Abstract
There is converging evidence for genetic, biochemical, and neuropsychological factors to increase the risk for anxiety and anxiety disorders. The pathogenesis of anxiety disorders is assumed to be influenced by a complex interaction of these individual risk factors on several levels, affecting intermediate phenotypes of anxiety such as the startle reflex. Thus, in the present double-blind, placebo-controlled study we attempted to paradigmatically investigate a multi-level pathogenetic model of anxiety by testing the effect of 300 mg caffeine citrate as an antagonist at the adenosine A2A receptor vs placebo on the emotion-potentiated (unpleasant, neutral, and pleasant International Affective Picture System pictures) startle reflex in 110 healthy individuals (male=56, female=54) stratified for the adenosine A2A receptor (ADORA2A) 1976T>C polymorphism (rs5751876). In addition to the expected main effect of picture category (highest startle amplitude for unpleasant, lowest for pleasant pictures) groups across all ADORA2A 1976T>C genotype and intervention (caffeine vs placebo) groups, an interaction effect of genotype, intervention, and picture category was discerned: In ADORA2A 1976TT risk genotype carriers, highest startle magnitudes were observed after caffeine administration in response to unpleasant pictures, with this effect arising particularly from the female subgroup. Our data point to a complex, multi-level, and potentially gender-specific pathogenetic model of anxiety, with genetic and biochemical factors interactively increasing the risk of maladaptive emotional processing and thereby possibly also anxiety disorders. The present findings may eventually aid in improving primary and secondary prevention by sharpening the risk profiles of anxiety-prone individuals.
Keywords: adenosine, caffeine, ADORA2A, panic disorder, emotional processing, startle
INTRODUCTION
Anxiety disorders such as panic disorder, generalized anxiety disorder, social phobia, and specific phobias are common diseases with a 12-month prevalence of about 12–14% (cf. Wittchen and Jacobi, 2005; Wittchen et al, 2011). The pathogenesis of pathological anxiety and anxiety disorders, respectively, has been suggested to be multifactorial, with a complex interaction of genetic risk factors, biochemical alterations, for example, of the adenosinergic system, and neuropsychological factors such as perception and processing of anxiety-relevant emotional stimuli. Association of each of these single risk factors with anxiety or anxiety disorders has repeatedly been demonstrated in previous studies:
(1) Family and twin studies report a strong genetic contribution to the development of anxiety disorders, with an estimated heritability between 28 and 48% (Hettema et al, 2001). Association studies of anxiety disorders and panic disorder have yielded evidence for several genes to increase the disease risk (for review see Jacob et al, 2010). Particularly, the adenosine A2A receptor gene (ADORA2A) has been suggested to have a pivotal role in the pathogenesis of anxiety and anxiety disorders in that the T allele of a silent polymorphism in exon-2 of the ADORA2A gene located on chromosome 22q11.23 (SNP rs5751876, 1976T>C, formerly 1083T>C, Tyr/Tyr) has been found to be associated with panic disorder mainly in Caucasian populations (Deckert et al, 1998; Hamilton et al, 2004; Lam et al, 2005; but Yamada et al, 2001). Additionally, the ADORA2A 1976T allele was associated with sympathetic psychophysiological indicators of anxiety-related arousal in blood-injury phobia (Hohoff et al, 2009). ADORA2A gene variation has furthermore been found to influence anxiety levels also in other psychiatric phenotypes such as autism spectrum disorder (Freitag et al, 2010) and in healthy individuals (Hohoff et al, 2010). These findings are in line with animal studies, where ADORA2A-knockout mice show increased anxiety levels (Ledent et al, 1997), suggesting a genetically driven reduced adenosine A2A receptor-mediated neurotransmission as a risk factor for anxiety (cf. Deckert, 1998).
(2) Caffeine exerts its arousal-promoting and potentially also anxiogenic effect through antagonism at adenosine A2A receptors (Huang et al, 2005). Consistently, caffeine has been shown to act as a potent anxiogenic challenge substance in the rodent model (eg, Bhattacharya et al, 1997) as well as in patients with anxiety disorders, first-degree relatives of patients with panic disorders, and healthy subjects (Boulenger et al, 1984; Bruce et al, 1992; Charney et al, 1985; Lee et al, 1998; Nardi et al, 2007, 2008, 2009; Nickell and Uhde, 1994). Also, an increase in acoustic startle reflex amplitude and a delayed habituation of acoustic startle blink amplitude as physiological correlates of anxiety have been observed after caffeine administration (Andrews et al, 1998; Blumenthal et al, 2005; Schicatano and Blumenthal, 1995). Reciprocally, caffeine abstinence has been reported to be beneficial as an adjunct in the treatment of anxiety disorders (eg, Bruce and Lader, 1989; Smith, 1988). In several twin studies, caffeine response has been shown to be considerably heritable (eg, Kendler and Prescott, 1999). Indeed, first molecular genetic studies investigating the impact of ADORA2A gene variation on anxiety after caffeine intervention demonstrated higher self-reported anxiety levels upon caffeine administration dependent on the ADORA2A 1976T risk allele (Alsene et al, 2003; Childs et al, 2008; Rogers et al, 2010). The ADORA2A 1976 genotype has furthermore been shown to drive subjective and objective responses to caffeine during sleep (Retey et al, 2007) as well as to amphetamines (Hohoff et al, 2005; for review see Yang et al, 2010).
(3) Converging evidence points toward a crucial role of unflexible and maladaptive processing of emotional stimuli in the pathogenesis of anxiety and anxiety disorders: anxiety-related emotional stimuli are rated as more threatening by patients than by control individuals and seem to be processed differently within the limbic-medial prefrontal circuit, partly driven by genetic factors (eg, Domschke et al, 2006, 2008b; Domschke and Dannlowski, 2010; Etkin, 2010; Lang and Cuthbert, 1984; Mathews, 1993; Mühlberger et al, 2006, 2007; Pauli et al, 1996; Wiedemann et al, 1999).
While there is support for each of those individual risk factors in anxiety as well as first evidence for two-way interactions between ADORA2A gene variation and caffeine consumption (eg, Alsene et al, 2003; Childs et al, 2008; Rogers et al, 2010), the complex interplay between all of these factors, including emotionally relevant stimuli, remains to be elucidated. Also, previous studies have mainly focused on subjective measures of anxiety as their primary outcome measure. It has been proposed, though, that intermediate phenotypes (synonymously used with the term endophenotypes), which are more narrowly and precisely defined than the complex overall phenotype (cf. Flint and Munafo, 2007; Gottesman and Gould, 2003), might be more apt to objectively capture the effect of risk factors in complex genetic phenotypes such as anxiety and anxiety disorders. The emotion-potentiated startle reflex with its characteristic amplification by unpleasant emotional stimuli (for review see Filion et al, 1998; Grillon and Baas, 2003; Grillon, 2008) has repeatedly been suggested as a promising objective intermediate phenotype of anxiety and anxiety disorders (eg, Butler et al, 1990; Filion et al, 1998; Grillon, 2002, 2008; Grillon et al, 1994, 1998; Grillon and Baas, 2003).
Thus, given evidence from previous studies for (1) a pivotal role of the ADORA2A gene in the pathogenesis of anxiety and anxiety disorders; (2) an anxiogenic effect of caffeine, which seems to be moderated by variation in the ADORA2A gene; and in addition (3) a maladaptive emotional reactivity in the development of pathological anxiety, the present study aims at investigating the multi-level interaction of these three risk factors on a genetic level (ADORA2A 1976T>C genotype), a biochemical level (placebo-controlled caffeine intervention), and the neuropsychological level of emotional processing (emotional stimuli) on the startle reflex as an objective intermediate phenotype of anxiety disorders. Our a priori hypothesis was that all three individual risk factors would interact synergistically in increasing startle response.
