Abstract
Impulsive action, the failure to withhold an inappropriate response, is treated clinically with dopamine agonists such as amphetamine. Despite the therapeutic efficacy, these drugs have inconsistent effects on impulsive action in rodents, causing improvements or disruptions in different tasks. Thus, we hypothesized that amphetamine is producing an effect by altering distinct cognitive processes in each task. To test this idea, we used the response inhibition (RI) task and trained rats to withhold responding for sucrose until a signal is presented. We then varied the duration that subjects were required to inhibit responding (short=4 s; long=60 s; or variable=1–60 s) and examined whether this influenced the pattern of premature responses. We also tested the effects of amphetamine (0.0, 0.125, 0.25, 0.5, and 1.0 mg/kg) on each task variant. The probability of premature responding varied across the premature interval with a unique pattern of time-dependent errors emerging in each condition. Amphetamine also had distinct effects on each version: the drug promoted premature responding when subjects expected a consistent delay, regardless of its duration, but reduced premature responding when the delay was unpredictable. We propose that the ability to inhibit a motor response is controlled by a different combination of cognitive processes in the three task conditions. These include timing, conditioned avoidance, and attention, which then interact with amphetamine to increase or decrease impulsive action. The effect of amphetamine on impulsive action, therefore, is not universal, but depends on the subject's experience and expectation of the task demands.
Keywords: impulsivity, cognition, disinhibition
INTRODUCTION
Impulsive actions, defined as the failure to withhold an inappropriate response (Winstanley et al, 2006), are a prominent feature of several psychiatric disorders (Moeller et al, 2001). Stimulants such as amphetamine and methylphenidate remain the primary pharmaceutical treatment for elevated impulsivity, particularly in attention-deficit hyperactivity disorder (Kollins, 2008), and act primarily by increasing dopaminergic tone, but also have effects on serotonin and noradrenaline (Sulzer et al, 2005). Animal models of impulsivity (Dalley et al, 2011) support a central role for these neurotransmitters (Pattij and Vanderschuren, 2008), although the effect of amphetamine on impulsive actions in rats remains inconclusive. Specifically, amphetamine increases premature responding in the five-choice serial reaction time task (5-CSRTT; Cole and Robbins, 1987; Harrison et al, 1997; van Gaalen et al, 2006) and produces a leftward shift of inter-response times (IRTs) using differential reinforcement of low rate (DRL) schedules (Seiden et al, 1979; Lobarinas and Falk, 1999; Bizot 1998; Fowler et al, 2009), both indicative of increased impulsivity. In contrast, amphetamine improves performance (ie, decreases impulsive action) in the stop task (Feola et al, 2000; Eagle and Robbins, 2003), but only in subjects with high baseline (ie, slow) stop-signal reaction times. This raises the important point that many pharmacological agents can produce distinct outcomes when drug effects are compared in normal and diseased populations.
The differential effects of amphetamine on impulsive action in rats may be explained by the variety of tasks employed to study this process. Each animal model places unique demands on cognitive mechanisms, such as timing, discrimination, and attention (Hayton and Olmstead, 2009); disruption of a particular mechanism, therefore, may alter performance in one task, but not another. For example, in both the DRL and 5-CSRTT, animals are required to withhold responding during a set delay (although the delay may be varied in the 5-CSRTT, it is typically held constant; Robbins, 2002). Performance in both tasks would be improved by the ability to time intervals, whereas disruption of this ability would promote anticipatory responses, the primary measure of impulsive action. Given that dopamine is a critical factor in interval timing (Meck et al, 2008), alterations in dopaminergic function may affect impulsivity, indirectly, in tasks such as these that include a set delay.
