Abstract
Alterations of the serotonergic system are involved in the pathophysiology of mood disorders and represent an important target for its pharmacological treatment. Genetic deletion of the serotonin transporter (SERT) in rodents leads to an anxious and depressive phenotype, and is associated with reduced neuronal plasticity as indicated by decreased brain-derived neurotrophic factor (Bdnf) expression levels. One of the transcription factors regulating Bdnf is the neuronal PAS domain protein 4 (Npas4), which regulates activity-dependent genes and neuroprotection, and has a critical role in the development of GABA synapses. On the basis of these premises, we investigated the expression of Npas4 and GABAergic markers in the hippocampus and prefrontal cortex of homozygous (SERT−/−) and heterozygous (SERT+/−) knockout rats, and analyzed the effect of long-term duloxetine treatment on the expression of these targets. We found that Npas4 expression was reduced in both the brain structures of adult SERT+/− and SERT−/− animals. This effect was already present in adolescent SERT−/−, and could be mimicked by prenatal exposure to the antidepressant fluoxetine. Moreover, SERT−/− rats showed a strong impairment of the GABAergic system, as indicated by the reduction of several markers, including the vesicular transporter (Vgat), glutamic acid decarboxylase-67 (Gad67), the receptor subunit GABA A receptor, gamma 2 (GABAA-γ2), and calcium-binding proteins that label subgroups of the GABAergic neurons. Interestingly, chronic treatment with the antidepressant duloxetine was able to restore the physiological levels of Npas4 and GABAergic markers in SERT−/− rats, although some differences in the modulation of GABAergic genes exist between hippocampus and prefrontal cortex. Our results demonstrate that SERT knockout rats, an animal model of mood disorders, have reduced Npas4 expression that correlates with decreased expression of Bdnf exon I and IV. These changes lead to an impairment of the GABAergic system that may contribute to the anxious and depressive phenotype associated with inherited SERT downregulation.
Keywords: depression, duloxetine, SERT, transcription factors, GABA, development
INTRODUCTION
It is widely accepted that alterations in the serotonergic system are involved in the pathophysiology and treatment of mood disorders (Neumeister et al, 2002). Indeed, serotonin (5-HT) transmission is implicated both in the onset of depression and in the mechanism of action of several antidepressant drugs (Jans et al, 2007). Moreover, findings from genetical and pharmacological studies indicate that serotonin signaling during early life is critically involved in the development of brain circuits that modulate adult emotional behavior (Ansorge et al, 2008; Ansorge et al, 2004).
Gene variants of the 5-HT system have been associated directly to depression and may enhance disease susceptibility, following interaction with stressful life events (Caspi et al, 2010; Caspi et al, 2003; Karg et al, 2011; Munafo et al, 2009). In this context, the 5-HT transporter (5-HTT in humans; serotonin transporter (SERT) in rodents) is particularly relevant, due to the presence of a human functional polymorphism within its promoter region that modulates the susceptibility to different neuropsychiatric disorders, and that may also affect antidepressant response (Caspi et al, 2003; Huezo-Diaz et al, 2009; Serretti et al, 2007; Uher and McGuffin, 2008). As the SERT polymorphism is not present in rodents (Caspi et al, 2010), its role has been extensively investigated using animal models with a genetic deletion of the transporter, a manipulation that leads to an anxious and depressive phenotype (Homberg and Lesch, 2011; Kalueff et al, 2010; Murphy and Lesch, 2008; Olivier et al, 2008). Accordingly, we used target-directed mutagenesis to generate SERT-knockout rats (Smits et al, 2006), which present an impaired serotonergic system and are characterized by anxiety- and depression-like behavioral alterations (Homberg et al, 2007; Kalueff et al, 2010; Olivier et al, 2008). We have previously demonstrated that SERT knockout (SERT−/−) rats have altered neuronal plasticity, as indicated by the reduction of activity-regulated cytoskeleton associated protein (Arc) and of brain-derived neurotrophic factor (Bdnf) expression levels in the hippocampus and prefrontal cortex (Molteni et al, 2009b; Molteni et al, 2010). Interestingly, long-term treatment with the antidepressant duloxetine was able to normalize the Bdnf expression in both the brain regions (Calabrese et al, 2010).
The Bdnf gene has a very complex structure that gives rise to multiple transcripts, which are regulated through the cooperation and interaction of different transcription factors (Aid et al, 2007; Greer and Greenberg, 2008; Pruunsild et al, 2011). Among them is the neuronal PAS domain protein 4 (Npas4), which specifically controls the activity-dependent Bdnf mRNA levels by acting on promoters I and IV. Indeed, the Bdnf expression is reduced by almost two-fold in cultures expressing Npas4-RNA interference, when compared with control cultures (Lin et al, 2008). Npas4 belongs to the basic helix-loop-helix-PAS transcription factors family, which is involved in functional regulation of neurons (Ooe et al, 2009), in the adaptation of cells to external stimuli, such as environmental stress (Gu et al, 2000), and in neuroprotection (Ooe et al, 2009). Npas4 is expressed predominantly in excitatory neurons and is selectively induced by Ca2+ influx. Recently, Npas4 has been shown to control GABAergic synapse development through a program of activity-dependent gene development; in particular, Npas4-RNA interference reduced the density of GABA A receptor, gamma 2 (GABAA-γ2) and GABA-β2/3 receptors, and of GABA-producing enzymes glutamic acid decarboxylase (Gad)65 and Gad67 (Lin et al, 2008).
Several clinical and preclinical studies support a central and causal role of the GABAergic system in the etiology of depressive disorders (Luscher et al, 2011; Sanacora et al, 1999). This led us to hypothesize that the transcription factor Npas4 may be altered in animal models of depression, and may eventually link alterations of neuroplastic markers, such as Bdnf, with an impaired function of the GABAergic system. On this basis, we investigated Npas4 expression in the hippocampus and prefrontal cortex of SERT+/− and SERT−/− rats, which model depression- and anxiety-related dysfunctions in humans, and we analyzed the expression of different GABAergic markers that may represent downstream targets of Npas4 and Bdnf signaling (Lin et al, 2008; Sakata et al, 2010). Moreover, we investigated the ability of long-term treatment with the serotonin–norepinephrine reuptake inhibitor (SNRI) duloxetine to normalize molecular defects associated with genetic deletion of SERT.
MATERIALS AND METHODS
General reagents were purchased from Sigma–Aldrich (Milan, Italy), and molecular biology reagents were obtained from Applied Biosystem Italia (Monza, Italy), Bio-Rad Laboratories S.r.l. Italia (Segrate, Italy), Eurofins MWG-Operon (Ebersberg, Germany), Tebu-bio (Magenta, Italy), GE Healthcare Europe GmbH (Pero, Italy).
Animals And Pharmacological Treatments
Serotonin transporter-knockout rats
SERT-knockout rats (Slc6a41Hubr) were generated by ENU-induced mutagenesis (Smits et al, 2006). All subjects were bred and reared in the Central Animal Laboratory of the Radboud University Nijmegen Medical Centre in Nijmegen, The Netherlands. Experimental animals were derived from crossing heterozygous (SERT+/−) knockout rats that were out crossed for eight generations. After weaning at the age of 21 days, ear cuts were taken for genotyping. Animals were supplied with food and water ad libitum and were kept on a 12 h : 12 h dark–light cycle (lights on at 0600 h).
