCa metastases: 56.3±21.5, 0; p<0.008 PCa metastases vs. primary lesions). Taken together, the data demonstrate that DKK-1 protein expression increased during PCa development but decreased as the tumor progressed.
Figure 2. DKK-1 protein is expressed at low levels within bone metastases compared to soft tissue metastases.
A. Box plot of DKK-1 Expression Index (EI) by tumor stage from the progression TMA where the EI is the product of the staining intensity and percent expression for each core. Graph key: box marks the 25–75th percentiles; whiskers are the maximum and minimum observation between the 25th and 75th inter-quartile range; the X’s mark values outside the IQR; the bar is the data median and the plus the data mean. *p<0.008 vs. primary PCa. B. Box plot of DKK-1 expression index (EI) within PCa skeletal and soft tissue metastases from the autopsy TMAs.
DKK-1 protein is expressed at low levels within bone metastases compared to soft tissue metastases
To evaluate the impact of organ site on DKK-1 protein expression, autopsy arrays composed of soft tissue and skeletal metastases were evaluated. The autopsy TMAs were comprised of 9 types of metastatic lesions from 30 total PCa patients. We have shown that DKK-1 protein was higher in PCa primary lesions compared to the pooled PCa metastases of the lymph node and liver (Figure 2B). Evaluated individually, DKK-1 protein in PCa primary lesions was greater than each of PCa metastases of the lung, liver, bone, adrenal, and lymph node (Figure 2B) (mean±SEM, median; primary PCa: 105.9±15.6, 60; lung: 99.5±17.9, 20; liver: 83.9±15.4, 0; bone: 66.7±13.4, 0; adrenal: 62.5±49.2, 0; lymph node, 53.8±12.7, 0). DKK-1 was also found to be increased over PCa primary lesions in a number of soft tissue metastases including the bladder, dura, and seminal vesicles (Figure 2B) (mean±SEM, median; dura: 154.3±36.2, 170; bladder: 201.7±37.5, 180; seminal vesicles: 200±70.7, 250). These data confirm that DKK-1 protein expression is reduced in PCa skeletal metastases compared to PCa primary lesions.
Nuclear β-catenin and DKK-1 concomitantly decrease during PCa progression
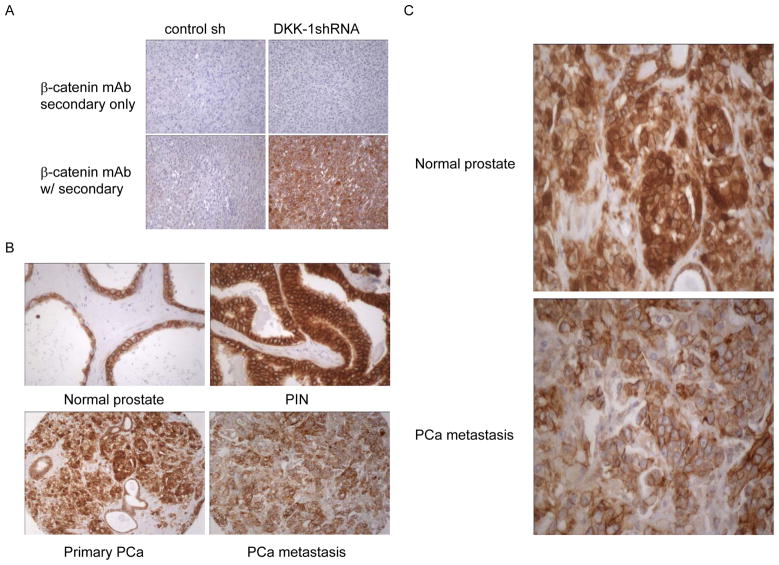
Aberrant β-catenin signal transduction has been implicated in the development of several types of cancer including PCa (21). We therefore evaluated β-catenin protein expression within prostate epithelial cells on the progression TMA and related these data to DKK-1 protein expression. In addition to determining the staining intensity and percent expression, the distribution of β-catenin (membranous, cytoplasmic, or nuclear) was also recorded. As with the DKK-1 antibody, the specificity of the β-catenin antibody was confirmed by control IHC studies of tumor tissue produced byβ-catenin− PC-3 shRNA control transfected PCa cells or β-catenin+ PC-3 DKK-1shRNA transfected PCa cells. Primary antibody was also excluded on identical sections to evaluate non-specific binding of secondary antibody. In these control studies, secondary antibody alone did not stain the human PCa tumor tissue (figure 3A, top row). β-catenin was detected in only those tumors formed by PC-3 shRNA control transfected cells (figure 3A, bottom row) thus confirming the antibody selectively detected human β-catenin protein.