This investigation aimed to clarify the role of amphetamine on impulsive action by testing rats under conditions in which they could, and could not, time the interval during which they must inhibit a response. We hypothesized that amphetamine would produce distinct effects when the delay to respond was either predictable or unpredictable. To that end, we trained rats in the response inhibition (RI) task (Befort et al, 2011; Hayton et al, 2010), which requires subjects to withhold pressing a lever until signaled to respond. Separate groups were trained with 4-s, 60-s, or variable (1–60-s) delays. To better understand how timing influences impulsive responses, we divided the delay period (ie, premature phase) into time bins and then examined whether subject was more likely to make premature responses at a particular time. This measure, the probability of response, showed distinct patterns of responding in each of the conditions (4-s, 60-s, and 1–60-s delays). Amphetamine produced a dose-dependent increase in impulsive actions when the delay was predictable, but a decrease when it was unpredictable. Amphetamine also produced time-dependent changes in probability of responding. These findings emphasize that impulsive actions are influenced by the subjects' expectation of the duration that a response must be withheld, and that amphetamine interacts with this process.
SUBJECTS AND METHODS
Subjects
Male Long-Evans rats (N=72; Charles River, Quebec), weighing 175–225 g at arrival, were pair housed on a reverse light–dark cycle with testing conducted during the dark cycle. Three days before training, rats were food restricted to 120 min of free access (Lab Diet: PMI Nutritional International) per day; water was freely available. Animal care was conducted in accordance with Canadian Council on Animal Care guidelines and the experiments were approved by the Queen's University Animal Care Committee.
Apparatus
Training and testing were conducted in operant conditioning chambers (26.5 × 22.0 × 20.0 cm), housed in a sound-attenuating chamber (constructed in-house). Each box was fitted with two retractable levers. Dustless sucrose pellets (45 mg; Bio-Serv, NJ) were delivered to a food magazine, located between the levers, via a pellet dispenser (Med Associates, VT). A houselight could indirectly illuminate the chamber and three signal lights were situated 4 cm above each lever and the magazine. A standard computer (Dell, Canada) controlled the equipment and was used for data collection (software written in-house using ECBASIC).
Behavioral Training
Rats were magazine trained for 1 session, receiving 20 sucrose pellets on a random time 90-s schedule. The houselight was illuminated until a food pellet was dispensed. At this point, the magazine light turned on and the houselight turned off for 1 s and the next trial commenced.
Rats were then trained to lever press for sucrose pellets on a continuous reinforcement (CRF) schedule; lever assignment (left vs right) was counterbalanced across rats and remained consistent for the remainder of the experiment. The houselight and discriminative stimulus (lever light) were turned on throughout these sessions, except during delivery of the reward, which was signaled by illumination of the magazine light (1 s). There was no time limit for responding on each trial; training continued until subjects earned a minimum of 80 pellets in a 60-min session for 2 consecutive days. Following CRF training, the response phase (houselight and discriminative stimulus on) was shortened to 10 s following lever insertion. If 10 s elapsed, the lever was retracted for a 10-s inter-trial interval (ITI) and the trial was scored as an omission. Training continued until animals reached a criterion of fewer than 20% omitted trials for two consecutive sessions. Over 90% of animals reached this criterion in 2 days, with the remaining completing this stage of training within 4 days. In all phases of the task, lever presses on the non-reinforced lever (NRL) were recorded, but had no programmed consequence.
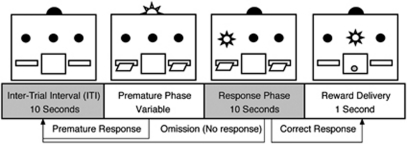
Subjects were then trained on the RI task (Figure 1), which requires subjects to withhold responding until signaled. Each trial of the task commenced with a 10-s ITI in which the levers were retracted and the houselight off. At the end of the ITI, trials progressed to the premature phase, during which both levers were inserted and the houselight illuminated. Lever presses during the premature phase (ie, premature responses) produced no reward and reinstated the ITI. If subjects did not press the lever in the premature phase, trials progressed to the correct phase, during which the houselight was extinguished and the discriminative stimulus (light above lever) illuminated. Responses in the correct phase were reinforced with a sucrose pellet (and illumination of the magazine light for 1 s) and initiated the next trial. Failure to lever press within 10 s of illumination of the discriminative stimulus resulted in an omission and initiated the next trial. Failure to lever press within 10 s of illumination of the discriminative stimulus resulted in an omission and initiated the next trial. Rats in the Fixed 4-s condition (n=16) received 21 sessions of baseline training with a 4-s premature phase and 100 trials/session. Subjects in the Fixed 60-s condition (n=32) were initially trained for 5 sessions with a 4-s, then 15-s, then 60-s premature phase (100 trials/session), followed by 14 sessions of baseline training with a 60-s premature phase (50 trials/session). Subjects in the Variable condition (n=24) were initially trained for 5 sessions with a 4-s, then 15-s, then 60-s, premature phase (100 trials/session), followed by 14 baseline sessions with 20 trials at premature phases of 1, 4, 15, 30, and 60 s (100 trials in total, with delays delivered randomly).