For basal analysis, a cohort of SERT+/+ and SERT−/− rats, and their wild-type controls (SERT+/+), were killed around 1100 h at postnatal day (PND) 35, whereas another cohort of SERT+/+, SERT+/− and SERT−/− rats were killed at adulthood (∼PND 100).
Drug treatments
Fluoxetine administration during gestation in wild-type rats
To establish the contribution of SERT during early life in the modulation of Npas4, pregnant Wistar rats (Harlan Laboratories, Horst, The Netherlands) were daily orally treated with methylcellulose or fluoxetine (12 mg/kg) from gestation day 11 until birth. Animals were weaned at PND 22, and they were killed at 3 months of age.
Fluoxetine administration at adulthood in wild-type rats
A group of Wistar rats were treated at adulthood with methylcellulose (by gavage) or fluoxetine (12 mg/kg by gavage) for 3 weeks and were killed 24 h after the last injection.
Duloxetine treatment in SERT+/+, SERT+/− and SERT−/− rats
Adult SERT+/+ and SERT−/− rats were treated chronically (21 days) with saline (by gavage) or duloxetine (10 mg/kg by gavage) and killed 24 h after the last injection.
Brain regions of interest (hippocampus, prefrontal cortex) were rapidly dissected. Prefrontal cortex (defined as Cg1, Cg3, and IL sub-regions corresponding to the plates 6 to 10 according to the atlas of Paxinos and Watson (1996)) was dissected from 2-mm thick slices, whereas hippocampus was dissected from the whole brain. The brain specimens were frozen on dry ice and stored at −80 °C for further analysis. All experiments were carried out in accordance with the guidelines laid down by the European Communities Council Directive of 24 November 1986 (86/609/EEC).
RNA Preparation And Gene Expression Analysis By Quantitative Real-Time PCR
Total RNA was isolated by a single step of guanidinium isothiocyanate/phenol extraction, using PureZol RNA isolation reagent (Bio-Rad Laboratories s.r.l. Italia) according to the manufacturer's instructions, and quantified by spectrophotometric analysis. Following total RNA extraction, the samples were processed for real-time PCR to assess Npas4, aryl hydrocarbon receptor nuclear translocator 2 (Arnt2), vesicular GABA transporter (Vgat), Gad67, GABAA-γ2, parvalbumin (Pvalb), calbindin (Calb1) mRNA levels. An aliquot of each sample was treated with DNase to avoid DNA contamination. RNA was analyzed by TaqMan qRT–PCR instrument (CFX384 real-time system, Bio-Rad Laboratories) using the iScript one-step RT–PCR kit for probes (Bio-Rad Laboratories). Samples were run in 384-well formats in triplicates as multiplexed reactions with a normalizing internal control (36B4). Probe and primer sequences used (Table 1) were purchased from Eurofins MWG-Operon.
Table 1. Sequences of Forward and Reverse Primers Used in Real-Time PCR Analysis.
| Gene | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| Npas4 | 5′-GGAAGTTGCTATACCTGTCGG-3′ | 5′-GTCGTAAATACTGTCACCCTGG-3′ | 5′-CATAGAATGGCCCAGATGCTCGCT-3′ |
| Arnt2 | 5′-AAGTGCTGTCGGTCATGTAC-3′ | 5′-GCTGAAGTTGCTTGACGTTG-3′ | 5′-AGCTTCACCTTCCAGAACCCCTACT-3′ |
| GABAAγ2 | 5′-ACTCATTGTGGTTCTGTCCTG-3′ | 5′-GCTGTGACATAGGAGACCTTG-3′ | 5′-ATGGTGCTGAGAGTGGTCATCGTC-3′ |
| Gad67 | 5′-ATACTTGGTGTGGCGTAGC-3′ | 5′-AGGAAAGCAGGTTCTTGGAG-3′ | 5′-AAAACTGGGCCTGAAGATCTGTGGT-3′ |
| Vgat | 5′-ACGACAAACCCAAGATCACG-3′ | 5′-GTAGACCCAGCACGAACATG-3′ | 5′-TTCCAGCCCGCTTCCCACG-3′ |
| Pvalb | 5′-CTGGACAAAGACAAAAGTGGC-3′ | 5′-GACAAGTCTCTGGCATCTGAG-3′ | 5′-CCTTCAGAATGGACCCCAGCTCA-3′ |
| Calb1 | 5′-AGAACTTGATCCAGGAGCTTC-3′ | 5′-CTTCGGTGGGTAAGACATGG-3′ | 5′-TGGGCAGAGAGATGATGGGAAAATAGGA-3′ |
| 36B4 | 5′-TTCCCACTGGCTGAAAAGGT-3′ | 5′-CGCAGCCGCAAATGC-3′ | 5′-AAGGCCTTCCTGGCCGATCCATC-3′ |
Abbreviations: Arnt2, aryl hydrocarbon receptor nuclear translocator 2; Calb1, calbindin; GABAA-γ2, GABA A receptor, gamma 2; Gad67, glutamic-acid decarboxylase; Npas4, neuronal PAS domain protein 4; Pvalb, Parvalbumin; Vgat, vesicular GABA transporter.
Thermal cycling was initiated with incubation at 50 °C for 10 min (RNA retrotranscription), and then at 95 °C for 5 min (TaqMan polymerase activation). After this initial step, 39 cycles of PCR were performed. Each PCR cycle consisted of heating the samples at 95 °C for 10 s to enable the melting process, and then for 30 s at 60 °C for the annealing and extension reactions. A comparative cycle threshold (Ct) method was used to calculate the relative target gene expression.
Statistical Analyses
All the analyses were carried out in individual animals (independent determinations)
The effect of genotype and/or antidepressant treatment on mRNA levels was analyzed with a two-way analysis of variance (ANOVA) followed by single contrast post-hoc test (SCPHT), or with one-way ANOVA, followed by Fisher's protected least significant difference post-hoc test or Student's t-test. Pearson product moment correlations (r) between levels of Npas4 mRNA and Bdnf exon I, Bdnf exon IV, and GABAA-γ2 mRNAs were performed to evaluate the correlation between the expression levels of all the genes in single animals. Significance for all tests was assumed for p<0.05. Data are presented as means±SEM.
RESULTS
Disruption of SERT Affects Npas4 mRNA Expression Levels
Npas4 is a brain-specific transcription factor with neuroprotective properties (Ooe et al, 2009) involved in the formation and/or the maintenance of GABAergic synapses on excitatory neurons (Lin et al, 2008). As clinical and preclinical evidence suggest that GABAergic deficits may be relevant for the etiology and manifestation of mood disorders (Luscher et al, 2011), we decided to investigate the expression of Npas4 in the hippocampus of SERT+/− and SERT−/− rats, and their controls, SERT+/+ rats. The SERT-knockout rat represents an animal model for anxiety and depression (Olivier et al, 2008), and thereby offers an important tool to investigate the mechanisms underlying the pathological condition in humans.