Figure 3. Totalβ-catenin and DKK-1 concomitantly decrease during PCa progression.
A. Specificity of β-catenin antibody. Serial sections from paraffin embedded tumor tissue produced byβ-catenin− PC-3 shRNA control transfected PCa cells or β-catenin+ PC-3 DKK-1shRNA transfected PCa cells were incubated using the protocol described in the Materials and Methods section with secondary only or primary antibody to β-catenin. B. A progression TMA was stained for β-catenin using routine immunohistochemistry. Shown are tissue cores that represent the median of β-catenin percent expression of normal prostate, PIN, primary PCa and PCa metastases presented in Figure 4A (200x). Brown color indicates β-catenin staining. C. High power (400x) magnification of primary PCa and metastatic lesions showing reduced nuclear β-catenin staining.
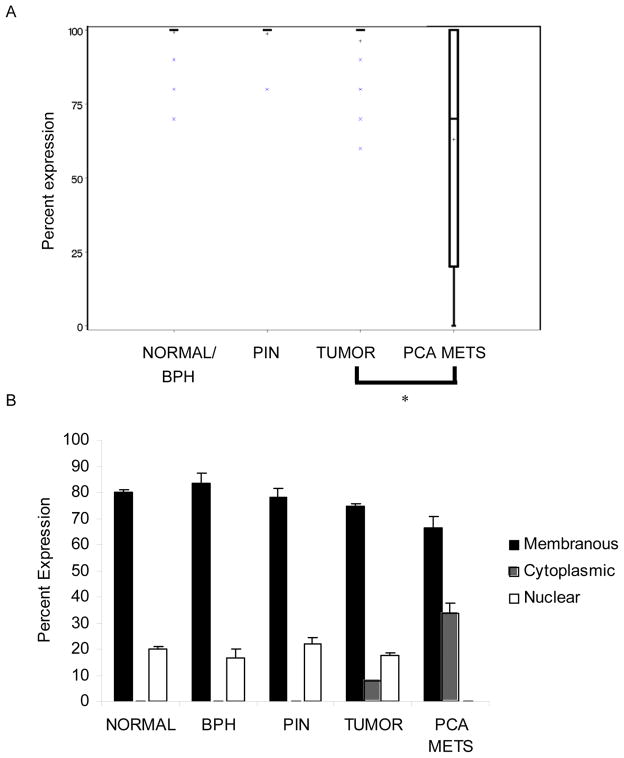
Qualitative evaluation of β-catenin staining on the progression TMA showed that the staining intensity was strong and percent expression approached 100% in the epithelial cells of normal, BPH, PIN, and primary PCa lesions (Figure 3B). However, in epithelial cells of PCa metastases both the total and nuclear staining of β-catenin protein appeared to decrease (Figure 3B & 3C). Quantitative analysis of β-catenin protein percent expression confirmed β-catenin staining in 100% of normal, PIN, and primary PCa samples (Figure 4A). Further, the distribution in these samples was 80% membranous with 20% nuclear staining (Figure 4B). However in epithelial cells of PCa metastases, total β-catenin protein decreased to 64.5% and was distributed between the membrane and the cytoplasm, 66.3% vs. 33.7%, respectively (Figure 4B). DKK-1 was recently described as a gene target of β-catenin (22). The fact that nuclear β-catenin was high in epithelial cells of PCa primary lesions but declines in epithelial cells of PCa metastases is consistent with the observation that β-catenin regulates DKK-1.
Figure 4. Nuclear β-catenin decreases in PCa metastases.