Figure 1.
Visual schematic of the response inhibition (RI) task with arrows indicating possible outcomes. The RI task requires subjects to withhold responding until the correct phase. Responses during the correct phase result in a sucrose pellet reward and reinstate the inter-trial interval (ITI). Responses during the premature phase restore the ITI with no reward. Failure to respond during the correct phase results in an omission and reinstates the ITI.
Drugs
-Amphetamine sulfate (Sigma-Aldrich, Canada) was dissolved in 0.9% physiological saline and was prepared at doses of 0.0, 0.125, 0.25, 0.5, and 1.0 mg/kg/ml. Amphetamine was injected 30 min before behavioral testing and each subject received two injections at each dose (10 injections in total), in either ascending then descending, or descending then ascending order, counterbalanced across rats. Doses were separated by 48 h, and rats were tested drug-free on the intervening days.
Data Analysis and Statistics
Premature responding was assessed as the percentage of trials in which rats pressed the reinforced lever before the discriminative stimulus (ie, during the premature phase). This dependent measure was calculated as (premature responses/(premature+correct responses)) × 100. Latencies to correct responses, the number of responses on the NRL and trial omissions were also recorded. Latencies were calculated as the time from discriminative stimulus presentation to the first lever press on the reinforced lever. NRL pressing was calculated as the rate of responses per second. All behavioral measures were calculated by pooling the data from both sessions at each dose of amphetamine.
Data were analyzed using analysis of variance (ANOVA), computed with the Statistical Program for the Social Sciences (SPSS; V.18.0). Degrees of freedom for repeated measures were adjusted using the Greenhouse–Geisser correction if assumptions of sphericity were violated (Greenhouse and Geisser 1959). When significant main effects were observed in between-subjects analyses, post hoc tests were performed with a Bonferroni correction. When appropriate, within-subjects significant main effects were further analyzed for simple effects, also using a Bonferroni correction for the number of comparisons made.
The probability of responding measure reflects the likelihood of a lever press occurring during a set period (1 or 5 s bin), allowing the identification of specific time points that animals are more or less likely to make a premature response. This was calculated as the number of responses during a bin, divided by the number of trials that reached that bin. For example, all subjects in the Variable condition had 100 trials/session (20 at each delay). If an individual subject made two premature responses in the 0–1-s bin when the delay was set at 30 s, the probability of responding during this bin would be 2/100=0.02. For this subject, there were 78 trials at the 1–2-s bin (all 20 trials at the 1-s delay had progressed to the correct phase and two 30-s delay trials had been terminated due to the previous errors). If this subject made four premature responses between 1 and 2 s, the probability of responding in this bin would be 4/78=0.051. Probability of responding data in the Fixed 4-s condition was analyzed using four 1-s bins, whereas the Fixed 60-s and Variable conditions were analyzed over twelve 5-s bins.