MATERIALS AND METHODS
Sample
A sample of 126 (male=60, female=66; mean age: 26.01 years, SD: 5.75) unrelated healthy subjects was consecutively recruited at the Department of Psychiatry, University of Muenster, and Department of Psychiatry, University of Wuerzburg, Germany, between 2009 and 2010. In order to minimize the risk of ethnic stratification, Caucasian descent was ascertained by Caucasian background of both parents. Current or prior diagnosis of DSM-IV axis-I disorders were excluded by using the Mini-International Neuropsychiatric Interview (M.I.N.I.; Sheehan et al, 1998). Additionally, anxiety sensitivity (AS), the general tendency to fear anxiety-related symptoms, was recorded by the German version of the Anxiety Sensitivity Index (ASI, German version; Alpers and Pauli, 2002; Reiss et al, 1986). To exclude any neurological or other somatic disorders, subjects underwent a physical and neurological examination in a screening session 1 week before the experiment, where additionally heart activity (electrocardiogram) and basic blood parameters were checked. Further exclusion criteria comprised caffeine or lactose intolerance, high and frequent caffeine consumption (more than three cups of coffee per day), illegal drug consumption (assessed by a urine drug screening for amphetamine, barbiturates, benzodiazepines, cocaine, ecstasy, methamphetamine, methadone, opiates, tricyclic antidepressants, tetrahydrocannabinol), alcohol consumption of more than 140 g per week (equivalent to about 15–20 U of alcohol), daily smoking of more than 20 cigarettes a day (smoking reduces the half-life of caffeine; Hart et al, 1976), daily use of any medication (except for hormonal contraception), pregnancy (assessed by a rapid urine pregnancy test) or breast feeding, less than a high school education, age under 18 and over 50 years, and left handedness. The subjects were asked to refrain from caffeine or tea consumption for 1 week prior to caffeine intervention and not to smoke, consume alcohol (assessed by a breath test), or take any medication for at least 24 h prior to the investigation. The protocol was approved by the ethics committees of the University of Muenster, and the University of Wuerzburg, Germany, and written informed consent from all subjects was obtained during the screening session. The design of the study is illustrated in Figure 1.
Figure 1.
Study design. EMG, electromyogram; ITI, inter-trial interval; POMS, Profile of Mood States; SAM, Self-Assessment Manikin; VAS, Visual Analogue Scale.
Genotyping
Subjects were genotyped for the ADORA2A 1976T>C (rs5751876) polymorphism according to published protocols (see Alsene et al, 2003; Deckert et al, 1998). According to previous findings, subjects were stratified into risk (TT) and non-risk (CT/CC) genotype carriers (cf. Alsene et al, 2003; Childs et al, 2008; Deckert et al, 1998; Rogers et al, 2010).
Caffeine Intervention
The study used a one-session, double-blind, placebo-controlled, between-subject design. All experimental sessions were conducted from 0815 to 1130 hours. After a negative drug and pregnancy urine test all electrodes were fixed and checked for impedances below 5 kΩ. Caffeine intervention was performed by oral administration of 300 mg caffeine citrate (Fagron, Barsbuettel, Germany; equivalent to 150 mg freebase caffeine), which has been shown to be close to the threshold for producing anxiogenic effects and as such might be the optimal dose to detect subtle genotype effects (cf. Alsene et al, 2003; Childs et al, 2008; Rogers et al, 2010). Caffeine citrate was administered in white opaque gelatin capsules; placebo capsules contained mannitol and aerosil (99.5 : 0.5) according to the German Drug Law (Arzneimittelgesetz; AMG). At about 0900 hours—after the first self-report anxiety measurement—subjects were administered a placebo or caffeine capsule with a glass of water. To assure correct implementation of the caffeine/placebo intervention, caffeine levels were determined by saliva test (0.5 ml) 55 min after caffeine administration. All subjects with a concentration differing more than one and a half interquartile ranges from the respective median (placebo or verum distribution) were excluded from further analysis.
Saliva samples were immediately frozen and analyzed not until all subjects were tested. Saliva caffeine levels were analyzed by means of reversed-phase, high-performance liquid chromatography.
Emotionally Relevant Stimuli
Twenty-four emotionally threatening unpleasant images taken from the International Affective Picture System (IAPS; Lang et al, 2005) were selected as anxiety-relevant emotional cues along with 24 neutral and 24 pleasant IAPS pictures (unpleasant IAPS pictures: 3000, 3053, 3170, 3102, 9410, 3080, 6313, 3120, 3130, 3071, 3100, 3010, 3060, 3064, 3140, 3110, 3150, 9300, 2800, 3030, 6540, 9252, 9250, 9040; neutral IAPS pictures: 2200, 2880, 5510, 5531, 7002, 7004, 7006, 7009, 7010, 7025, 7034, 7050, 7080, 7090, 7100, 7130, 7175, 7185, 7217, 7224, 7950, 2215, 5535, 7031; pleasant IAPS pictures: 1710, 2091, 2160, 2216, 2340, 2345, 4608, 4626, 4641, 8120, 5623, 5831, 5833, 8041, 8370, 8200, 8210, 8461, 8496, 5814; for men: 4220, 4290, 4607, 4680; for women: 4550, 4658, 4687, 5631). Ninety-five percent of all pictures were exactly the same for both genders, whereas different erotic pictures were chosen for men and women to ensure comparable valence and arousal levels.
Objective Outcome Measure: Emotion-Potentiated Startle Paradigm
At the assumed maximum plasma level of caffeine and the described time to peak increases in self-report ratings of anxiety, respectively (60 min after administration; Alsene et al, 2003; Childs et al, 2008; Rogers et al, 2010), the emotion-potentiated startle experiment was started (at 1000 hours). In order to get subjects used to the startle stimulus (50 ms of 95-dB white noise with an instantaneous rise-time presented through Bose Around-Ear Headphones) and to prevent outlier startle responses during the critical trials, eight startle stimuli at random intervals of 1–12 s were presented. The startle experiment per se consisted of three blocks of 24 anxiety-relevant, neutral, or pleasant IAPS pictures, respectively, as described above, and 3-min breaks between the blocks. An experimental block contained eight pictures of each of the three categories in random order, with the constraint that no two of the same type (unpleasant, neutral, or pleasant) were presented successively. During the experiment, in a dimly lit room subjects sat in a recliner, which was separated by a room divider from the experimenter. Visual stimuli were presented for 8 s (inter-trial interval (ITI): mean=21 s, range=16.5–25.5 s) on a 19″ LCD computer screen approximately 1 m away from the subject. Startle probes were administered 2.5, 4, or 5.5 s after picture onset during picture presentation, and 10 as well as 12 s after picture offset during the ITI. In each block as well as in the overall experiment, 75% of all trials contained startle probes during picture presentation (evenly distributed across each picture category), 12.5% of all trials contained startle probes during the ITI, and 12.5% of the trials did not contain any startle probe.
Electromyogram activity of the M. orbicularis oculi, which is responsible for eyelid closure, was measured as a commonly used variable for recording startle reactions in humans (Blumenthal et al, 2005). For this purpose, two transparent pediatric 13-mm electrodes were placed under the left eye. The reference electrode was placed on the forehead, 2 cm beneath the hairline, and the ground electrode was placed on the processus mastoideus behind the left ear (sintered Ag/AgCl electrodes). Electromyogram activity was recorded by using V-Amp 16 (Brain Products GmbH, Gilching, Germany), a 16-channel DC amplifier system using the BrainVision Recorder Software (V-Amp Edition 1.10; Brain Products GmbH). Sampling rate was 1000 Hz and an online notch filter of 50 Hz was applied. Stimuli were presented by using the software package Presentation (v13.0; Neurobehavioral Systems, Albany, CA). BrainVision Analyzer 2 (Brain Products GmbH) was used as an offline analyzing software with which the signals were rectified, filtered (low cut-off, 28 Hz; high cut-off, 500 Hz; notch, 50 Hz), and smoothed (using a time constant of 50 ms). Startle magnitude was quantified as the difference between the highest peak 21–200 ms after and the average during 50 ms before startle probe presentation. Startle data were checked for zero responses and artifacts in each subject. Startle reactions with no detectable responses (<5 μV) were scored as 0. Artifacts were defined as spontaneous eye blinks during baseline or within 20 ms after startle probe onset, and were scored as missing values. Subjects were excluded from data analysis when having too many zero responses (more than 2.5 SDs above mean number of zero responses) or less than three valid startle responses in one picture category. All startle magnitudes were T-transformed within subjects in order to assure comparability of data (for methodical overview Blumenthal et al, 2005; Mühlberger et al, 2008; Pauli et al, 2010).