The expression of β-catenin was evaluated on a progression TMA by immunohistochemistry. The percent of positive stained cells was determined by counting 100 cells in 2 random fields. A. Box plot of β-catenin protein staining vs. tumor stage. Graph key: box marks the 25–75th percentiles; whiskers are the maximum and minimum observation between the 25th and 75th inter-quartile range; the X’s mark values outside the IQR; the bar is the data median and the plus the data mean. *p<0.005 vs. primary PCa. B. graph of β-catenin percent expression in three cellular locations: membrane, cytoplasm, and nucleus.
High DKK-1 levels are associated with shorter overall survival
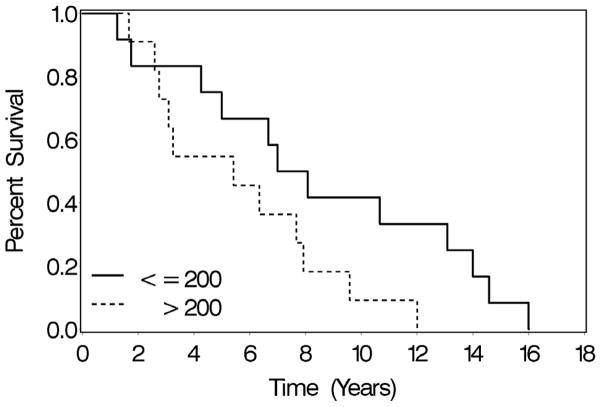
To determine whether DKK-1 protein expression correlated with patient survival, the time interval from diagnosis to death was plotted against DKK-1 protein in the metastases from the autopsy arrays. The prognostic value of DKK-1 expression could not be evaluated in primary tumors because of insufficient follow-up on patients who appear on the progression TMA. However, the autopsy arrays contained suitable temporal data to correlated DKK-1 protein expression in metastatic lesions with patient survival. For this analysis, the metastatic lesion with the highest DKK-1 levels was determined for each patient. The median of the maxscores (200 EI) was then used separate patients into two groups. DKK-1 EI values greater than 200 were considered high expression whereas DKK-1 EI values less than 200 were considered low expression. Of the 30 patients represented in the autopsy array, survival data was available for 23 patients. Median overall survival in high DKK-1 expression group was 60 months which was less than the low DKK-1 expression group at 90.5 months, p<0.07, indicating that DKK-1 protein expression inversely correlated with patient survival (Figure 5). To our knowledge this is the first report to show that high levels of a Wnt inhibitor are associated with poor patient survival.
Figure 5. Elevated DKK-1 protein levels are associated with shorter overall survival.
Kaplan-Meier plot of patient survival. The metastatic lesion with the greatest DKK-1 expression index (EI) was determined for each patient. The median of the DKK-1 maxscores (200 EI) was used as a cutoff to separate patients into two groups; high DKK-1 expression (values greater than 200) or low DKK-1 expression (values less than or equal to 200). DKK-1 EI was plotted against patient survival from diagnosis to death, n=23, p<0.07.
Discussion
In the present study, we demonstrated that DKK-1 protein increases during PCa development but decreases in skeletal metastases compared to primary tumors. We further provide evidence that elevated levels of DKK-1 protein in PCa metastases were associated with shorter patient survival. These results support a model in which DKK-1 is a potential therapeutic target and a molecular switch between osteolytic and osteoblastic disease.
We previously reported that osteolytic PCa cells express abundant DKK-1 protein where osteoblastic PCa cells do not (4). Blocking DKK-1 expression in osteolytic PCa cells stimulated osteoblast differentiation and mineralization in vitro (4). Conversely, DKK-1 over-expression in osteoblastic PCa cells suppressed bone formation and induced osteolytic lesions in vivo (4). We concluded that Wnts mediate PCa osteoblastic response and that DKK-1 expression should decrease in clinical PCa bone metastases to allow Wnt-mediated bone formation. The clinical observations are in agreement with our experimental data in that DKK-1 protein is decreased in PCa skeletal metastases compared to PCa primary lesions and other soft tissue metastases. During the preparation of this manuscript, Li et al published an analysis of DKK-1 expression in six samples of non-neoplastic prostate, nine samples of primary PCa, and 38 bone metastasis samples (23). They report DKK-1 was negative in normal PCa, primary PCa, and 74% of osteoblastic bone metastases but was detected in two of three osteolytic bone lesions. The discrepancy between this and our data is likely because the published study was not sufficiently powered. However, these data are in agreement that DKK-1 protein is found at low levels in the majority of PCa bone metastases thus supporting a role of Wnts in PCa osteoblastic activity.