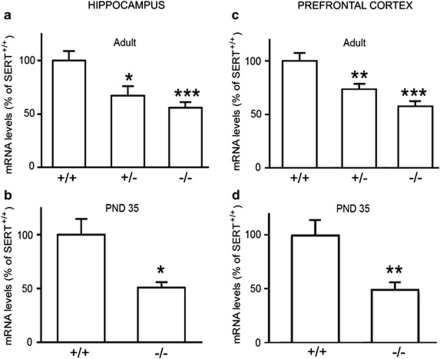
As shown in Figure 1, we found that the expression of the transcription factor Npas4 was significantly reduced in the hippocampus and prefrontal cortex of adult SERT−/− (−44%, p<0.001; −42%, p<0.001, respectively), as well as of SERT+/− rats (−33%, p<0.05; −26%, p<0.01, respectively), when compared with SERT+/+ rats (one-way ANOVA with Fisher's protected least significant difference test; Figures 1a–c). Npas4 mRNA levels were already reduced in both the brain regions of SERT−/− rats at puberty (PND 35, −49%, p<0.05 in hippocampus; −51%, p<0.01 in prefrontal cortex; Student's t–test; Figures 1b–d), suggesting that the reduced expression of the transcription factor in SERT-knockout rats may be due to impaired SERT function during development.
Figure 1.
The gene expression of neuronal PAS domain protein 4 (Npas4) is altered in rat hippocampus and prefrontal cortex of serotonin transporter (SERT) mutant rats. Npas4 mRNA levels were measured in the hippocampus (a, b) and prefrontal cortex (c, d) of adult (a, c) and adolescent (b, d) SERT mutant rats (heterozygous, SERT+/−; and homozygous, SERT−/−), as compared with their wild-type (SERT+/+) counterparts. The data, expressed as a percentage of SERT+/+ animals (set at 100%), are the mean±SEM of at least seven independent determinations. *p<0.05, **p<0.01, ***p<0.001 vs SERT+/+ rats (one-way analysis of variance (ANOVA) with Fischer's protected least significant difference for adult rats; Student t-test for postnatal day (PND) 35).
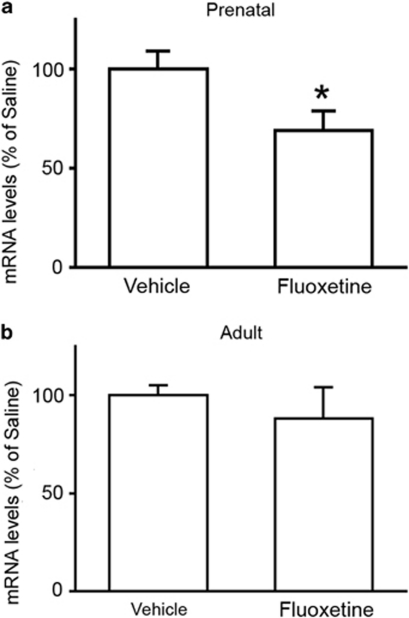
To confirm this possibility, we chronically treated pregnant rats, starting from embryonic day 11, with the antidepressant fluoxetine to mimic the lack of SERT in developing fetuses. We found that the Npas4 gene expression was significantly decreased in animals that were exposed to chronic fluoxetine during gestation (−31%, p<0.05; Student's t–test; Figure 2). Conversely, when we treated adult rats with the SSRI antidepressant, we did not observe any significant change in the mRNA levels of the transcription factor (−12%, p>0.05; Student's t–test; Figure 2). This implies that altered expression of Npas4 in SERT-knockout rats does not originate from the lack of SERT in adulthood, but is probably due to its impaired function during fetal life.
Figure 2.
Fluoxetine treatment during gestation, but not at adulthood, influences neuronal PAS domain protein 4 (Npas4) gene expression in the hippocampus of adult rats. Npas4 mRNA levels were measured in the hippocampus of adult rats that were treated chronically with fluoxetine during gestation (a) or at adulthood (b). The data, expressed as a percentage of animals treated with vehicle (set at 100%), are the mean±SEM from at least seven independent determinations. *p<0.05 vs vehicle-treated rats (Student's t-test).
Effect of Chronic Duloxetine Treatment on Npas4 and Arnt2 mRNA Levels in SERT−/− Rats
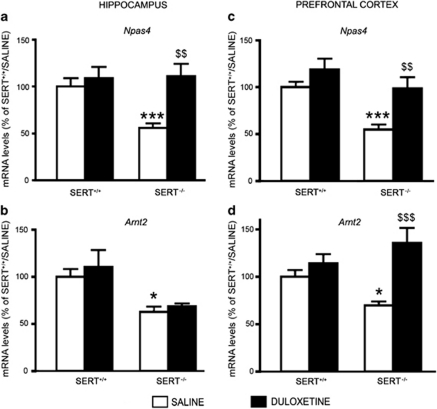
As SERT-knockout rats display anxiety- and depressive-like behaviors (Kalueff et al, 2010; Olivier et al, 2008), we tested whether antidepressant treatment may restore the expression of Npas4 in these animals. Therefore, we chronically treated adult SERT−/− rats with the SNRI duloxetine, (Carter and McCormack, 2009), and measured Npas4 mRNA levels in the hippocampus and prefrontal cortex. In the hippocampus, we found a significant drug treatment effect (F1,36=9.806, p<0.01) and a significant genotype-X drug-treatment interaction (F1,36=4.941, p<0.05). Although duloxetine did not change the Npas4 expression in wild-type animals (+9%, p>0.05), long-term administration of the antidepressant was able to normalize the reduced Npas4 mRNA levels observed in SERT−/− rats (+98% vs SERT−/− treated with saline, p<0.01; two-way ANOVA with SCPHT; Figure 3a). In the prefrontal cortex, we found a significant drug treatment effect (F1,42=13.645, p<0.001). Chronic duloxetine treatment normalized the Npas4 reduction in SERT−/− rats (+69% vs SERT−/− treated with vehicle, p<0.01), without affecting its levels in wild-type animals (+19%, p>0.05; Figure 3c).
Figure 3.
Chronic duloxetine treatment modulates the levels of neuronal PAS domain protein 4 (Npas4) and aryl hydrocarbon receptor nuclear translocator 2 (Arnt2) gene expression in serotonin transporter (SERT)−/− rats. Npas4 and Arnt2 mRNA levels were measured in the hippocampus (a, b) and in prefrontal cortex (c, d) of wild-type (SERT+/+), and of SERT−/− rats treated for 21 days with saline or duloxetine, and killed 24 h after the last injection. The data, expressed as a percentage of SERT+/+/saline (set at 100%), are the mean±SEM from at least five independent determinations. *p<0.05, ***p<0.001 vs SERT+/+/saline; $$p<0.01, $$$p<0.001 vs SERT−/−/saline (two-way analysis of variance (ANOVA) with single contrast post-hoc test (SCPHT)).
To further the analysis of the transcription factors that may contribute to the Bdnf changes present in SERT−/− rats, we investigated the expression of Arnt2, a member of the Arnt family, that specifically cooperate with Npas4 in the transcriptional control of Bdnf exons (Ooe et al, 2009; Pruunsild et al, 2011). In the hippocampus, we found a significant genotype effect (F1,24=13.696, p<0.01) as demonstrated by the reduction of Arnt2 mRNA levels in SERT−/− rats (−37%, p<0.05; two-way ANOVA with SCPHT), although duloxetine treatment was not able to modulate the Arnt2 expression in wild-type, as well as in SERT−/− rats (Figure 3b). In the prefrontal cortex, we found a significant drug treatment effect (F1,41=15.955, p<0.001) and a significant genotype-X drug-treatment interaction (F1,41=6.632, p<0.05). In fact, SERT−/− rats displayed a significant reduction of Arnt2 mRNA levels (−30%, p<0.05), which were significantly upregulated by chronic antidepressant treatment (+94% vs SERT−/− treated with vehicle, p<0.001; Figure 3d).
