Abstract
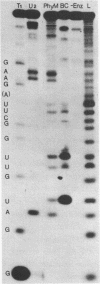
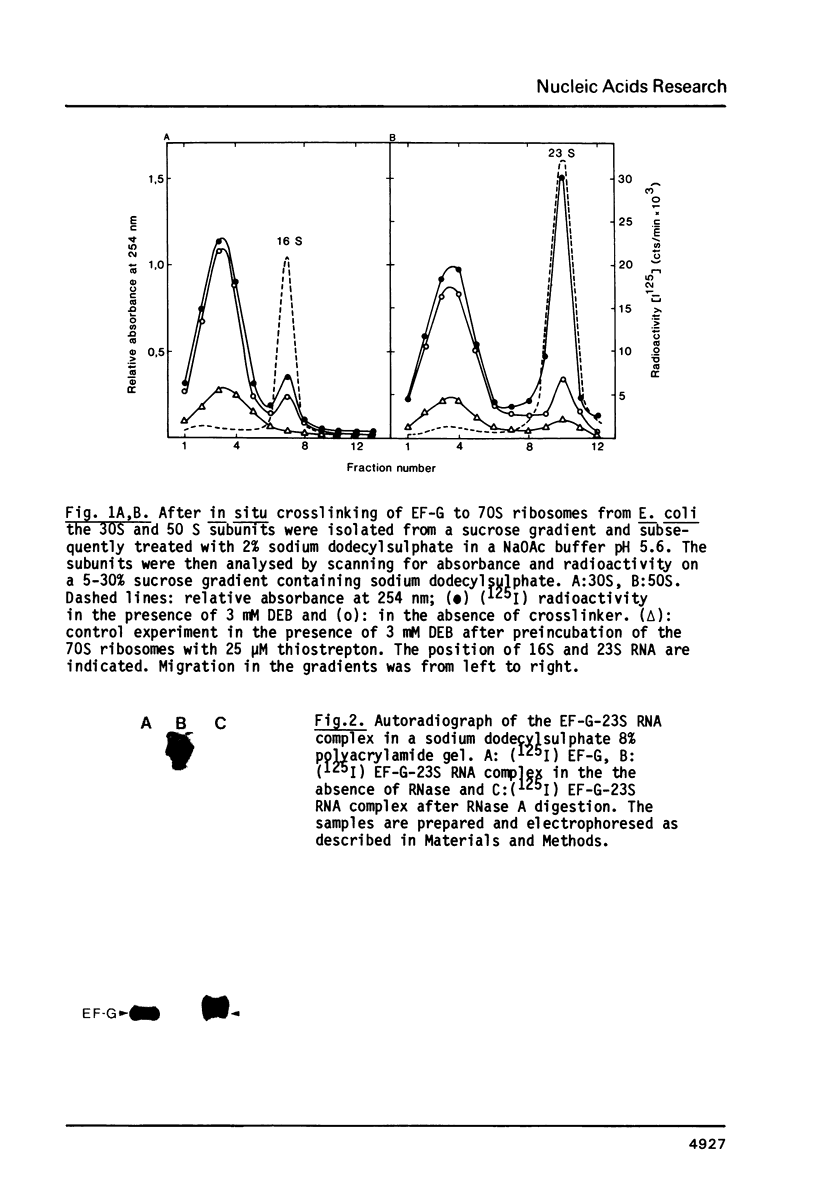
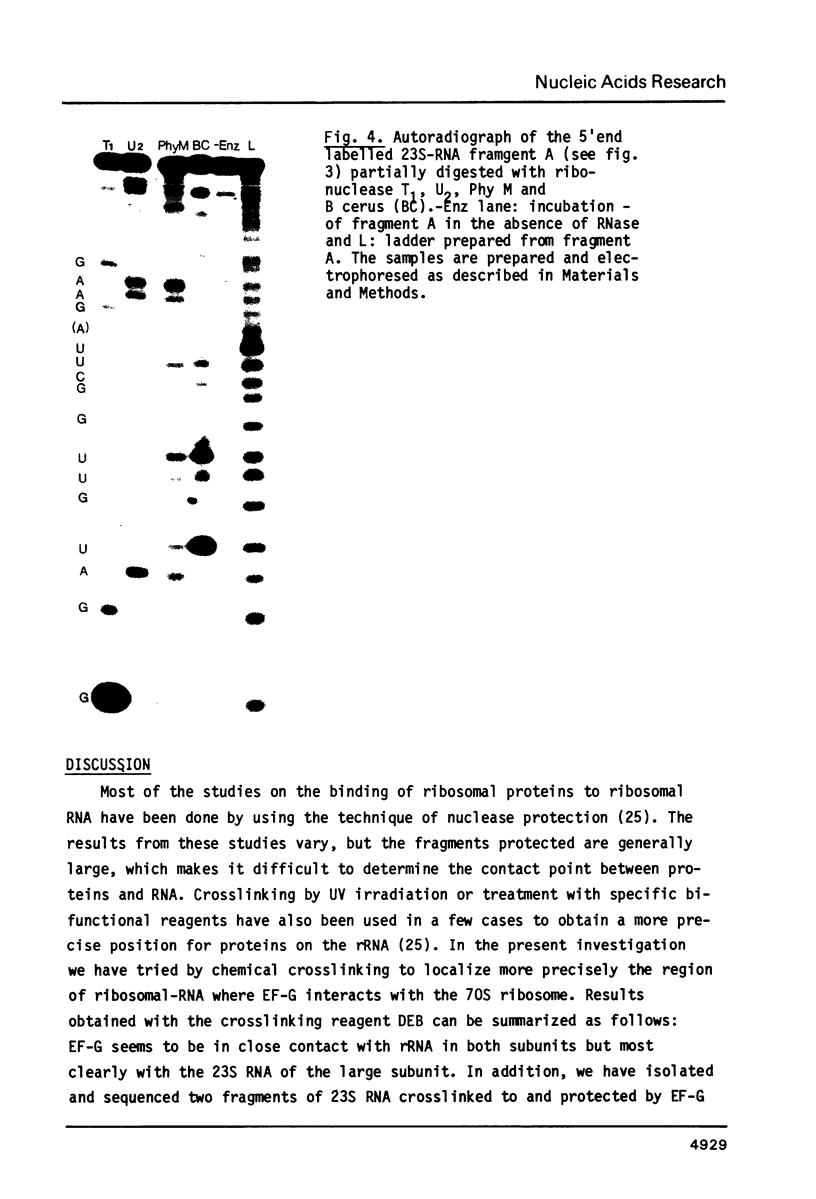
Elongation factor G was crosslinked to the 23S RNA of 70S Escherichia coli ribosomes with the bifunctional, cleavable reagent diepoxybutane (DEB). The EF-G-23S RNA complex was isolated and digested with ribonuclease A. After digestion, an RNA fragment, protected by EF-G was cleaved from the complex and isolated. The nucleotide sequence of this RNA fragment was determined by partial ribonuclease digestion. It proved to be 27 nucleotides long and it could be identified with residues 1055 to 1081 of the nucleotide sequence of E. coli 23S RNA. In the presence of thiostrepton, which prevents binding of EF-G to the ribosome, there was a dramatic decrease in the yield of this complex.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya A. S., Moore P. B., Richards F. M. Cross-linking of elongation factor EF-G to the 50S ribosomal subunit of Escherichia coli. Biochemistry. 1973 Jul 31;12(16):3108–3114. doi: 10.1021/bi00740a026. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumert H. G., Sköld S. E., Kurland C. G. RNA-protein neighbourhoods of the ribosome obtained by crosslinking. Eur J Biochem. 1978 Sep 1;89(2):353–359. doi: 10.1111/j.1432-1033.1978.tb12536.x. [DOI] [PubMed] [Google Scholar]
- Dijk J., Garrett R. A., Müller R. Studies on the binding of the ribosomal protein complex L7/12-L10 and protein L11 to the 5'-one third of 23S RNA: a functional centre of the 50S subunit. Nucleic Acids Res. 1979 Jun 25;6(8):2717–2729. doi: 10.1093/nar/6.8.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H. Phy M: an RNase activity specific for U and A residues useful in RNA sequence analysis. Nucleic Acids Res. 1980 Jul 25;8(14):3133–3142. doi: 10.1093/nar/8.14.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douthwaite S., Garrett R. A. Secondary structure of prokaryotic 5S ribosomal ribonucleic acids: a study with ribonucleases. Biochemistry. 1981 Dec 8;20(25):7301–7307. doi: 10.1021/bi00528a039. [DOI] [PubMed] [Google Scholar]