A role of Wnt signaling in cancer development has been well documented. The bulk of the literature describe a role of autocrine Wnt signaling resulting from mutations in the β-catenin degrading complex (such as APC or GSK3β) or in β-catenin itself that promote tumor cell proliferation (for review see (5). Both APC and β-catenin mutations lead to the stabilization ofβ-catenin and the inappropriate expression of TCF regulated genes within the tumor cell, including the transcription factor c-Myc (24), the cell cycle regulatory protein cyclin-D1 (25), the angiogenic factor and chemokine IL-8 (26), and the proteases matrilysin and MMP7 (27,28) which have obvious implications to tumor development and progression. In the present study, β-catenin protein was found at high levels in normal prostate, BPH, PIN, and primary lesions. β-catenin protein was also found to simultaneously decrease and redistribute from the nucleus to the cytoplasm in PCa metastases. Although DKK-1 is a β-catenin target gene, expression of β-catenin likely does not account for the increase in DKK-1 within primary lesions as β-catenin levels are similar in both normal and primary lesion. However, the reduction in DKK-1 in PCa metastases could be mediated in part by β-catenin as nuclear expression of β-catenin decreases in PCa metastases. Although nuclear β-catenin levels should increase in response to declining DKK-1 levels, this effect would not necessarily be observed in PCa epithelial cells where the expression was scored. β-catenin levels in the surrounding stroma were not evaluated. The direct role of β-catenin in both DKK-1 regulation and progression of PCa requires further study.
We observed that elevated DKK-1 protein levels in PCa metastases were associated with shorter patient survival. On the surface this observations seems inconsistent with the role of canonical Wnt signaling in promoting tumorigenesis. Our retrospective study could not determine whether the increased DKK-1 protein resulted from or contributed to primary tumor development. However, we can speculate that high DKK-1 expression may reflect a feed-back mechanism whose purpose is to stem unchecked intra-cellular Wnt signaling. We can further speculate that persistent high levels of DKK-1 expression in PCa soft tissue metastases may suggest a functional role of DKK-1 in the formation of these lesions. These data are supported by published reports that show an elevated expression of Wnt inhibitors sFRP1, sFRP2, and DKK-1 in human tumors and that their expression promotes tumor growth in animal models (29–32). Conversely, low levels of DKK-1 in soft tissue metastases of the liver, lung, lymph node and adrenal may suggest that canonical Wnt signaling contributes more heavily to the development of lesions at these sites. The effect of low levels of DKK-1 expression within bone metastases could also contribute to the formation of osteoblastic lesions through Wnts (4,33). Initial high DKK-1 protein levels within bone lesions could promote tumor establishment by inducing bone resorption through DKK-1’s ability to block Wnt-mediated increase in osteoprotegerin which would suppress osteoclastogenesis (34). Once the tumor has established within the bone, subsequent reductions in DKK-1 levels, as observed in the autopsy array, could permit Wnt-mediated bone formation and the characteristic osteoblastic lesion. In this way, modulation of DKK-1 expression could explain the presence of both osteolytic and osteoblastic disease in PCa osseous lesions. Although the association between DKK-1 protein and tumor stage does not provide a mechanism for an osteoblastic switch, the analyses presented here are essential for the design of appropriate mechanism-based studies.
In conclusion, we provide evidence that DKK-1 protein increases during PCa development but decreases as the tumor progresses to bone metastases. We further observe that elevated DKK-1 protein levels in PCa metastases is associated with shorter patient survival. These data suggest that DKK-1 expression in PCa is an early event necessary to support osteolysis and early tumor establishment. However as the tumor progresses, DKK-1 is lost allowing Wnt mediated osteogenic effect to predominate. The data further support DKK-1 as a potential prognostic marker and therapeutic target for PCa.