The Reduced Npas4 mRNA Levels are Associated with Impaired Expression of GABAergic Markers in SERT−/− Rats
Given that Npas4 expression turns on a program of gene expression that triggers the formation and/or maintenance of inhibitory synapses (Lin et al, 2008), we investigated the expression of three key elements of the GABAergic synapses, the Vgat, the GABA-producing enzyme Gad67, and the postsynaptic GABAA-γ2, in the hippocampus and prefrontal cortex of SERT−/− rats under basal conditions, as well as after antidepressant treatment.
We found a significant decrease of Vgat expression levels in the hippocampus (−37%, p<0.05; two-way ANOVA with SCPHT), but not in the prefrontal cortex of SERT−/− rats (Figures 4a–d). Chronic duloxetine treatment was able to restore the physiological expression of Vgat in the hippocampus (+52% vs SERT−/− treated with vehicle, p<0.05; two-way ANOVA with SCPHT; Figure 4a) as demonstrated by the significant genotype-X drug-treatment interaction (F1,25=5.046, p<0.05), without affecting its levels in the prefrontal cortex (Figure 4d).
Figure 4.
Serotonin transporter (SERT)−/− rats show impaired expression of GABAergic markers, modulation by chronic duloxetine treatment. Vesicular transporter (Vgat; a, d), glutamic acid decarboxylase-67 (Gad67; b, e), and GABA A receptor, gamma 2 (GABAA-γ2; c, f) mRNA levels were measured in the hippocampus (a–c), and prefrontal cortex (d–f) of SERT+/+ and SERT−/− rats treated for 21 days with saline or duloxetine, and killed 24 h after the last injection. The data, expressed as a percentage of SERT+/+/saline (set at 100%), are the mean±SEM from at least six independent determinations. *p<0.05, **p<0.01 vs SERT+/+/saline; $p<0.05, $$$p<0.001 vs SERT−/−/saline (two-way analysis of variance (ANOVA) with single contrast post-hoc test (SCPHT)).
Gad67 expression levels were significantly reduced in the hippocampus of SERT−/− rats (−29%, p<0.05). Chronic duloxetine treatment had a genotype-specific effect on Gad67, as confirmed by the significant genotype-X drug-treatment interaction (F1,26=6.382, p<0.05). Indeed, chronic duloxetine treatment in SERT−/− rats was able to normalize the reduction of Gad67 to control levels (+31% vs SERT−/− treated with vehicle, p<0.05), without affecting its expression in SERT+/+ rats (−14%, p>0.05; Figure 4b). The expression of Gad67 was also reduced in the prefrontal cortex (−18%, p<0.05) as demonstrated by the significant genotype effect (F1,40=9.690, p<0.01). However, differently from the hippocampus, duloxetine treatment was not able to normalize the Gad67 expression in the prefrontal cortex of SERT−/− rats (−4% vs SERT−/− treated with vehicle, p>0.05; Figure 4e).
To have an indication of inhibitory synapse number in the mutant rat, we also investigated the expression of the GABAA-γ2-receptor subunit. In the hippocampus, the receptor expression levels were significantly reduced in SERT−/− rats (−38%, p<0.05) and normalized by long-term antidepressant treatment (+80% vs SERT−/− treated with vehicle, p<0.05; two-way ANOVA with SCPHT; Figure 4c). The effect of duloxetine was limited to SERT−/− rats, as confirmed by the significant genotype-X drug-treatment interaction (F1,26=8.774, p<0.01). In prefrontal cortex, we found a genotype specific effect (F1,41=5.996, p<0.05) and a significant drug treatment effect (F1,41=20.7971, p<0.001). Indeed SERT−/− rats showed a reduction of GABAA-γ2 mRNA levels (−35%, p<0.05), whereas duloxetine treatment was able to normalize this deficit (+91% vs SERT−/− treated with vehicle, p<0.001) and to increase the receptor mRNA levels also in wild-type animals (+45%, p<0.05; two-way ANOVA with SCPHT; Figure 4f).
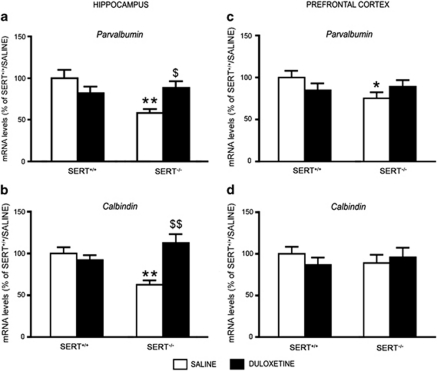
The impairment of the GABAergic system in SERT-knockout rats was also associated with a dysfunction of two calcium-binding proteins, parvalbumin and calbindin, which label subgroups of GABAergic interneurons in the hippocampus and prefrontal cortex (Schwaller et al, 2002).
Parvalbumin expression was significantly decreased in hippocampus (−42%, p<0.01), and to a less extent in the prefrontal cortex (−25%, p<0.05) of SERT−/− rats. Duloxetine had a different impact on its expression in the two brain regions, as chronic antidepressant treatment in SERT−/− rats had a significant effect in the hippocampus (F1,26=8.653, p<0.01), where parvalbumin levels were restored to its physiological levels (+52% vs SERT−/− treated with vehicle, p<0.05; Figure 5a), but not in prefrontal cortex (+19% vs SERT−/− treated with vehicle, p>0.05; two-way ANOVA with SCPHT; Figure 5c). With respect to calbindin, its expression was significantly decreased in the hippocampus (−37%, p<0.01), but not in the prefrontal cortex of SERT−/− rats. Chronic treatment with the antidepressant duloxetine was able to restore the physiological expression of calbindin in hippocampus (+79% vs SERT−/− treated with vehicle, p<0.05; two-way ANOVA with SCPHT; Figure 5b) as demonstrated by the significant genotype-X drug-treatment interaction (F1,26=14.566, p<0.001), without affecting its expression levels in prefrontal cortex (Figure 5d).
Figure 5.
Analysis of parvalbumin and calbindin gene expression in serotonin transporter (SERT)−/− rats, modulation by chronic duloxetine treatment. Parvalbumin (a, c) and Calbindin (b, d) mRNA levels were measured in the hippocampus (a, b) and prefrontal cortex (c, d) of SERT+/+ and SERT−/− rats treated for 21 days with saline or duloxetine, and killed 24 h after the last injection. The data, expressed as a percentage of SERT+/+/saline (set at 100%), are the mean±SEM from at least six independent determinations. *p<0.05, **p<0.01 vs SERT+/+/saline; $p<0.05, $$p<0.01 vs SERT−/−/saline (two-way analysis of variance (ANOVA) with single contrast post-hoc test (SCPHT)).