After the experiment, electrodes were removed and subjects were given the possibility to clean their faces from the electrode paste and have a short break. Finally, each subject had to rate all pictures in a free-viewing condition by valence (1=highly pleasant, 9=highly unpleasant) and arousal (1=excited, 9=calm) by using Self-Assessment-Manikin (SAM) scales (Lang, 1980). At about 1130 hours subjects were discharged by a physician and paid an allowance of 100€.
Subjective Outcome Measures: VAS and POMS
Subjects were asked to rate their anxiety level by placing a mark on a visual analogue scale (VAS) consisting of a 100 mm horizontal line labeled ‘not at all anxious' (score of 0) and ‘extremely anxious' (score of 100). Additionally, the 35-item German version of the Profile of Mood States (POMS) was administered, on which subjects report their current mood on a seven-point scale from ‘not at all' (score of 0) to ‘extremely' (score of 6) (Biehl et al, 1986; McNair et al, 1992). Four categories have been distinguished within the German version of the POMS: (1) ‘Depression–Anxiety,' (2) ‘Fatigue,' (3) ‘Vigor,' and (4) ‘Hostility' (cf. Albani et al, 2005). In the present study, the subscale ‘Depression–Anxiety' was used as a subjective measure of anxiety. VAS and POMS measurements were taken at three points in the course of the study: (1) before the respective intervention (caffeine/placebo); (2) at maximum caffeine plasma level and the described time to peak increases in self-report anxiety, respectively, (60 min; Alsene et al, 2003); and (3) after emotion-potentiated startle.
Statistical Analysis
Sample characteristics were evaluated by χ2 tests for genotype (ADORA2A 1976TT risk vs. 1976CC/CT non-risk genotypes) and gender with intervention (caffeine versus placebo) as between-subjects-factor as well as by one-way ANOVAs for caffeine consumption, age and anxiety sensitivity with intervention, genotype, and gender as between-subjects-factors.
VAS and POMS ratings were analyzed by ANOVAs for repeated measures, with genotype and intervention (caffeine vs placebo) as between-subject factors, and measurement time (three measurement times: before substance intake, 1 h after substance intake, and after the startle experiment) as a within-subject factor. Baseline startle response (assessed in the ITIs) was analyzed by ANOVA for repeated measures, with measurement time (the 12 ITI startle responses were divided into four measurement times (T1–T4) each being the mean of three consecutive startle responses) as a within-subject factor and genotype and intervention as between-subject factors. Picture ratings, which were conducted at the end of the startle experiment (see Figure 1), and picture viewing times—defined as time periods between picture onset and consecutive ratings—were analyzed by ANOVA for repeated measures, with genotype and intervention as between-subject factors, and picture category (unpleasant, neutral, and pleasant) as a within-subject factor. Pairwise comparisons of picture valence or time of measurement were assessed by post-hoc t-tests.
According to a priori hypotheses, the main multi-level analysis of emotion-potentiated startle response was performed by ANOVA for repeated measures, with genotype and intervention as between-subject factors, and picture category (unpleasant, neutral, and pleasant) as a within-subject factor. Picture categories were further analyzed by post-hoc univariate ANOVAs.
Further explorative analyses were performed by using gender and AS (median split) as additional factors for all above mentioned ANOVAs.
Alpha level was set at 5% using Greenhouse–Geisser corrections where appropriate.
RESULTS
Sample Characteristics
Eight subjects of initially 126 recruited subjects showed too many zero startle responses (>35; mean zero responses per subject in the whole group: 5.7, SD: 11.5; see Materials and Methods) and were therefore excluded from further analyses. Eight additional subjects were excluded because of unexpected saliva caffeine concentrations (placebo group: 7 subjects, mean concentration: 72.2 mg/l, SD: 31.9; verum group: 1 subject, mean concentration: 173.4 mg/l; see Materials and Methods) not consistent with caffeine abstinence prior to the experiment.
The remaining sample of 110 subjects was equally distributed regarding genotype (ADORA2A 1976TT vs 1976CC/CT) and gender across intervention groups (caffeine vs placebo; both χ2(1)<0.17, p>0.68; see Table 1).
Table 1. Sample Characteristics.
| Intervention | ADORA2A 1976T>C genotype |
Gender |
Total | |
|---|---|---|---|---|
| Men | Women | |||
| Placebo | Risk (TT) | n=13 | n=13 | n=26 |
| Age=28.0 (8.68) | Age=24.1 (3.25) | Age=26.1 (6.72) | ||
| ASI=14.2 (7.89) | ASI=15.6 (6.85) | ASI=14.9 (7.27) | ||
| cc=151.7 mg/day (141.76) | cc=111.5 mg/day (96.08) | cc=131.6 mg/day (120.39) | ||
| Non-risk (CC/CT) | n=15 | n=12 | n=27 | |
| Age=25.7 (4.77) | Age=27.3 (7.68) | Age=26.4 (6.16) | ||
| ASI=13.9 (5.93) | ASI=12.6 (7.35) | ASI=13.3 (6.50) | ||
| cc=140.0 mg/day (82.81) | cc=91.7 mg/day (70.17) | cc=118.5 mg/day (79.84) | ||
| Verum | Risk (TT) | n=14 | n=13 | n=27 |
| (300 mg caffeine citrate) | Age=27.2 (3.26) | Age=25.3 (7.30) | Age=26.3 (5.55) | |
| ASI=13.5 (5.93) | ASI=14.2 (4.36) | ASI=13.9 (5.15) | ||
| cc=113.2 mg/day (103.34) | cc=111.5 mg/day (129.35) | cc=112.4 mg/day (114.29) | ||
| Non-risk (CC/CT) | n=14 | n=16 | n=30 | |
| Age=28.8 (6.17) | Age=25.3 (2.73) | Age=27.2 (4.84) | ||
| ASI=12.4 (6.57) | ASI=11.0 (7.49) | ASI=11.7 (6.99) | ||
| cc=137.4 mg/day (108.02) | cc=112.5 mg/day (95.74) | cc=123.7 mg/day (100.35) | ||
| Total | n=56 | n=54 | n=110 | |
| Age=27.4 (5.93) | Age=25.6 (5.48) | Age=26.5 (5.76) | ||
| ASI=13.5 (6.44) | ASI=13.2 (6.72) | ASI=13.4 (6.55) | ||
| cc=135.3 mg/day (107.73) | cc=107.4 mg/day (97.81) | cc=121.5 mg/day (103.41) | ||
Abbreviations: ASI, anxiety sensitivity index; cc, caffeine consumption.
Standard deviation in parentheses.
One-way ANOVA of mean caffeine consumption (calculated in mg/day, with one cup of coffee corresponding to 100 mg of caffeine) revealed no differences between genotypes, intervention groups, or gender (all F(1, 109)<2.01, p>0.16). Comparing caffeine consumers (n=83) with caffeine non-consumers (n=26) a marginally significant association between caffeine consumption and genotype was identified (χ2(1)=3.84, p=0.05), with a 2.45 higher odds of being an ADORA2A 1976TT risk genotype carrier in caffeine non-consumers than in caffeine consumers, and a trend for an association between caffeine consumption and gender (χ2(1)=3.42, p=0.06), with the odds of being a non-consumer being 2.35 times higher in women than in men. No association was seen between caffeine consumption and intervention condition (χ2(1)=0.08, p=0.77).
Factorial ANOVA revealed that neither genotype nor intervention condition or gender groups differed in age (all F(1, 102)<2.64, p>0.11) or AS (AS; F(1, 102)<2.13, p>0.15). Mean AS was 13.4 (SD: 6.6; range: 3–35; median: 13), which is lower than the expected mean AS of about 18 in a non-clinical population (Peterson and Reiss, 1992).