Acknowledgments
Supported in part by National Cancer Institute Grants P01 CA093900 and R01 CA071672 (E. T. Keller) and P50 CA069568 (K. J. Pienta).
References
- 1.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 2.Logothetis CJ, Lin SH. Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer. 2005;5(1):21–28. doi: 10.1038/nrc1528. [DOI] [PubMed] [Google Scholar]
- 3.Dai J, Keller J, Zhang J, Lu Y, Yao Z, Keller ET. Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer Res. 2005;65(18):8274–8285. doi: 10.1158/0008-5472.CAN-05-1891. [DOI] [PubMed] [Google Scholar]
- 4.Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005;65(17):7554–7560. doi: 10.1158/0008-5472.CAN-05-1317. [DOI] [PubMed] [Google Scholar]
- 5.Hall CL, Kang S, Macdougald OA, Keller ET. Role of wnts in prostate cancer bone metastases. J Cell Biochem. 2006;97(4):661–672. doi: 10.1002/jcb.20735. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y. Wnts and wing: Wnt signaling in vertebrate limb development and musculoskeletal morphogenesis. Birth Defects Res C Embryo Today. 2003;Part C(4):305–317. doi: 10.1002/bdrc.10026. Embryo Today. [DOI] [PubMed] [Google Scholar]
- 7.Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148(6):2635–2643. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- 8.Morvan F, Boulukos K, Clement-Lacroix P, Roman Roman S, Suc-Royer I, Vayssiere B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21(6):934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 9.Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Belmonte JC, Westphal H. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1(3):423–434. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39(4):754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Bodine PV, Zhao W, Kharode YP, Bex FJ, Lambert AJ, Goad MB, Gaur T, Stein GS, Lian JB, Komm BS. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18(5):1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 12.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD., Jr The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349(26):2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 13.Ali I, Rafiee P, Hogan WJ, Jacob HJ, Komorowski RA, Haasler GB, Shaker R. Dickkopf homologs in squamous mucosa of esophagitis patients are overexpressed compared with Barrett’s patients and healthy controls. Am J Gastroenterol. 2006;101(7):1437–1448. doi: 10.1111/j.1572-0241.2006.00584.x. [DOI] [PubMed] [Google Scholar]
- 14.Forget MA, Turcotte S, Beauseigle D, Godin-Ethier J, Pelletier S, Martin J, Tanguay S, Lapointe R. The Wnt pathway regulator DKK1 is preferentially expressed in hormone-resistant breast tumours and in some common cancer types. Br J Cancer. 2007;96(4):646–653. doi: 10.1038/sj.bjc.6603579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Politou MC, Heath DJ, Rahemtulla A, Szydlo R, Anagnostopoulos A, Dimopoulos MA, Croucher PI, Terpos E. Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int J Cancer. 2006;119(7):1728–1731. doi: 10.1002/ijc.22033. [DOI] [PubMed] [Google Scholar]
- 16.Voorzanger-Rousselot N, Goehrig D, Journe F, Doriath V, Body JJ, Clezardin P, Garnero P. Increased Dickkopf-1 expression in breast cancer bone metastases. Br J Cancer. 2007;97(7):964–970. doi: 10.1038/sj.bjc.6603959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin MA, Putzi M, Mucci N, Smith DC, Wojno K, Korenchuk S, Pienta KJ. Rapid (“warm”) autopsy study for procurement of metastatic prostate cancer. Clin Cancer Res. 2000;6(3):1038–1045. [PubMed] [Google Scholar]
- 18.Tomlins SA, Mehra R, Rhodes DR, Cao X, Wang L, Dhanasekaran SM, Kalyana-Sundaram S, Wei JT, Rubin MA, Pienta KJ, Shah RB, Chinnaiyan AM. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39(1):41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- 19.Fu Z, Kitagawa Y, Shen R, Shah R, Mehra R, Rhodes D, Keller PJ, Mizokami A, Dunn R, Chinnaiyan AM, Yao Z, Keller ET. Metastasis suppressor gene Raf kinase inhibitor protein (RKIP) is a novel prognostic marker in prostate cancer. Prostate. 2006;66(3):248–256. doi: 10.1002/pros.20319. [DOI] [PubMed] [Google Scholar]