Npas4 Expression Changes Correlate With the mRNA Levels for Bdnf Exon I, Bdnf Exon IV, and GABAA- γ2 in Hippocampus and Prefrontal Cortex of Wild-Type and SERT−/− rats
We recently demonstrated that SERT−/− rats have reduced expression of the neurotrophin Bdnf, which is sustained by a decrease of several transcripts, including exon I and IV (Molteni et al, 2010), and that such changes could be normalized by chronic treatment with duloxetine (Calabrese et al, 2010). These alterations were confirmed in the animals investigated in the present study and a summary of the changes for total Bdnf mRNA, and for the two transcripts regulated by Npas4 (exon I and exon IV) are shown in Table 2.
Table 2. Analysis of the mRNA Levels for Npas4, Total Bdnf, Bdnf Exon I, and Bdnf Exon IV in Wild-Type and SERT−/− Rats, and their Modulation by Chronic Duloxetine Treatment.
| Gene | Hippocampus | Prefrontal cortex | ||||||
|---|---|---|---|---|---|---|---|---|
| |
+/+/SAL |
+/+/DLX |
−/−/SAL |
−/−/DLX |
+/+/SAL |
+/+/DLX |
−/−/SAL |
−/−/DLX |
| Npas4 | 100±13 | 109±12 | 56±5*** | 111±13$$ | 100±6 | 119±12 | 55±6*** | 98±12$$ |
| Bdnf total | 100±4 | 123±2*** | 86±2* | 117±3$$$ | 100±4 | 120±4*** | 72±8*** | 101±3$$$ |
| Bdnf exon I | 100±10 | 105±11 | 61±5* | 138±13$$$ | 100±7 | 162±16*** | 63±6** | 93±9$ |
| Bdnf exon IV | 100±7 | 95±8 | 66±8* | 138±15$$$ | 100±9 | 111±19 | 59±6** | 93±9$ |
Abbreviations: ANOVA, analysis of variance; Bdnf, brain-derived neurotrophic factor; DLX, duloxetine; Npas4, neuronal PAS domain protein 4; SAL, saline; SCPHT, single-contrast post-hoc test; SERT, serotonin transporter; Vgat, vesicular GABA transporter.
Npas4, total Bdnf, Bdnf exon I, and Bdnf exon IV mRNA levels were measured in the hippocampus and in the prefrontal cortex of wild-type (+/+) and of SERT−/− (−/−) rats treated for 21 days with SAL or with DLX, and killed 24 h after the last injection. The data, expressed as percentage of +/+/SAL (set at 100%), are the mean±SEM from at least six independent determinations.
*p<0.05.
**p<0.01.
***p<0.001 vs +/+/SAL.
$p<0.05.
$$p<0.01.
$$$p<0.001 vs −/−/SAL (two-way ANOVA with SCPHT).
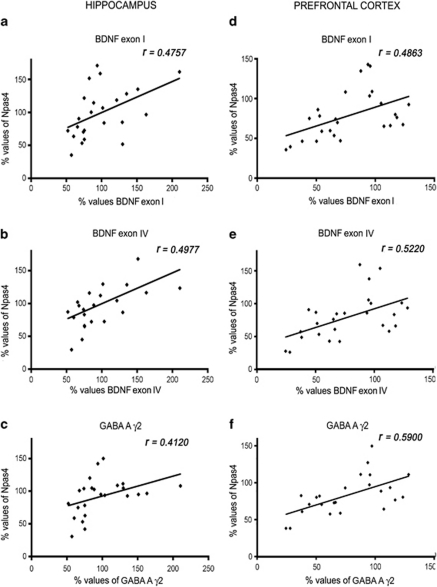
Hence, Npas4 mRNA data were examined for possible covariation within the gene expression of the two Bdnf transcripts in the hippocampus and in the prefrontal cortex. The analyses revealed that, Npas4 mRNA levels correlated positively with the expression of both Bdnf exons in the hippocampus (r=0.4757, n=24, p<0.05; r=0.4977, n=23, p<0.05; exon I and exon IV, respectively; Figures 6a and b), as well as in the prefrontal cortex (r=0.4863, n=26, p<0.05; r=0.5220, n=26, p<0.05; exon I and exon IV, respectively; Figures 6d and e). Moreover, to assess the possible correlation between the changes in Npas4 gene expression and the alteration in the GABAergic markers, we performed a similar comparison between Npas4 and the GABAA-γ2 mRNA levels. We found a positive correlation with the expression of the two genes in the hippocampus (r=0.4120, n=24, p<0.05), as well as in the prefrontal cortex (r=0.5900, n=24, p<0.01; Figures 6c–f).
Figure 6.
Correlation analyses between neuronal PAS domain protein 4 (Npas4), brain-derived neurotrophic factor (Bdnf) exon I (a, d), Bdnf exon IV (b, e), and GABA A receptor, gamma 2 (GABAA- γ2) (c, f) in the hippocampus (a, b, c) and prefrontal cortex (d, e, f) of wild-type and serotonin transporter (SERT)−/− rats. Data points in plots indicate the amount of Npas4, Bdnf exon I, Bdnf exon IV, and GABAA-γ2 mRNA levels in single rats. Analyses by Pearson's product–moment correlation (r). The data, from at least 11 independent determinations, are expressed as a percentage of SERT+/+/saline (set at 100%).
DISCUSSION
In this study, we provide evidence that the expression of the transcription factor Npas4 is significantly reduced in the hippocampus and prefrontal cortex of SERT−/− rats, that such changes are associated with an impairment of the GABAergic system in adulthood, and that these alterations may be normalized by chronic antidepressant treatment.
Npas4 is an activity-regulated transcription factor, whose neuronal expression is selectively induced by the Ca2+ influx, and has a critical role in the development of inhibitory synapses by regulating the expression of activity-dependent genes (Lin et al, 2008). As SERT−/− rats represent a genetic model of anxiety and depression, our findings raise the possibility that the reduction of Npas4 expression may be linked, or may contribute to the behavioral alterations found in these animals (Olivier et al, 2008). The reduction of Npas4 expression in SERT−/− rats appears to be the consequence of an impairment of SERT early in life, as it is already present at adolescence (PND 35). Moreover, we show that pharmacological blockade (fluoxetine) of SERT during gestation can mimic the reduced Npas4 expression found in the hippocampus of SERT−/− rats, providing further support to the developmental origin of the observed changes. During early life, serotonergic neurons are among the earliest to be generated, and 5-HT has an important role in different processes pivotal for neuronal growth and maturation (Gaspar et al, 2003). Indeed, blockade of SERT during fetal life of rats can lead to negative outcomes on brain function and behavior, including behavioral despair and anxiety-like behavior (Lisboa et al, 2007; Olivier et al, 2011). Moreover, pharmacological blockade of SERT during early post-natal life (PND 4–21) results in increased immobility time in the forced-swim test (Hansen et al, 1997), anxiety-related behavioral disturbances (Ansorge et al, 2008; Ansorge et al, 2004), and increased REM sleep (Popa et al, 2008) at adulthood. Collectively, these data show that SERT blockade exerts age-dependent effects on behavior (for review see Olivier et al (2010)), leading to a variety of unfavorable outcomes in rodents, which are opposite with respect to the effects produced by SSRIs during adulthood, but comparable to SERT knockout in rodents (Homberg et al, 2011).