Picture Ratings and Viewing Times
Valence ratings corresponded to a priori categories (F(2, 212)=3000.06, p<0.001; linear trend, F(1, 99)=3714.12, p<0.001; pleasant>neutral>unpleasant: all t(109)>∣33.02∣, p<0.001). There were no direct or interaction effects of genotype or intervention on valence ratings (all F(2, 212)<2.86, p>0.08).
Arousal ratings were affected by picture valence (F(2, 212)=1146.89, p<0.001), with highest ratings for unpleasant pictures, followed by relatively high ratings for pleasant pictures and low ratings for neutral pictures (all t(109)>∣18.85∣, p<0.001). There were no direct or interaction effects of genotype or intervention on arousal ratings (all F(2, 212)<1.18, p>0.30).
Analysis of picture viewing times revealed no significant effect of picture category or additional interactions of genotype or intervention (all F(2, 210)<0.77, p>0.46) (One additional subject had to be excluded from this analysis because of technical problems during recording of picture viewing times.). However, there was a main effect of intervention (F(2, 210)=5.28, p=0.02), with higher overall viewing times under caffeine as compared with placebo (t(107)=−2.35, p=0.02).
Habituation and Baseline Startle Response
Analysis of the startle response during the inter-trial interval revealed a significant effect of measurement time on startle magnitude (F(3, 306)=51.31, p<0.001): Mean baseline startle magnitudes declined across the first three measurement times (all t(105)>4.72, p<0.001), with no difference between the third and the fourth measurement time (t(105)=0.78, p=0.43) (Four additional subjects had to be excluded from this analysis because of consecutive zero responses, leading to a missing mean in one of the four measurement times (see Materials and Methods)). There was no genotype or intervention effect, or a genotype × intervention interaction effect, on baseline startle response times (all F(3, 306)<1.35, p>0.25).
Startle Modulation: Influence of Picture Category
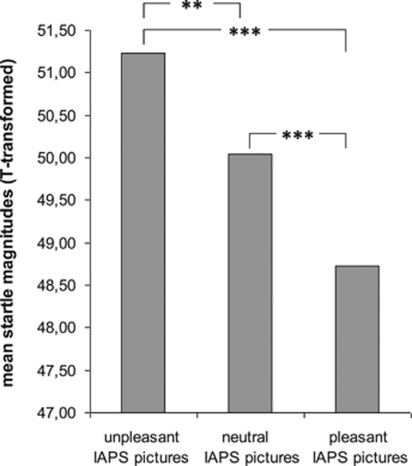
ANOVA revealed a significant main effect of picture category on startle response (F(2, 212)=23.51, p<0.001), which was due to increasing startle magnitudes from pleasant to neutral to unpleasant pictures (linear trend, F(1, 106)=44.99, p<0.001; each t(109)>2.81, p<0.007; see Figure 2).
Figure 2.
Mean startle magnitude modulated by picture category. Mean startle magnitude was significantly modulated by picture category (F(2, 212)=23.51, p<0.001), with decreasing magnitudes from unpleasant to neutral to pleasant pictures (linear trend, F(1, 106)=44.99, p<0.001; each t(109)>2.81, p<0.007). **, significant at a significance level of p⩽0.01; ***, significant at a significance level of p⩽0.001.
Genotype Effects on Startle Modulation
There was no significant main effect of genotype on mean startle magnitudes (F(1, 106)=0.65; p=0.42), and no significant interaction effect of genotype (ADORA2A 1976TT vs 1976CC/CT) and picture category could be discerned (F(2, 212)=0.003, p=0.99).
Effects of Intervention (Caffeine vs Placebo) on Startle Modulation
There was no significant main effect of intervention (caffeine vs placebo) on mean startle magnitudes (F(1, 106)=0.43; p=0.51). Also, no significant interaction effect of intervention (caffeine vs placebo) and picture category was observed (F(2, 212)=0.04, p=0.95).
Genotype × Intervention Effects on Startle Modulation
A significant interaction between genotype, intervention, and picture category was discerned (F(2, 212)=3.84, p=0.02; see Figure 3). Post-hoc separate analyses for each genotype revealed that under placebo ADORA2A TT risk genotype carriers did not show significant differences in startle magnitude between unpleasant and neutral pictures (t(25)=0.24, p=0.82), but between neutral and pleasant (t(25)=2.83, p=0.01), as well as between unpleasant and pleasant pictures (t(25)=3.07, p=0.005), whereas in the caffeine condition, subjects carrying the ADORA2A TT risk genotype showed significant differences in startle magnitude between unpleasant and neutral pictures (t(26)=2.38, p=0.03) as well as between unpleasant and pleasant pictures (t(26)=3.77, p=0.001), but not between neutral and pleasant pictures (t(26)=1.18, p=0.25).
Figure 3.
Multifactorial startle modulation by genotype (ADORA2A 1976T>C), intervention (caffeine vs placebo), and picture category. A significant interaction between genotype, intervention, and picture category was discerned (F(2, 212)=3.84, p=0.02). *, significant at a significance level of p⩽0.05; **, significant at a significance level of p⩽0.01; ***, significant at a significance level of p⩽0.001.
Vice versa, under placebo ADORA2A CC/CT non-risk genotype carriers showed significant differences between unpleasant and neutral (t(26)=3.19, p=0.004) as well as between unpleasant and pleasant pictures (t(26)=4.04, p<0.001), but not between neutral and pleasant pictures (t(26)=1.31, p=0.20), whereas in the caffeine condition ADORA2A CC/CT subjects showed no significant differences between unpleasant and neutral pictures (t(29)=0.23, p=0.82), but between neutral and pleasant (t(29)=3.69, p=0.001) as well as between unpleasant and pleasant pictures (t(26)=2.70, p=0.01).
Explorative Analysis of Gender and AS Effects on Startle Modulation
When using gender and AS (median split of the ASI) as additional factors in the analysis of startle modulation, ANOVA revealed significant interaction effects of picture category and gender (F(2, 188)=10.30, p<0.001), and of picture category, gender, and AS (F(2, 188)=5.55, p=0.006). Therefore, further analyses were conducted separately for both gender groups. In both men and women, a significant effect of picture category on startle magnitude emerged (women: F(2, 92)=23.66, p<0.001; men: F(2, 96)=9.03, p=0.001). In contrast to the overall effect of picture category, no significant differences between unpleasant and neutral pictures were discerned in men (t(55)=−0.25; p=0.80), and no significant differences between neutral and pleasant pictures in women (t(53)=1.75; p=0.09). In women, but not in men, ANOVA revealed the above mentioned genotype × intervention × picture category effect (women: F(2, 92)=4.60, p=0.01; men: F(2, 96)=1.04, p>0.34), with post-hoc univariate ANOVAs in women showing a significant interaction of genotype and intervention for unpleasant pictures (F(1, 46)=6.83, p=0.01; with higher startle magnitudes for TT risk genotype carriers in the caffeine condition and higher startle magnitudes in non-risk CC/CT genotype carriers in the placebo condition) and neutral pictures (F(1, 46)=6.26, p=0.03; with higher startle magnitudes for TT risk genotype carriers in the placebo condition and higher startle magnitudes in CC/CT non-risk genotype carriers in the caffeine condition), but not for pleasant pictures (F(1, 46)=0.49, p=0.49). In men, but not in women, a significant interaction effect of picture category and AS on startle magnitudes was observed (F(2, 96)=4.96, p=0.01): Only men with high AS showed—like women—the above mentioned overall pattern of startle magnitudes (unpleasant>neutral>pleasant; linear trend: F(1, 28)=23.67, p<0.001). Men with low AS, however, showed a different pattern (unpleasant<neutral>pleasant; quadratic trend: F(1, 26)=8.26, p=0.008; see Figure 4).
Figure 4.
Multifactorial startle modulation by gender, AS, and picture category. AS, anxiety sensitivity. In men, but not in women, there was a significant interaction effect of picture category and AS on startle magnitudes (F(2, 96)=4.96, p=0.01): Only men with high AS showed—like women—the overall pattern of startle magnitudes (unpleasant>neutral>pleasant; linear trend: F(1, 28)=23.67, p<0.001).