- 20.Yu J, Cao Q, Mehra R, Laxman B, Yu J, Tomlins SA, Creighton CJ, Dhanasekaran SM, Shen R, Chen G, Morris DS, Marquez VE, Shah RB, Ghosh D, Varambally S, Chinnaiyan AM. Integrative genomics analysis reveals silencing of beta-adrenergic signaling by polycomb in prostate cancer. Cancer Cell. 2007;12(5):419–431. doi: 10.1016/j.ccr.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Yardy GW, Brewster SF. Wnt signalling and prostate cancer. Prostate Cancer Prostatic Dis. 2005;8(2):119–126. doi: 10.1038/sj.pcan.4500794. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Sancho JM, Aguilera O, Garcia JM, Pendas-Franco N, Pena C, Cal S, Garcia de Herreros A, Bonilla F, Munoz A. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2005;24(6):1098–1103. doi: 10.1038/sj.onc.1208303. [DOI] [PubMed] [Google Scholar]
- 23.Li ZG, Yang J, Vazquez ES, Rose D, Vakar-Lopez F, Mathew P, Lopez A, Logothetis CJ, Lin SH, Navone NM. Low-density lipoprotein receptor-related protein 5 (LRP5) mediates the prostate cancer-induced formation of new bone. Oncogene. 2008;27(5):596–603. doi: 10.1038/sj.onc.1210694. [DOI] [PubMed] [Google Scholar]
- 24.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 25.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben Ze’ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96(10):5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy L, Neuveut C, Renard CA, Charneau P, Branchereau S, Gauthier F, Van Nhieu JT, Cherqui D, Petit-Bertron AF, Mathieu D, Buendia MA. Transcriptional activation of interleukin-8 by beta-catenin-Tcf4. J Biol Chem. 2002;277(44):42386–42393. doi: 10.1074/jbc.M207418200. [DOI] [PubMed] [Google Scholar]
- 27.Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol. 1999;155(4):1033–1038. doi: 10.1016/s0002-9440(10)65204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, Matrisian LM. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18(18):2883–2891. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- 29.Joesting MS, Perrin S, Elenbaas B, Fawell SE, Rubin JS, Franco OE, Hayward SW, Cunha GR, Marker PC. Identification of SFRP1 as a candidate mediator of stromal-to-epithelial signaling in prostate cancer. Cancer Res. 2005;65(22):10423–10430. doi: 10.1158/0008-5472.CAN-05-0824. [DOI] [PubMed] [Google Scholar]
- 30.Oshima T, Abe M, Asano J, Hara T, Kitazoe K, Sekimoto E, Tanaka Y, Shibata H, Hashimoto T, Ozaki S, Kido S, Inoue D, Matsumoto T. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005;106(9):3160–3165. doi: 10.1182/blood-2004-12-4940. [DOI] [PubMed] [Google Scholar]
- 31.Roth W, Wild-Bode C, Platten M, Grimmel C, Melkonyan HS, Dichgans J, Weller M. Secreted Frizzled-related proteins inhibit motility and promote growth of human malignant glioma cells. Oncogene. 2000;19(37):4210–4220. doi: 10.1038/sj.onc.1203783. [DOI] [PubMed] [Google Scholar]
- 32.Wirths O, Waha A, Weggen S, Schirmacher P, Kuhne T, Goodyer CG, Albrecht S, Von Schweinitz D, Pietsch T. Overexpression of human Dickkopf-1, an antagonist of wingless/WNT signaling, in human hepatoblastomas and Wilms’ tumors. Lab Invest. 2003;83(3):429–434. doi: 10.1097/01.lab.0000059926.66359.bd. [DOI] [PubMed] [Google Scholar]
- 33.Li ZG, Yang J, Vazquez ES, Rose D, Vakar-Lopez F, Mathew P, Lopez A, Logothetis CJ, Lin SH, Navone NM. Low-density lipoprotein receptor-related protein 5 (LRP5) mediates the prostate cancer-induced formation of new bone. Oncogene. 2007 doi: 10.1038/sj.onc.1210694. [DOI] [PubMed] [Google Scholar]
- 34.Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8(5):751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]