Baseline calculations of probability of responding were made by pooling the final seven sessions, and the effects of amphetamine on this measure were calculated by combining data from the two sessions at each dose. Baseline probabilities of responding were analyzed using repeated-measures ANOVA, with time (1-s or 5-s bins) as a within-subject factor and condition as a between-subjects factor. Drug testing data were analyzed using repeated-measures ANOVAs with dose as a within-subject factor and order of injection (ascending vs descending) as a between-subjects factor. ANOVAs conducted on data from the Variable condition also included delay (1, 4, 15, 30, and 60 s) as a within-subject factor. We included injection order as a factor in the ANOVA to determine if there was an effect of repeated drug exposure on behavioral measures. Subjects who showed unstable performance (>10% deviation from baseline) during drug-free days were excluded from the analysis of drug effects (this criterion applied to four subjects, two from the Variable condition, one from each of the Fixed conditions).
RESULTS
Fixed and Variable Conditions Produce Distinct Patterns of Premature Responses Across Time
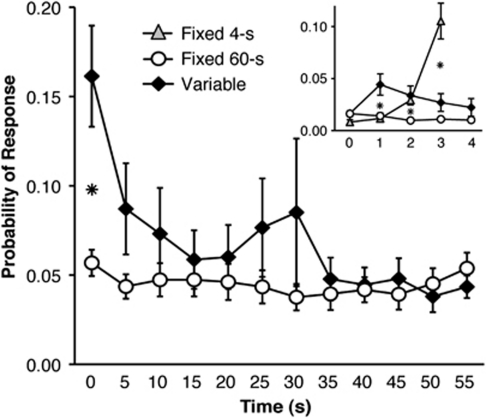
The probability of responding during the premature phase of the RI task followed distinct patterns under different training conditions (Figure 2). Analysis of these data in 1-s bins for the first 4 s of the premature phase (see Figure 2, inset) revealed a significant effect of Condition (F(2,69)=7.668, p<0.001), Time (F(3,207)=30.895, p<0.001), and a significant Time × Condition interaction (F(6,207)=33.315, p<0.005). Simple effects confirmed significant Condition effects at 1–3 s (all p<0.005), and post hoc t-tests revealed significantly more premature responding by the Variable group during the 1-s bin, significantly less premature responding by the Fixed 60-s group during the 2-s bin, and significantly more responding by the Fixed 4-s group during the 3-s bin (all p<0.05 vs other two conditions).
Figure 2.
Differences in the probability of responding across different conditions of the response inhibition (RI) task. The probability of responding during the final seven sessions of baseline training is displayed in 1-s bins for the first 0–5 s of all three conditions (inset) and 5-s bins across 0–60-s for the Fixed 60-s and Variable conditions (*p<0.05 vs all groups; error bars display standard error of the mean).
We also examined the probability of responding from 0 to 60 s for the Variable and Fixed 60-s conditions, which showed no significant effect of Condition (F(1,53)=0.672, p=0.42), but significant effects of Time (F(11,583)=20.120, p<0.001), and a Time × Condition interaction (F(11,583)=12.220, p<0.001). Post hoc t-tests revealed a significant effect of Condition, only during the first 5 s of the premature phase (p<0.01).