Subjective Measures of Anxiety
A significant main effect of measurement time on VAS anxiety ratings was observed (F(2, 212)=11.41, p<0.001), with significantly decreasing anxiety levels from point-1 (before capsule intake) to point-2 (1 h after capsule intake; t(109)=2.76, p=0.007) and significantly increasing anxiety levels from point-2 to point-3 (after the startle experiment; t(109)=−4.51, p<0.001) (Figure 2). However, there were no significant effects of genotype, intervention, or genotype × intervention on VAS ratings (all F(2, 212)<1.28, p>0.28).
Analysis of the POMS subscale ‘Depression–Anxiety' revealed a significant main effect of measurement time (F(2, 212)=22.52, p<0.001), with highest scores at the end of the experiment and lowest scores prior to the startle experiment (point-3>point-2 and point-1; point-2<point-1; all t(109)>∣2.76∣, p<0.008). There were no significant effects of genotype or intervention (both F(2, 212)<1.30, p>0.27), but a significant interaction effect of genotype × intervention (F(2, 212)=5.25, p=0.02). Further analysis revealed that the above mentioned pattern (point-1>point-2<point-3) was valid for the ADORA2A non-risk genotype group under both conditions and for the risk genotype group only under verum (all t(26)>∣2.26∣, p<0.03), but not under placebo (all t(25)<∣1.75∣, p>0.09). In risk genotype carriers, increase in POMS ‘Depression–Anxiety' ratings from point-2 to point-3 was significant in the verum condition (t (26)=−5.20, p<0.001) but not in the placebo condition (t (25)=−1.10, p=0.28).
DISCUSSION
In the present study, we observed an interaction of genetic factors (ADORA2A 1976TT risk genotype), biochemical factors (caffeine intervention), and anxiety-related emotional stimuli to increase psychophysiological parameters of anxiety: The ADORA2A 1976TT risk genotype seems to be generally linked to maladaptive emotional processing in that under the placebo condition risk genotype carriers show equally increased startle magnitudes for unpleasant and neutral pictures as compared with pleasant pictures. This finding pointing to an undifferentiated potentiation of anxiety-related measures in high-risk populations is paralleled by a number of studies showing increased startle or brain activation levels not only during processing of negative but also of neutral stimuli (Armbruster et al, 2010; Bernat et al, 2006; Bradley, 2001; Rosen and Donley, 2006), particularly in patients with anxiety disorders (Yoon and Zinbarg, 2007), who perceive these stimuli as ambiguous or uncertain. Additionally, according to our a priori hypothesis of a synergistic effect of the ADORA2A risk genotype and caffeine on startle response to negative emotional stimuli, in ADORA2A 1976TT risk genotype carriers highest startle magnitudes were observed after caffeine administration in response to unpleasant emotional material, with caffeine now eliciting a significant contrast between unpleasant and neutral emotional stimuli. Reciprocally, ADORA2A 1976CC/CT low-risk genotype carriers challenged with caffeine show equally high startle magnitudes for unpleasant and neutral pictures, whereas under placebo—as expected in an unmodulated emotion-potentiated startle paradigm in healthy probands—a gradual increase in startle response from pleasant to neutral to unpleasant emotional material was observed. Thus, subjects carrying either the ADORA2A anxiety 1976TT risk genotype or receiving caffeine, respectively, as single risk-increasing factors react to unpleasant and neutral stimuli in an equally aroused manner as reflected by comparable startle magnitudes, whereas in a multi-level risk model caffeine in synergy with genotype further increases startle reaction specifically for unpleasant emotional stimuli.
The present study extends previous genetic and pharmacological findings of (1) reports of association of the ADORA2A 1976T allele with panic disorder (Deckert et al, 1998; Hamilton et al, 2004; Hohoff et al, 2010) and (2) reports of caffeine exerting an anxiogenic effect (eg, Boulenger et al, 1984; Charney et al, 1985) partially conferred by the ADORA2A 1976TT risk genotype (Alsene et al, 2003; Childs et al, 2008; Rogers et al, 2010) by including emotional stimuli as a third risk factor and by integrating these individual risk factors into a multi-level risk model of anxiety. Also, besides subjective measures of anxiety, in the present study startle reflex potentiation during unpleasant stimuli, which has previously been linked to fearfulness and behavioral inhibition as dimensional phenotypes of anxiety disorders, and therefore has been suggested as a promising intermediate phenotype of anxiety (Cook et al, 1992; Grillon and Baas, 2003; Hawk and Kowmas, 2003; Koch, 1999), was used as an objective outcome measure. Based on this experimental model of anxiety, the present results for the first time suggest a multi-level interaction effect between three risk factors on a genetic level (ADORA2A 1976TT genotype), a biochemical level (caffeine), and the neuropsychological level of emotional processing (anxiety-related emotional stimuli) on the startle reflex as an objective intermediate phenotype of anxiety disorders.
The observed female-predominant influence of the ADORA2A 1976TT genotype on increased startle response dependent on caffeine intervention and anxiety-related emotional stimulation is in accordance with a higher prevalence and also a higher heritability of AS or anxiety disorders, respectively, in female patients (Jang et al, 1999; Weissman et al, 1997) as well as with previous genetic findings restricted to female patients with anxiety and affective disorders (eg, Deckert et al, 1999; Domschke et al, 2004, 2007, 2008a, in press). Also, there are first reports of a potentially gender-differential effect of caffeine in animal models as well as in humans, with, however, still inconsistent conclusions (eg, Botella and Parra, 2003; Fisher and Guillet, 1997; Noschang et al, 2009). Thus, a potential gender specificity of the presently investigated multi-level risk model of anxiety warrants further investigation in future studies preferably involving additional potential mediators, particularly, as we observed AS to apparently mediate startle response to emotional stimuli in a gender-differential manner.
We did not discern a significant influence of caffeine (150 mg freebase) on VAS or POMS measures of anxiety as opposed to previous studies (Alsene et al, 2003; Childs et al, 2008). There might be several reasons for that: First, the presently applied 35-item German version of the POMS scale is not fully comparable to the American version (65 items; Albani et al, 2005; McNair et al, 1992). A lower than average mean AS in the present sample (cf. Peterson and Reiss, 1992) might further account for low anxiety ratings on VAS or POMS, respectively. Also, the presently administered dose of caffeine might have been too low to elicit increased self-report anxiety, as Childs et al (2008) observed increases in VAS anxiety ratings only after administration of 450 mg caffeine, but not at the equivalent of 150 mg freebase caffeine, and other studies have shown that only high doses of caffeine increase anxiety (cf. Evans and Griffiths, 1991; Griffiths and Woodson, 1988). Finally, the lack of a cross-over design in the present study might have prevented detection of effects on self-report data.
The present results have to be interpreted in the light of some considerations and limitations: The sample size is modest, however, within the range of comparable previous studies applying the startle reflex paradigm or measures of neuronal activation as an intermediate phenotype approach (eg, Giakoumaki et al, 2008; Mattay et al, 2003; Pauli et al, 2010). Furthermore, in the present study caffeine non-users as well as light-to-intermediate caffeine users were enrolled, who were asked to refrain from caffeine consumption for 1 week prior to the investigation, which was controlled only at the experimental day, thus not excluding shorter abstinence periods. A potentially confounding effect of withdrawal and/or tolerance therefore cannot be completely excluded. Also, a potential selection bias has to be taken into consideration given that ADORA2A 1976T allele carriers have been reported to be more likely to be lighter caffeine consumers in the first place (Cornelis et al, 2007), which tended to be true also in the present sample. Furthermore, only a single dose of caffeine was administered so that no conclusions can be drawn on the effects of a chronic caffeine medication. Almost all of the female probands had been on hormonal contraceptives during participation in the present study. The actual hormonal status, however, was not considered in the present study, which might have introduced a potential confounder based on case reports suggesting oral contraceptives to potentially induce panic attacks (Deci et al, 1992; Ushiroyama et al, 1992) and animal as well as human studies providing evidence for gonadal hormones and menstrual cycle to modulate AS (Nillni et al, 2011; Toufexis et al, 2006). Furthermore, the functional relevance of the silent ADORA2A 1976T>C polymorphism is still unknown and therefore the mechanism by which this variant might confer susceptibility to anxiety remains to be elucidated. However, in analogy to synonymous mutations, that is, mutations not changing the amino-acid sequence in the human dopamine D2 receptor (DRD2) gene (Duan et al, 2003), it may have drastic functional effects by altering mRNA stability or translation. Alternatively, the associated polymorphism might not constitute the actual causative variant, but rather reflect association of other polymorphisms in linkage disequilibrium with this locus such as the ADORA2A 2592T/− (rs35320474) polymorphism (cf. Alsene et al, 2003). Finally, owing to the limited sample size the present results have not been controlled for influence of other relevant genetic risk factors such as the DRD2 rs1110976 polymorphism (Childs et al, 2008). This could have constituted a potentially confounding factor, as a mutual functional relationship between the adenosinergic and the dopaminergic system has been suggested: Adenosine A2A receptors form functional heteromeric complexes with DRD2 receptors interacting at multiple levels to control cell function, with activation of A2A reducing DRD2 signaling (Fuxe et al, 2007). Furthermore, in DRD2-knockout mice the behavioral properties of caffeine are altered and DRD2 antagonists attenuate the discriminative stimulus effects of low caffeine doses (Powell et al, 1999), and DRD2 receptors in the amygdala have been suggested to have a role in setting up adaptive responses to cope with aversive environmental stimuli (de la Mora et al, 2010).