It is interesting to note that Npas4 is a transcription factor associated with promoters I and IV of the Bdnf gene (Pruunsild et al, 2011), suggesting that it may directly regulate activity-dependent expression of the neurotrophin (Lin et al, 2008). Interestingly, we have previously demonstrated that SERT−/− rats have reduced Bdnf expression in the hippocampus and prefrontal cortex, and that this occurs through the modulation of different Bdnf transcripts, including exon I and exon IV (Molteni et al, 2010). A large body of evidence demonstrates that a reduction of Bdnf levels is associated with deficits or impairment of neuronal plasticity, which can have a role in anxiety and major depression (Calabrese et al, 2009; Calabrese et al, 2011; Castren, 2005; Krishnan and Nestler, 2008; Martinowich et al, 2007; Pittenger and Duman, 2008). Our data demonstrate that there is a significant correlation between the levels of Npas4 in the hippocampus and prefrontal cortex, and those of Bdnf exon I and IV (see Figure 6), suggesting that neurotrophic abnormalities may be causally linked to alterations of the transcription factor. One system lying downstream from Npas4 and Bdnf that may be relevant for the phenotype of SERT mutants is GABA. We demonstrate for the first time that SERT−/− rats show a strong impairment of the GABAergic system in the hippocampus and prefrontal cortex, although some differences may exist between these two structures. In fact, the expression of Vgat, is significantly reduced only in the hippocampus of SERT−/− rats, suggesting that these animals may display a reduced number of GABAergic terminals, whereas the expression of the GABA synthesizing enzyme Gad67 or the postsynaptic GABAA-γ2 was similarly reduced in the hippocampus and prefrontal cortex of mutant rats. Moreover, genetic deletion of SERT may influence the sub-population of GABAergic neurons, as the expression of parvalbumin was decreased in both structures, whereas calbindin, which labels a small sub-group of interneurons, was reduced only in the hippocampus. Parvalbumin alteration in hippocampal interneurons may lead to a loss of perisomatic inhibition of pyramidal neurons, which in turn affects network synchronization and memory formation (Bartos et al, 2007; Lewis et al, 2005).
Disturbances in the anatomy and function of the GABAergic system have been postulated in animal models of depression and in different stress-related psychiatric disorders (Benes and Berretta, 2001; Brambilla et al, 2003; Krystal et al, 2002; Luscher et al, 2011; Sanacora et al, 1999). In fact, GABAA receptors can be downregulated in different brain regions of rats exposed to the learned helplessness paradigm (Drugan et al, 1992), whereas lower CSF and plasma GABA levels have been found in depressed patients, as compared with control subjects (Gerner et al, 1984; Gerner and Hare, 1981; Kasa et al, 1982; Petty et al, 1992). Mice that lack the GABAAγ2 receptor subunit show an anxious, depressive phenotype that is related to HPA axis hyperactivity (Sen et al, 2008). Furthermore, parvalbumin immunoreactive neurons are reduced in the hippocampus of tree shrews after chronic psychosocial stress (Czeh et al, 2005), as well as in Octodon degus after repeated separation stress (Seidel et al, 2008), whereas calbindin immunoreactive neurons were significantly reduced in the CA1 of the hippocampus of mice after inescapable electric foot shocks paradigm (Huang et al, 2010). On these bases, the defects of hippocampal GABAergic markers in SERT−/− animals may contribute to the anxious/depressive phenotype observed in these animals (Olivier et al, 2008). It remains to be established if different subgroups of interneurons expressing neuropeptides, such as somatostatin, neuropeptide Y, cholecystokinin, or tachykinin-1, which may also be regulated by Bdnf (Glorioso et al, 2006; Mellios et al, 2009), are altered in SERT KO rats, considering that some of these neuropeptides may be altered in depression (Tripp et al, 2011).
It is important to point out that Npas4 can regulate the development of GABAergic synapses, as silencing of Npas4 gene leads to reduced expression of GABAergic markers, including the presynaptic GABA-producing enzymes Gad65, Gad67, and the GABAA-γ2 receptor subunit (Lin et al, 2008). Furthermore, a strong relationship exists between Bdnf and GABA (Yamada et al, 2002). It has recently been demonstrated that promoter IV-driven Bdnf transcription has a critical role in GABAergic transmission (Sakata et al, 2009), and mice with a selective deficiency of promoter-IV-dependent expression of Bdnf show depression-like behavior (Sakata et al, 2010). Collectively, these data support the potential link between Npas4, Bdnf, and GABA in contributing to the phenotypic changes observed in SERT−/− rats.
We also provide evidence that chronic antidepressant treatment may normalize the molecular alterations found in SERT−/− rats. We had previously demonstrated that chronic treatment with the SNRI antidepressant duloxetine is able to normalize Bdnf deficits in these animals, also through the modulation of Bdnf exon I and IV (Calabrese et al, 2010, present results). Such data are in agreement with the possibility that repeated, but not acute, treatment with major classes of antidepressants may improve neuronal plasticity through the modulation of Bdnf expression (Calabrese et al, 2007; Castren et al, 2007; Molteni et al, 2009a; Russo-Neustadt and Chen, 2005), and that this effect may contribute to their therapeutic action (Berton and Nestler, 2006; Calabrese et al, 2009; Calabrese et al, 2011; Groves, 2007; Martinowich et al, 2007).
We show here that chronic duloxetine treatment does normalize the reduced expression of Npas4 in the hippocampus and prefrontal cortex of SERT−/− rats, without altering transcription factor levels in wild-type animals, suggesting that the effect of the antidepressant may be considered a restorative mechanism rather than a general potentiation of Npas4-dependent transcription. This effect has strong similarity with the changes produced by duloxetine on the expression of Bdnf exon IV in SERT−/− rats, which is significantly upregulated by chronic antidepressant treatment only in SERT−/− rats (Calabrese et al, 2010). On the basis of the lack of SERT function in mutant animals, it is feasible to hypothesize that the modulation of Bdnf transcripts and Npas4 by duloxetine may be due to the ‘noradrenergic component' of the antidepressant. Because, as mentioned above, Npas4 can regulate the expression of Bdnf exon IV, our data suggest that chronic duloxetine may restore the correct expression of Bdnf in SERT−/− rats via modulation of the transcription factor. Moreover, we show that the expression of Arnt2, another transcription factor that cooperate with Npas4 in the regulation of Bdnf exon IV (Pruunsild et al, 2011) is also significantly reduced in SERT−/− rats, although its expression can be restored by duloxetine treatment in the prefrontal cortex, but not in the hippocampus.
These results provide further support to the notion that transcriptional mechanisms may be an important component of the long-term mechanisms set in motion by antidepressant therapy. It is well established that the cAMP-response element-binding protein (Creb) is implicated in depression and in the response to antidepressant treatment (Gass and Riva, 2007), as a decrease in Creb phosphorylation was found in the hippocampus of chronically stressed rats (Qi et al, 2008), whereas several classes of antidepressants increase the levels of Creb expression and function in the rat hippocampus (Nibuya et al, 1996; Thome et al, 2000). However, Creb does not appear to have a role in SERT−/− abnormalities and in the action of duloxetine (data not shown), suggesting that, although Bdnf represents a downstream target of several antidepressant drugs, its regulation may occur through different intracellular pathways.