Amphetamine Increases Premature Responding in the Fixed 4-s Condition
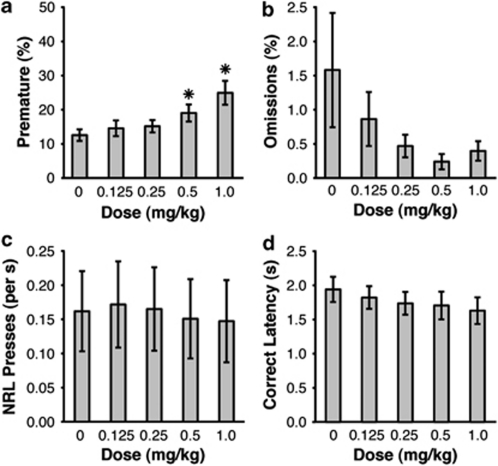
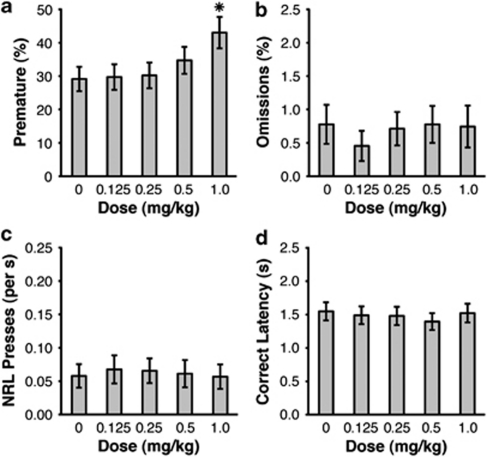
In animals trained with a 4-s premature phase, amphetamine dose dependently increased premature responding (Figure 3a; F(4,52)=16.639, p<0.001), with post hoc tests confirming significantly more premature responses at 1.0 mg/kg than all other doses, as well as significantly more premature responses at 0.5 mg/kg than the 0.0 and 0.25 mg/kg doses (all p<0.05). Amphetamine did not affect omissions (F(4,52)=1.857, p=0.179; Figure 3b) or NRL rate (F(4,52)=1.590, p=0.224; Figure 3c). There was a mild, but non-significant, trend toward quicker latencies to respond in the correct phase following amphetamine injections (F(4,52)=2.689, p=0.076; Figure 3d). For all measures, there was no significant effect of Dosing Order, nor were there any significant interactions between this factor and Dose (all p>0.05).
Figure 3.
Effect of amphetamine on performance in the Fixed 4-s condition of the response inhibition (RI) task. Amphetamine increased premature responding (a), but had no effect on Omissions (b), NRL rate (c), or Correct Latency (d) (*p<0.05 vs 0.0 mg/kg dose; error bars display standard error of the mean).
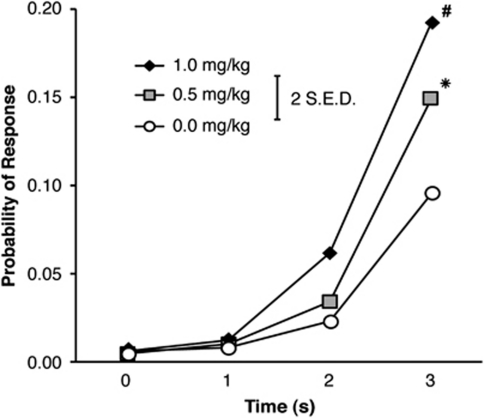
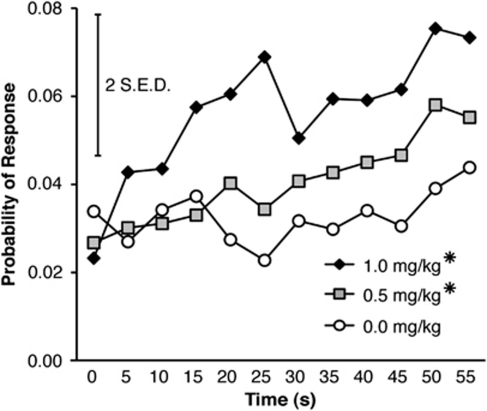
There was also a time-dependent effect of amphetamine on probability of responding during the 4-s premature phase (Figure 4), manifested as significant effects of Time (F(3,42)=52.846, p<0.001) and Dose (F(4,56)=17.566, p<0.001), as well as a Time × Dose interaction (F(12,168)=11.312, p<0.001). Simple effects confirmed a significant drug effect during the final bin (3–4 s) (p<0.05), with post hoc t-test showing a significantly higher probability of responding after 0.5 or 1.0 mg/kg amphetamine compared with all other doses, and a significantly higher probability of responding after 1.0 mg/kg than 0.5 mg/kg amphetamine (all p<0.05).
Figure 4.
Effect of amphetamine on the probability of responding in the Fixed 4-s condition of the response inhibition (RI) task. Amphetamine increased the probability of responding during the final 1-s bin of the trials. (For clarity, data for the 0.125 and 0.25 mg/kg doses are not displayed, but are statistically identical to the 0.0 mg/kg dose; *p<0.05 vs 0.0 mg/kg dose; #p<0.05 vs all groups; error bar displays two standard errors of the difference).