In summary, this study—exemplarily focusing on the adenosinergic system—provides evidence for a multi-level pathogenetic model of anxiety involving genetic factors and biochemical alterations interactively increasing the risk of maladaptive emotional processing as reflected by potentiation of the startle response as an objective intermediate phenotype of anxiety. Provided replication in independent studies, these data could help to define subtypes of anxiety-prone individuals and therefore finally may aid in improving primary and secondary prevention of anxiety disorders.
Acknowledgments
This study was supported by grants from the Deutsche Forschungsgemeinschaft (DFG; SFB-TRR-58 project C2 to KD and JD; project Z2 to JD, AR, and PP; project B1 to PP and AM; and project C1 to PZ). We acknowledge the technical support by Max Hilscher, Heike Ewald, Thilo Rattay, and Julian Conrad.
All affiliations mentioned below have no relevance to the work covered in the manuscript: AR has received a research grant from Astra Zeneca. KD has received speaker fees from Pfizer, Lilly, and Bristol-Myers Squibb; she is a consultant for Johnson & Johnson and has received funding from Astra Zeneca. In the past 3 years, JD has received speaker's honoraria by Janssen, Bristol-Myers Squibb, Wyeth, Lundbeck, Astra Zeneca, and Pfizer, and grant support from Medice. PZ has received speaker fees from Pfizer, Servier, Lilly, Astra Zeneca, and Bristol-Myers Squibb; he is on the advisory board of Pfizer, is a consultant for Ironwood Pharmaceuticals, and has received funding from AstraZeneca. VA is member of advisory boards and/or gave presentations for the following companies: Astra-Zeneca, Janssen-Organon, Lilly, Lundbeck, Servier, Pfizer, and Wyeth. He chaired the committee for the ‘Wyeth Research Award Depression and Anxiety'. All other authors have no conflicts of interest to declare, financial or otherwise.
References
- Albani C, Blaser G, Geyer M, Schmutzer G, Brahler E, Bailer H, et al. [The German short version of ‘Profile of Mood States' (POMS): psychometric evaluation in a representative sample] Psychother Psychosom Med Psychol. 2005;55:324–330. doi: 10.1055/s-2004-834727. [DOI] [PubMed] [Google Scholar]
- Alpers GW, Pauli P. Angstsensitivitäts-Index. PsychScience: Würzburg; 2002. [Google Scholar]
- Alsene K, Deckert J, Sand P, de Wit H. Association between A2a receptor gene polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology. 2003;28:1694–1702. doi: 10.1038/sj.npp.1300232. [DOI] [PubMed] [Google Scholar]
- Andrews SE, Blumenthal TD, Flaten MA. Effects of caffeine and caffeine-associated stimuli on the human startle eyeblink reflex. Pharmacol Biochem Behav. 1998;59:39–44. doi: 10.1016/s0091-3057(97)00331-6. [DOI] [PubMed] [Google Scholar]
- Armbruster D, Mueller A, Strobel A, Kirschbaum C, Lesch KP, Brocke B. Influence of functional tryptophan hydroxylase 2 gene variation and sex on the startle response in children, young adults, and older adults. Biol Psychol. 2010;83:214–221. doi: 10.1016/j.biopsycho.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Bernat E, Patrick CJ, Benning SD, Tellegen A. Effects of picture content and intensity on affective physiological response. Psychophysiology. 2006;43:93–103. doi: 10.1111/j.1469-8986.2006.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya SK, Satyan KS, Chakrabarti A. Anxiogenic action of caffeine: an experimental study in rats. J Psychopharmacol. 1997;11:219–224. doi: 10.1177/026988119701100304. [DOI] [PubMed] [Google Scholar]
- Biehl B, Dangel S, Reiser A.1986Profile of mood statesIn: CIPS (ed).Internationale Skalen für Psychiatrie Beltz: Weinberg [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Botella P, Parra A. Coffee increases state anxiety in males but not in females. Hum Psychopharmacol. 2003;18:141–143. doi: 10.1002/hup.444. [DOI] [PubMed] [Google Scholar]
- Boulenger JP, Uhde TW, Wolff EA, III, Post RM. Increased sensitivity to caffeine in patients with panic disorders. Preliminary evidence. Arch Gen Psychiatry. 1984;41:1067–1071. doi: 10.1001/archpsyc.1983.01790220057009. [DOI] [PubMed] [Google Scholar]
- Bradley SJ. Anxiety and mood disorders in children and adolescents: a practice update. Paediatr Child Health. 2001;6:459–463. doi: 10.1093/pch/6.7.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M, Scott N, Shine P, Lader M. Anxiogenic effects of caffeine in patients with anxiety disorders. Arch Gen Psychiatry. 1992;49:867–869. doi: 10.1001/archpsyc.1992.01820110031004. [DOI] [PubMed] [Google Scholar]
- Bruce MS, Lader M. Caffeine abstention in the management of anxiety disorders. Psychol Med. 1989;19:211–214. doi: 10.1017/s003329170001117x. [DOI] [PubMed] [Google Scholar]
- Butler RW, Braff DL, Rausch JL, Jenkins MA, Sprock J, Geyer MA. Physiological evidence of exaggerated startle response in a subgroup of Vietnam veterans with combat-related PTSD. Am J Psychiatry. 1990;147:1308–1312. doi: 10.1176/ajp.147.10.1308. [DOI] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Jatlow PI. Increased anxiogenic effects of caffeine in panic disorders. Arch Gen Psychiatry. 1985;42:233–243. doi: 10.1001/archpsyc.1985.01790260027003. [DOI] [PubMed] [Google Scholar]
- Childs E, Hohoff C, Deckert J, Xu K, Badner J, de Wit H. Association between ADORA2A and DRD2 polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology. 2008;33:2791–2800. doi: 10.1038/npp.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EW, III, Davis TL, Hawk LW, Spence EL, Gautier CH. Fearfulness and startle potentiation during aversive visual stimuli. Psychophysiology. 1992;29:633–645. doi: 10.1111/j.1469-8986.1992.tb02038.x. [DOI] [PubMed] [Google Scholar]
- Cornelis MC, El-Sohemy A, Campos H. Genetic polymorphism of the adenosine A2A receptor is associated with habitual caffeine consumption. Am J Clin Nutr. 2007;86:240–244. doi: 10.1093/ajcn/86.1.240. [DOI] [PubMed] [Google Scholar]
- de la Mora MP, Gallegos-Cari A, Arizmendi-García Y, Marcellino D, Fuxe K. Role of dopamine receptor mechanisms in the amygdaloid modulation of fear and anxiety: structural and functional analysis. Prog Neurobiol. 2010;90:198–216. doi: 10.1016/j.pneurobio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Deci PA, Lydiard RB, Santos AB, Arana GW. Oral contraceptives and panic disorder. J Clin Psychiatry. 1992;53:163–165. [PubMed] [Google Scholar]
- Deckert J. The adenosine A(2A) receptor knockout mouse: a model for anxiety. Int J Neuropsychopharmacol. 1998;1:187–190. doi: 10.1017/S1461145798001217. [DOI] [PubMed] [Google Scholar]
- Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, et al. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet. 1999;8:621–624. doi: 10.1093/hmg/8.4.621. [DOI] [PubMed] [Google Scholar]
- Deckert J, Nothen MM, Franke P, Delmo C, Fritze J, Knapp M, et al. Systematic mutation screening and association study of the A1 and A2a adenosine receptor genes in panic disorder suggest a contribution of the A2a gene to the development of disease. Mol Psychiatry. 1998;3:81–85. doi: 10.1038/sj.mp.4000345. [DOI] [PubMed] [Google Scholar]