- Girshovich A. S., Bochkareva E. S., Ovchinnikov Y. A. Elongation factor G and protein S12 are the nearest neighbours in the Escherichia coli ribosome. J Mol Biol. 1981 Sep 15;151(2):229–243. doi: 10.1016/0022-2836(81)90513-1. [DOI] [PubMed] [Google Scholar]
- Girshovich A. S., Kurtskhalia T. V. Elongation factor G interacts with both ribosomal subparticles. FEBS Lett. 1978 Aug 15;92(2):203–206. doi: 10.1016/0014-5793(78)80754-6. [DOI] [PubMed] [Google Scholar]
- Girshovich A. S., Kurtskhalia T. V., Ovchinnikov YuA, Vasiliev V. D. Localization of the elongation factor G on Escherichia coli ribosome. FEBS Lett. 1981 Jul 20;130(1):54–59. doi: 10.1016/0014-5793(81)80664-3. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Randerath K. Rapid print-readout technique for sequencing of RNA's containing modified nucleotides. Nucleic Acids Res. 1979 Aug 10;6(11):3443–3458. doi: 10.1093/nar/6.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Chapman N. M., Noller H. F. Mechanism of ribosomal subunit association: discrimination of specific sites in 16 S RNA essential for association activity. J Mol Biol. 1979 Jun 5;130(4):433–449. doi: 10.1016/0022-2836(79)90433-9. [DOI] [PubMed] [Google Scholar]
- Highland J. H., Lin L., Bodley J. W. Protection of ribosomes from thiostrepton inactivation by the binding of G factor and guanosine diphosphate. Biochemistry. 1971 Nov 23;10(24):4404–4409. doi: 10.1021/bi00800a009. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maassen J. A., Möller W. Elongation factor G-dependent binding of a photoreactive GTP analogue to Escherichia coli ribosomes results in labeling of protein L11. J Biol Chem. 1978 Apr 25;253(8):2777–2783. [PubMed] [Google Scholar]