The impairment of the GABAergic system in the hippocampus and in the prefrontal cortex of SERT−/− rats can also be normalized by long-term treatment with the SNRI antidepressant. Duloxetine appears to be highly effective on hippocampal alterations, where all GABAergic abnormalities in SERT−/− rats (Vgat, Gad67, GABAA-γ2, parvalbumin, and calbindin) are normalized by antidepressant treatment, whereas within the prefrontal cortex, duloxetine appears to restore only the expression of GABAA-γ2. As Npas4 changes in SERT−/− rats are normalized by duloxetine in both brain regions, these results suggest that factors, other than Npas4, may contribute to GABAergic dysfunction in the prefrontal cortex of SERT mutant animals.
Interestingly, there is recent evidence that treatment with antidepressants can revert the reduction of Gad67 in human depressed subjects (Karolewicz et al, 2010), which parallels our findings. These findings suggest that antidepressant treatment can normalize the dysfunction of the GABAergic system (Luscher et al, 2011), which may lead to the increase in GABA release observed in patients (Carter and McCormack, 2009; Sanacora et al, 2002). Collectively, animal and clinical studies indicate that a deficit for GABAergic activity may be crucial in the pathophysiology of mood disorders, and that effective modulation of GABAergic transmission may represent another important mechanism through which antidepressant drugs exert their therapeutic activity (Luscher et al, 2011).
In summary, our results demonstrate that animals with a genetic deletion of SERT show a reduction of Npas4 expression and an impairment of the GABAergic system, suggesting that these defects may contribute to SERT−/− behavioral traits, particularly those associated with anxiety and depression. Given that SERT-knockout rodents model the common SERT promoter polymorphism in humans (Caspi et al, 2010; Hariri and Holmes, 2006; Homberg et al, 2011), it may be inferred that the pharmacological modulation of Npas4 may provide a valuable strategy aimed at improving GABAergic function, as well as neuroplastic mechanisms closely related to the neurotrophin Bdnf. Furthermore, the characterization of genes downstream from the transcription factor Npas4 may prove useful to identify novel systems that may be also affected in mood disorders.
Acknowledgments
We are grateful to Juliet Richetto for language editing. This publication was made possible by grants to MA Riva from the Ministry of University and Research (PRIN 2007STRNHK), from the Ministry of Health (Ricerca Finalizzata RF 2007 conv/42), from Regione Lombardia (Accordo Quadro 2010), and by a liberal contribution from Eli Lilly Italia.
The authors Guidotti G, Calabrese F, Auletta F, Olivier J, and Homberg J have no financial interest or potential conflict of interest. Racagni G has received compensation as speaker/consultant for Eli Lilly, InnovaPharma, Servier. Riva MA has received compensation as speaker/consultant for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Servier, Takeda.
References
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Perez J, Barale F, Schettini G, Soares JC.2003GABAergic dysfunction in mood disorders Mol Psychiatry 8721–737.715. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Cattaneo A, Macchi F, Racagni G, Gennarelli M, et al. Long-Term duloxetine treatment normalizes altered brain-derived neurotrophic factor expression in serotonin transporter knockout rats through the modulation of specific neurotrophin isoforms. Mol Pharmacol. 2010;77:846–853. doi: 10.1124/mol.109.063081. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Maj PF, Cattaneo A, Gennarelli M, Racagni G, et al. Chronic duloxetine treatment induces specific changes in the expression of BDNF transcripts and in the subcellular localization of the neurotrophin protein. Neuropsychopharmacology. 2007;32:2351–2359. doi: 10.1038/sj.npp.1301360. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Racagni G, Riva MA. Neuronal plasticity: a link between stress and mood disorders. Psychoneuroendocrinology. 2009;34 (Suppl 1:S208–S216. doi: 10.1016/j.psyneuen.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Riva MA. Antistress properties of antidepressant drugs and their clinical implications. Pharmacol Ther. 2011;132:39–56. doi: 10.1016/j.pharmthera.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Carter NJ, McCormack PL. Duloxetine: a review of its use in the treatment of generalized anxiety disorder. CNS Drugs. 2009;23:523–541. doi: 10.2165/00023210-200923060-00006. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Castren E. Is mood chemistry. Nat Rev Neurosci. 2005;6:241–246. doi: 10.1038/nrn1629. [DOI] [PubMed] [Google Scholar]
- Castren E, Voikar V, Rantamaki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Czeh B, Simon M, van der Hart MG, Schmelting B, Hesselink MB, Fuchs E. Chronic stress decreases the number of parvalbumin-immunoreactive interneurons in the hippocampus: prevention by treatment with a substance P receptor (NK1) antagonist. Neuropsychopharmacology. 2005;30:67–79. doi: 10.1038/sj.npp.1300581. [DOI] [PubMed] [Google Scholar]
- Drugan RC, Scher DM, Sarabanchong V, Guglielmi A, Meng I, Chang J, et al. Controllability and duration of stress alter central nervous system depressant-induced sleep time in rats. Behav Neurosci. 1992;106:682–689. doi: 10.1037//0735-7044.106.4.682. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gass P, Riva MA. CREB, neurogenesis and depression. Bioessays. 2007;29:957–961. doi: 10.1002/bies.20658. [DOI] [PubMed] [Google Scholar]
- Gerner RH, Fairbanks L, Anderson GM, Young JG, Scheinin M, Linnoila M, et al. CSF neurochemistry in depressed, manic, and schizophrenic patients compared with that of normal controls. Am J Psychiatry. 1984;141:1533–1540. doi: 10.1176/ajp.141.12.1533. [DOI] [PubMed] [Google Scholar]
- Gerner RH, Hare TA. CSF GABA in normal subjects and patients with depression, schizophrenia, mania, and anorexia nervosa. Am J Psychiatry. 1981;138:1098–1101. doi: 10.1176/ajp.138.8.1098. [DOI] [PubMed] [Google Scholar]
- Glorioso C, Sabatini M, Unger T, Hashimoto T, Monteggia LM, Lewis DA, et al. Specificity and timing of neocortical transcriptome changes in response to BDNF gene ablation during embryogenesis or adulthood. Mol Psychiatry. 2006;11:633–648. doi: 10.1038/sj.mp.4001835. [DOI] [PubMed] [Google Scholar]
- Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Groves JO. Is it time to reassess the BDNF hypothesis of depression. Mol Psychiatry. 2007;12:1079–1088. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Sanchez C, Meier E. Neonatal administration of the selective serotonin reuptake inhibitor Lu 10-134-C increases forced swimming-induced immobility in adult rats: a putative animal model of depression. J Pharmacol Exp Ther. 1997;283:1333–1341. [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Lesch KP. Looking on the bright side of serotonin transporter gene variation. Biol Psychiatry. 2011;69:513–519. doi: 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Olivier JD, Blom T, Arentsen T, van Brunschot C, Schipper P, et al. Fluoxetine exerts age-dependent effects on behavior and amygdala neuroplasticity in the rat. PLoS One. 2011;6:el6646. doi: 10.1371/journal.pone.0016646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, Olivier JD, Smits BM, Mul JD, Mudde J, Verheul M, et al. Characterization of the serotonin transporter knockout rat: a selective change in the functioning of the serotonergic system. Neuroscience. 2007;146:1662–1676. doi: 10.1016/j.neuroscience.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Huang HJ, Liang KC, Chang YY, Ke HC, Lin JY, Hsieh-Li HM. The interaction between acute oligomer Abeta(1-40) and stress severely impaired spatial learning and memory. Neurobiol Learn Mem. 2010;93:8–18. doi: 10.1016/j.nlm.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Huezo-Diaz P, Uher R, Smith R, Rietschel M, Henigsberg N, Marusic A, et al. Moderation of antidepressant response by the serotonin transporter gene. Br J Psychiatry. 2009;195:30–38. doi: 10.1192/bjp.bp.108.062521. [DOI] [PubMed] [Google Scholar]