- Domschke K, Braun M, Ohrmann P, Suslow T, Kugel H, Bauer J, et al. Association of the functional -1019C/G 5-HT1A polymorphism with prefrontal cortex and amygdala activation measured with 3 T fMRI in panic disorder. Int J Neuropsychopharmacol. 2006;9:349–355. doi: 10.1017/S1461145705005869. [DOI] [PubMed] [Google Scholar]
- Domschke K, Dannlowski U. Imaging genetics of anxiety disorders. Neuroimage. 2010;53:822–831. doi: 10.1016/j.neuroimage.2009.11.042. [DOI] [PubMed] [Google Scholar]
- Domschke K, Deckert J, O'Donovan MC, Glatt SJ. Meta-analysis of COMT val158met in panic disorder: ethnic heterogeneity and gender specificity. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:667–673. doi: 10.1002/ajmg.b.30494. [DOI] [PubMed] [Google Scholar]
- Domschke K, Freitag CM, Kuhlenbaumer G, Schirmacher A, Sand P, Nyhuis P, et al. Association of the functional V158M catechol-O-methyl-transferase polymorphism with panic disorder in women. Int J Neuropsychopharmacol. 2004;7:183–188. doi: 10.1017/S146114570400416X. [DOI] [PubMed] [Google Scholar]
- Domschke K, Hohoff C, Jacob C, Maier W, Fritze J, Bandelow B, et al. Chromosome 4q31–34 panic disorder risk locus: association of neuropeptide Y Y5 receptor variants. Am J Med Genet B Neuropsychiatr Genet. 2008a;147B:510–516. doi: 10.1002/ajmg.b.30629. [DOI] [PubMed] [Google Scholar]
- Domschke K, Hohoff C, Mortensen LS, Roehrs T, Deckert J, Arolt V, et al. MAO-A variant influences antidepressant treatment response in female patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:224–228. doi: 10.1016/j.pnpbp.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Domschke K, Ohrmann P, Braun M, Suslow T, Bauer J, Hohoff C, et al. Influence of the catechol-O-methyltransferase val158met genotype on amygdala and prefrontal cortex emotional processing in panic disorder. Psychiatry Res. 2008b;163:13–20. doi: 10.1016/j.pscychresns.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Duan J, Wainwright MS, Comeron JM, Saitou N, Sanders AR, Gelernter J, et al. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum Mol Genet. 2003;12:205–216. doi: 10.1093/hmg/ddg055. [DOI] [PubMed] [Google Scholar]
- Etkin A. Functional neuroanatomy of anxiety: a neural circuit perspective. Curr Top Behav Neurosci. 2010;2:251–277. doi: 10.1007/7854_2009_5. [DOI] [PubMed] [Google Scholar]
- Evans SM, Griffiths RR. Dose-related caffeine discrimination in normal volunteers: individual differences in subjective effects and self-reported cues. Behav Pharmacol. 1991;2:345–356. [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM. The psychological significance of human startle eyeblink modification: a review. Biol Psychol. 1998;47:1–43. doi: 10.1016/s0301-0511(97)00020-3. [DOI] [PubMed] [Google Scholar]
- Fisher S, Guillet R. Neonatal caffeine alters passive avoidance retention in rats in an age- and gender-related manner. Brain Res Dev Brain Res. 1997;98:145–149. doi: 10.1016/s0165-3806(96)00158-7. [DOI] [PubMed] [Google Scholar]
- Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag CM, Agelopoulos K, Huy E, Rothermundt M, Krakowitzky P, Meyer J, et al. Adenosine A(2A) receptor gene (ADORA2A) variants may increase autistic symptoms and anxiety in autism spectrum disorder. Eur Child Adolesc Psychiatry. 2010;19:67–74. doi: 10.1007/s00787-009-0043-6. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Ferre S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Giakoumaki SG, Roussos P, Bitsios P. Improvement of prepulse inhibition and executive function by the COMT inhibitor tolcapone depends on COMT Val158Met polymorphism. Neuropsychopharmacology. 2008;33:3058–3068. doi: 10.1038/npp.2008.82. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Woodson PP. Caffeine physical dependence: a review of human and laboratory animal studies. Psychopharmacology (Berl) 1988;94:437–451. doi: 10.1007/BF00212836. [DOI] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biol Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology (Berl) 2008;199:421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Goddard A, Woods SW, Davis M. Baseline and fear-potentiated startle in panic disorder patients. Biol Psychiatry. 1994;35:431–439. doi: 10.1016/0006-3223(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, III, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biol Psychiatry. 1998;44:1027–1036. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Hamilton SP, Slager SL, De Leon AB, Heiman GA, Klein DF, Hodge SE, et al. Evidence for genetic linkage between a polymorphism in the adenosine 2A receptor and panic disorder. Neuropsychopharmacology. 2004;29:558–565. doi: 10.1038/sj.npp.1300311. [DOI] [PubMed] [Google Scholar]
- Hart P, Farrell GC, Cooksley WG, Powell LW. Enhanced drug metabolism in cigarette smokers. Br Med J. 1976;2:147–149. doi: 10.1136/bmj.2.6028.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk LW, Jr, Kowmas AD. Affective modulation and prepulse inhibition of startle among undergraduates high and low in behavioral inhibition and approach. Psychophysiology. 2003;40:131–138. doi: 10.1111/1469-8986.00013. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Hohoff C, Domschke K, Schwarte K, Spellmeyer G, Vogele C, Hetzel G, et al. Sympathetic activity relates to adenosine A(2A) receptor gene variation in blood-injury phobia. J Neural Transm. 2009;116:659–662. doi: 10.1007/s00702-008-0089-5. [DOI] [PubMed] [Google Scholar]
- Hohoff C, McDonald JM, Baune BT, Cook EH, Deckert J, de Wit H. Interindividual variation in anxiety response to amphetamine: possible role for adenosine A2A receptor gene variants. Am J Med Genet B Neuropsychiatr Genet. 2005;139B:42–44. doi: 10.1002/ajmg.b.30228. [DOI] [PubMed] [Google Scholar]
- Hohoff C, Mullings EL, Heatherley SV, Freitag CM, Neumann LC, Domschke K, et al. Adenosine A(2A) receptor gene: evidence for association of risk variants with panic disorder and anxious personality. J Psychiatr Res. 2010;44:930–937. doi: 10.1016/j.jpsychires.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, et al. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- Jacob C, Domschke K, Gajewska A, Warrings B, Deckert J. Genetics of panic disorder: focus on association studies and therapeutic perspectives. Expert Rev Neurother. 2010;10:1273–1284. doi: 10.1586/ern.10.76. [DOI] [PubMed] [Google Scholar]
- Jang KL, Stein MB, Taylor S, Livesley WJ. Gender differences in the etiology of anxiety sensitivity: a twin study. J Gend Specif Med. 1999;2:39–44. [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Caffeine intake, tolerance, and withdrawal in women: a population-based twin study. Am J Psychiatry. 1999;156:223–228. doi: 10.1176/ajp.156.2.223. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Lam P, Hong CJ, Tsai SJ. Association study of A2a adenosine receptor genetic polymorphism in panic disorder. Neurosci Lett. 2005;378:98–101. doi: 10.1016/j.neulet.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Lang PJ.1980Behavioral treatment and bio-behavioral assessment: computer applicationsIn: Sidowski JB, Johnson JH, Williams TA (eds).Technology in Mental Health Care Delivery Systems Ablex: Norwood, NJ; 119–l137. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. University of Florida: Gainesville; 2005. International affective picture system (IAPS): affective ratings of pictures and instruction manual. [Google Scholar]