Amphetamine Increases Premature Responding in the Fixed 60-s Condition
In animals trained with a 60-s premature phase, amphetamine increased premature responding (Figure 5a; F(4,116)=11.553, p<0.001), with post hoc tests confirming significantly more premature responses after 1.0 mg/kg of amphetamine than all other doses. Amphetamine did not produce a significant effect on omissions (F(4,116)=0.429, p=0.717; Figure 5b), NRL rate (F(4,116)=0.722, p=0.506; Figure 5c), or latency to respond in the correct phase (F(4,116)=1.554, p=0.205; Figure 5d). For all measures, there were no significant effects of Dosing Order or interaction with Dose (all p>0.05).
Figure 5.
Effect of amphetamine on performance in the Fixed 60-s condition of the response inhibition (RI) task. Amphetamine increased premature responding (a), but had no effect on Omissions (b), NRL rate (c), or Correct Latency (d) (*p<0.05 vs 0.0 mg/kg dose; error bars display standard error of the mean).
There was also a time-dependent effect of amphetamine on probability of responding during the premature phase (Figure 6). This was revealed by significant effects of Time (F(11,319)=3.768, p<0.05) and Dose (F(4,116)=9.630, p<0.001), and a trend toward a significant Time × Dose interaction (F(44,1276)=1.931, p=0.057). Post hoc tests revealed no significant differences between the 0, 0.125, and 0.25 mg/kg doses, but a significantly higher probability of responding across time for the 0.5 and 1.0 mg/kg doses, compared with the 0 mg/kg dose (p<0.05).
Figure 6.
Effect of amphetamine on the probability of responding in the Fixed 60-s condition of the response inhibition (RI) task. Amphetamine increased the probability of responding across time. (For clarity, the data for 0.125 and 0.25 mg/kg doses are not displayed, but are significantly identical from the 0.0 mg/kg dose; *p<0.05 vs 0.0 mg/kg dose; error bar displays two standard errors of the difference).
Amphetamine Reduces Premature Responding in the Variable Condition
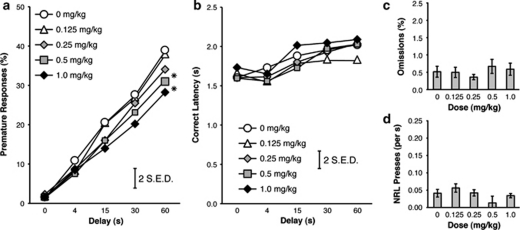
In animals trained with a variable (1–60 s) premature phase, amphetamine dose dependently decreased premature responding (Figure 7a), with significant effects of Dose (F(4,64)=3.68, p=0.009) and Delay (F(4,64)=50.44, p<0.001), and a Dose × Delay interaction (F(16,256)=2.90, p<0.05). Post hoc tests revealed significantly less premature responses after the 0.5 and 1.0 mg/kg doses, compared with the 0 mg/kg dose (p<0.05). Simple effects showed a significant effect of Dose at the 30-s and 60-s delays, and post hoc tests showed significantly fewer premature responses with 0.5 and 1.0 mg/kg amphetamine at the 60-s delay (p<0.05), and a trend toward a significant difference between 0 and 1 mg/kg at the 30-s delay (p=0.051).
Figure 7.
Effect of amphetamine on performance in the Variable condition of the response inhibition (RI) task. Amphetamine decreased premature responding at the 60-s delay (a), while having no effect on Correct Latency (b), Omissions (c), or NRL rate (d) (*p<0.05 vs 0.0 mg/kg dose; error bar displays two standard errors of the difference).