- Jans LA, Riedel WJ, Markus CR, Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry. 2007;12:522–543. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Olivier JD, Nonkes LJ, Homberg JR. Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci Biobehav Rev. 2010;34:373–386. doi: 10.1016/j.neubiorev.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The Serotonin Transporter Promoter Variant (5-HTTLPR), Stress, and Depression Meta-analysis Revisited: Evidence of Genetic Moderation. Arch Gen Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolewicz B, Maciag D, O'Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol. 2010;13:411–420. doi: 10.1017/S1461145709990587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasa K, Otsuki S, Yamamoto M, Sato M, Kuroda H, Ogawa N. Cerebrospinal fluid gamma-aminobutyric acid and homovanillic acid in depressive disorders. Biol Psychiatry. 1982;17:877–883. [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7 (Suppl 1:S71–S80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, et al. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisboa SF, Oliveira PE, Costa LC, Venancio EJ, Moreira EG. Behavioral evaluation of male and female mice pups exposed to fluoxetine during pregnancy and lactation. Pharmacology. 2007;80:49–56. doi: 10.1159/000103097. [DOI] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2009;65:1006–1014. doi: 10.1016/j.biopsych.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Molteni R, Calabrese F, Cattaneo A, Mancini M, Gennarelli M, Racagni G, et al. Acute stress responsiveness of the neurotrophin BDNF in the rat hippocampus is modulated by chronic treatment with the antidepressant duloxetine. Neuropsychopharmacology. 2009a;34:1523–1532. doi: 10.1038/npp.2008.208. [DOI] [PubMed] [Google Scholar]
- Molteni R, Calabrese F, Maj PF, Olivier JD, Racagni G, Ellenbroek BA, et al. Altered expression and modulation of activity-regulated cytoskeletal associated protein (Arc) in serotonin transporter knockout rats. Eur Neuropsychopharmacol. 2009b;19:898–904. doi: 10.1016/j.euroneuro.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Molteni R, Cattaneo A, Calabrese F, Macchi F, Olivier JD, Racagni G, et al. Reduced function of the serotonin transporter is associated with decreased expression of BDNF in rodents as well as in humans. Neurobiol Dis. 2010;37:747–755. doi: 10.1016/j.nbd.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Durrant C, Lewis G, Flint J. Gene X environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Konstantinidis A, Stastny J, Schwarz MJ, Vitouch O, Willeit M, et al. Association between serotonin transporter gene promoter polymorphism (5HTTLPR) and behavioral responses to tryptophan depletion in healthy women with and without family history of depression. Arch Gen Psychiatry. 2002;59:613–620. doi: 10.1001/archpsyc.59.7.613. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier JD, Blom T, Arentsen T, Homberg JR. The age-dependent effects of selective serotonin reuptake inhibitors in humans and rodents: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;35:1400–1408. doi: 10.1016/j.pnpbp.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Olivier JD, Valles A, van Heesch F, Afrasiab-Middelman A, Roelofs JJ, Jonkers M, et al. Fluoxetine administration to pregnant rats increases anxiety-related behavior in the offspring. Psychopharmacology (Berl) 2011;217:419–432. doi: 10.1007/s00213-011-2299-z. [DOI] [PubMed] [Google Scholar]
- Olivier JD, Van Der Hart MG, Van Swelm RP, Dederen PJ, Homberg JR, Cremers T, et al. A study in male and female 5-HT transporter knockout rats: an animal model for anxiety and depression disorders. Neuroscience. 2008;152:573–584. doi: 10.1016/j.neuroscience.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Ooe N, Saito K, Kaneko H. Characterization of functional heterodimer partners in brain for a bHLH-PAS factor NXF. Biochim Biophys Acta. 2009;1789:192–197. doi: 10.1016/j.bbagrm.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Academic Press, New York; 1996. The Rat Brain in Stereotaxis Coordinates. [Google Scholar]
- Petty F, Kramer GL, Gullion CM, Rush AJ. Low plasma gamma-aminobutyric acid levels in male patients with depression. Biol Psychiatry. 1992;32:354–363. doi: 10.1016/0006-3223(92)90039-3. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Popa D, Lena C, Alexandre C, Adrien J. Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior. J Neurosci. 2008;28:3546–3554. doi: 10.1523/JNEUROSCI.4006-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruunsild P, Sepp M, Orav E, Koppel I, Timmusk T. Identification of cis-elements and transcription factors regulating neuronal activity-dependent transcription of human BDNF gene. J Neurosci. 2011;31:3295–3308. doi: 10.1523/JNEUROSCI.4540-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Lin W, Li J, Li H, Wang W, Wang D, et al. Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiol Dis. 2008;31:278–285. doi: 10.1016/j.nbd.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Chen MJ. Brain-derived neurotrophic factor and antidepressant activity. Curr Pharm Des. 2005;11:1495–1510. doi: 10.2174/1381612053764788. [DOI] [PubMed] [Google Scholar]
- Sakata K, Jin L, Jha S. Lack of promoter IV-driven BDNF transcription results in depression-like behavior. Genes Brain Behav. 2010;9:712–721. doi: 10.1111/j.1601-183X.2010.00605.x. [DOI] [PubMed] [Google Scholar]
- Sakata K, Woo NH, Martinowich K, Greene JS, Schloesser RJ, Shen L, et al. Critical role of promoter IV-driven BDNF transcription in GABAergic transmission and synaptic plasticity in the prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106:5942–5947. doi: 10.1073/pnas.0811431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- Schwaller B, Meyer M, Schiffmann S. ‘New' functions for ‘old' proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum. 2002;1:241–258. doi: 10.1080/147342202320883551. [DOI] [PubMed] [Google Scholar]
- Seidel K, Helmeke C, Poeggel G, Braun K. Repeated neonatal separation stress alters the composition of neurochemically characterized interneuron subpopulations in the rodent dentate gyrus and basolateral amygdala. Dev Neurobiol. 2008;68:1137–1152. doi: 10.1002/dneu.20651. [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry. 2007;12:247–257. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- Smits BM, Mudde JB, van de Belt J, Verheul M, Olivier J, Homberg J, et al. Generation of gene knockouts and mutant models in the laboratory rat by ENU-driven target-selected mutagenesis. Pharmacogenet Genomics. 2006;16:159–169. doi: 10.1097/01.fpc.0000184960.82903.8f. [DOI] [PubMed] [Google Scholar]
- Thome J, Sakai N, Shin K, Steffen C, Zhang YJ, Impey S, et al. cAMP response element-mediated gene transcription is upregulated by chronic antidepressant treatment. J Neurosci. 2000;20:4030–4036. doi: 10.1523/JNEUROSCI.20-11-04030.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp A, Kota RS, Lewis DA, Sibille E. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis. 2011;42:116–124. doi: 10.1016/j.nbd.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, Nishiyama N, et al. Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci. 2002;22:7580–7585. doi: 10.1523/JNEUROSCI.22-17-07580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]