- Lang PJ, Cuthbert BN. Affective information processing and the assessment of anxiety. J Behav Assess. 1984;6:369–395. doi: 10.1007/BF01321326. [DOI] [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, et al. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- Lee H, Yeom H, Kim YG, Yoon CN, Jin C, Choi JS, et al. Structure-related inhibition of human hepatic caffeine N3-demethylation by naturally occurring flavonoids. Biochem Pharmacol. 1998;55:1369–1375. doi: 10.1016/s0006-2952(97)00644-8. [DOI] [PubMed] [Google Scholar]
- Mathews A. Anxiety and the processing of emotional information. Prog Exp Pers Psychopathol Res. 1993;16:254–280. [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. Profile of Mood States Manual (Rev) Educational and Industrial Testing Service: San Diego; 1992. [Google Scholar]
- Mühlberger A, Bulthoff HH, Wiedemann G, Pauli P. Virtual reality for the psychophysiological assessment of phobic fear: responses during virtual tunnel driving. Psychol Assess. 2007;19:340–346. doi: 10.1037/1040-3590.19.3.340. [DOI] [PubMed] [Google Scholar]
- Mühlberger A, Wiedemann G, Herrmann MJ, Pauli P. Phylo- and ontogenetic fears and the expectation of danger: differences between spider- and flight-phobic subjects in cognitive and physiological responses to disorder-specific stimuli. J Abnorm Psychol. 2006;115:580–589. doi: 10.1037/0021-843X.115.3.580. [DOI] [PubMed] [Google Scholar]
- Mühlberger A, Wieser MJ, Pauli P. Darkness-enhanced startle responses in ecologically valid environments: a virtual tunnel driving experiment. Biol Psychol. 2008;77:47–52. doi: 10.1016/j.biopsycho.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Nardi AE, Lopes FL, Freire RC, Veras AB, Nascimento I, Valenca AM, et al. Panic disorder and social anxiety disorder subtypes in a caffeine challenge test. Psychiatry Res. 2009;169:149–153. doi: 10.1016/j.psychres.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Nardi AE, Lopes FL, Valenca AM, Freire RC, Veras AB, de-Melo-Neto VL, et al. Caffeine challenge test in panic disorder and depression with panic attacks. Compr Psychiatry. 2007;48:257–263. doi: 10.1016/j.comppsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Nardi AE, Valenca AM, Nascimento I, Freire RC, Veras AB, de-Melo-Neto VL, et al. A caffeine challenge test in panic disorder patients, their healthy first-degree relatives, and healthy controls. Depress Anxiety. 2008;25:847–853. doi: 10.1002/da.20354. [DOI] [PubMed] [Google Scholar]
- Nickell PV, Uhde TW. Dose–response effects of intravenous caffeine in normal volunteers. Anxiety. 1994;1:161–168. doi: 10.1002/anxi.3070010403. [DOI] [PubMed] [Google Scholar]
- Nillni YI, Toufexis DJ, Rohan KJ. Anxiety sensitivity, the menstrual cycle, and panic disorder: a putative neuroendocrine and psychological interaction. Clin Psychol Rev. 2011;31:1183–1191. doi: 10.1016/j.cpr.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noschang CG, Krolow R, Pettenuzzo LF, Avila MC, Fachin A, Arcego D, et al. Interactions between chronic stress and chronic consumption of caffeine on the enzymatic antioxidant system. Neurochem Res. 2009;34:1568–1574. doi: 10.1007/s11064-009-9945-4. [DOI] [PubMed] [Google Scholar]
- Pauli P, Conzelmann A, Mucha RF, Weyers P, Baehne CG, Fallgatter AJ, et al. Affect-modulated startle reflex and dopamine D4 receptor gene variation. Psychophysiology. 2010;47:25–33. doi: 10.1111/j.1469-8986.2009.00923.x. [DOI] [PubMed] [Google Scholar]
- Pauli P, Montoya P, Martz GE. Covariation bias in panic-prone individuals. J Abnorm Psychol. 1996;105:658–662. doi: 10.1037//0021-843x.105.4.658. [DOI] [PubMed] [Google Scholar]
- Peterson RA, Reiss S.1992Anxiety Sensitivity Index Manual International Diagnostic Systems: Worthington, OH; vol. 2. [Google Scholar]
- Powell KR, Koppelman LF, Holtzman SG. Differential involvement of dopamine in mediating the discriminative stimulus effects of low and high doses of caffeine in rats. Behav Pharmacol. 1999;10:707–716. doi: 10.1097/00008877-199912000-00001. [DOI] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Retey JV, Adam M, Khatami R, Luhmann UF, Jung HH, Berger W, et al. A genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin Pharmacol Ther. 2007;81:692–698. doi: 10.1038/sj.clpt.6100102. [DOI] [PubMed] [Google Scholar]
- Rogers PJ, Hohoff C, Heatherley SV, Mullings EL, Maxfield PJ, Evershed RP, et al. Association of the anxiogenic and alerting effects of caffeine with ADORA2A and ADORA1 polymorphisms and habitual level of caffeine consumption. Neuropsychopharmacology. 2010;35:1973–1983. doi: 10.1038/npp.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB, Donley MP. Animal studies of amygdala function in fear and uncertainty: relevance to human research. Biol Psychol. 2006;73:49–60. doi: 10.1016/j.biopsycho.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Schicatano EJ, Blumenthal TD. The effects of different doses of caffeine on habituation of the human acoustic startle reflex. Pharmacol Biochem Behav. 1995;52:231–236. doi: 10.1016/0091-3057(95)00110-i. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. 1998The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10 J Clin Psychiatry 59(Suppl 2022–33.quiz 34–57. [PubMed] [Google Scholar]
- Smith GA. Caffeine reduction as an adjunct to anxiety management. Br J Clin Psychol. 1988;27:265–266. doi: 10.1111/j.2044-8260.1988.tb00785.x. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Davis M. The effect of gonadal hormones and gender on anxiety and emotional learning. Horm Behav. 2006;50:539–549. doi: 10.1016/j.yhbeh.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Ushiroyama T, Okamoto Y, Toyoda K, Sugimoto O. A case of panic disorder induced by oral contraceptive. Acta Obstet Gynecol Scand. 1992;71:78–80. doi: 10.3109/00016349209007956. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, et al. The cross-national epidemiology of panic disorder. Arch Gen Psychiatry. 1997;54:305–309. doi: 10.1001/archpsyc.1997.01830160021003. [DOI] [PubMed] [Google Scholar]
- Wiedemann G, Pauli P, Dengler W, Lutzenberger W, Birbaumer N, Buchkremer G. Frontal brain asymmetry as a biological substrate of emotions in patients with panic disorders. Arch Gen Psychiatry. 1999;56:78–84. doi: 10.1001/archpsyc.56.1.78. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Jacobi F. Size and burden of mental disorders in Europe—a critical review and appraisal of 27 studies. Eur Neuropsychopharmacol. 2005;15:357–376. doi: 10.1016/j.euroneuro.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Yamada K, Hattori E, Shimizu M, Sugaya A, Shibuya H, Yoshikawa T. Association studies of the cholecystokinin B receptor and A2a adenosine receptor genes in panic disorder. J Neural Transm. 2001;108:837–848. doi: 10.1007/s007020170033. [DOI] [PubMed] [Google Scholar]
- Yang A, Palmer AA, de Wit H. Genetics of caffeine consumption and responses to caffeine. Psychopharmacology (Berl) 2010;211:245–257. doi: 10.1007/s00213-010-1900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KL, Zinbarg RE. Threat is in the eye of the beholder: social anxiety and the interpretation of ambiguous facial expressions. Behav Res Ther. 2007;45:839–847. doi: 10.1016/j.brat.2006.05.004. [DOI] [PubMed] [Google Scholar]