- Noller H. F., Chaires J. B. Functional modification of 16S ribosomal RNA by kethoxal. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3115–3118. doi: 10.1073/pnas.69.11.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F., Kop J., Wheaton V., Brosius J., Gutell R. R., Kopylov A. M., Dohme F., Herr W., Stahl D. A., Gupta R. Secondary structure model for 23S ribosomal RNA. Nucleic Acids Res. 1981 Nov 25;9(22):6167–6189. doi: 10.1093/nar/9.22.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F. J., Thompson J., Lee K., Dijk J., Cundliffe E. The binding site for ribosomal protein L11 within 23 S ribosomal RNA of Escherichia coli. J Biol Chem. 1981 Dec 10;256(23):12301–12305. [PubMed] [Google Scholar]
- Schrier P. I., Möller W. The involvement of 50S ribosomal protein l11 in the EF-G dependent GTP hydrolysis of E. coli ribosomes. FEBS Lett. 1975 Jun 15;54(2):130–134. doi: 10.1016/0014-5793(75)80059-7. [DOI] [PubMed] [Google Scholar]
- Sköld S. E. RNA-protein complexes identified by crosslinking of polysomes. Biochimie. 1981 Jan;63(1):53–60. doi: 10.1016/s0300-9084(81)80146-0. [DOI] [PubMed] [Google Scholar]
- Thompson J., Cundliffe E., Stark M. Binding of thiostrepton to a complex of 23-S rRNA with ribosomal protein L11. Eur J Biochem. 1979 Jul;98(1):261–265. doi: 10.1111/j.1432-1033.1979.tb13184.x. [DOI] [PubMed] [Google Scholar]
- Thompson J., Schmidt F., Cundliffe E. Site of action of a ribosomal RNA methylase conferring resistance to thiostrepton. J Biol Chem. 1982 Jul 25;257(14):7915–7917. [PubMed] [Google Scholar]
- Toots I., Metspalu A., Villems R., Saarma M. Location of single-stranded and double-stranded regions in rat liver ribosomal 5S RNA and 5.8S RNA. Nucleic Acids Res. 1981 Oct 24;9(20):5331–5343. doi: 10.1093/nar/9.20.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann H. G. Components of bacterial ribosomes. Annu Rev Biochem. 1982;51:155–183. doi: 10.1146/annurev.bi.51.070182.001103. [DOI] [PubMed] [Google Scholar]
- Yamada T., Mizugichi Y., Nierhaus K. H., Wittmann H. G. Resistance to viomycin conferred by RNA of either ribosomal subunit. Nature. 1978 Oct 5;275(5679):460–461. doi: 10.1038/275460a0. [DOI] [PubMed] [Google Scholar]