Amphetamine had no effect on response latency in this condition (Figure 7b; F(4,80)=1.075, p=0.351). These was a significant effect of Delay (F(4,80)=23.419, p<0.001) with a decreased latency to respond after a 1-s and 4-s delay, compared with the 30-s and 60-s delays (p<0.05), but no Dose × Delay interaction (F(16,320)=0.653, p=0.700). Amphetamine had no effect on the number of omissions (F(4,80)=0.711, p=0.533; Figure 7c) and there was no effect of Delay (F(4,80)=1.498, p=0.221) or Dose × Delay (F(16,320)=0.872, p=0.517). Similarly, amphetamine had no effect on responding on the NRL (Figure 7d; F(4,80)=1.41, p=0.256), but there was an effect of Delay (F(4,80)=8.411, p<0.01), with a higher rate of responding after the 1-s and 4-s delays than the 30-s and 60-s delays (p<0.05), but no Dose × Delay interaction (F(16,320)=1.44, p=0.249). For all measures, there were no significant effects of Dosing Order, nor were there any significant interactions with Dose (all p>0.05).
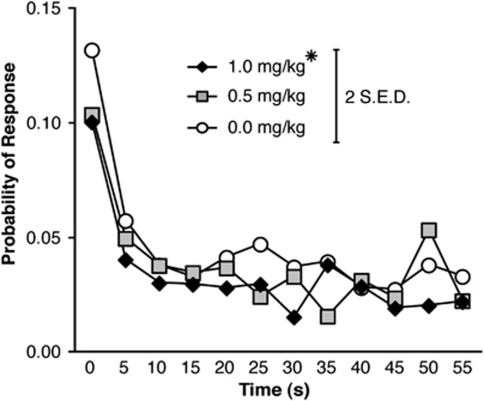
Figure 8 shows the effect of amphetamine on probability of responding during the premature phase in the Variable condition. Responding during the final 30 s of the premature phase was variable as each subject only received, at most, 40 trials at this delay. There was a significant effect of Time (F(11,231)=29.304, p<0.001), a trend toward a significant effect of Dose (F(4,84)=2.733, p=0.054), but no Time × Dose interaction (F(44,924)=0.907, p=0.522). Post hoc tests confirmed a significant reduction in the probability of responding across time after 1.0 mg/kg amphetamine, compared with the 0.0 mg/kg dose (p<0.05).
Figure 8.
Effect of amphetamine on the probability of responding in the Variable condition of the response inhibition (RI) task. Amphetamine decreased the probability of responding across time. (For clarity, data for 0.125 and 0.25 mg/kg doses are not displayed, but are statistically identical to 0.0 mg/kg dose; *p<0.05 vs 0.0 mg/kg dose; error bar displays two standard errors of the difference).
DISCUSSION
A primary objective of this study was to investigate how amphetamine altered the mechanisms that control responding in a rat model of impulsive action. Our analysis of time-dependent changes in the probability of responding revealed that subjects make distinct patterns of errors when they can and cannot time the RI interval. By examining the effect of amphetamine on different conditions of the same task, we demonstrate that the drug increases or decreases impulsive actions when subjects must withhold responding for a fixed or variable delay, respectively. These results are particularly striking because, at its simplest, the requirements for each variation of the RI task are identical: Do not press the lever until the signal light illuminates. Therefore, the animal's expectation for delay length directly influenced the drug's effect, which may explain the apparently contradictory effects of amphetamine on impulsive action in previous studies.
Expectation of Delay Duration Alters the Probability of Impulsive Responding Across Time
Impulsive action is commonly measured in rodent models as the number or proportion of premature responses per session. This metric provides a global estimate of a subject's inability to withhold a response. We examined this measure in greater depth by analyzing the probability of impulsive responding across time. This analysis revealed that the likelihood of making an impulsive response at a particular time point varied, depending on the subjects' expectation of the premature phase. Specifically, training in the Fixed 4-s condition resulted in far more responses in the final second of the premature phase, reminiscent of the scalloped pattern of responding under fixed interval schedules (Fester and Skinner, 1957). A likely explanation for this profile is that subjects are timing the interval length and premature errors are anticipatory responses. This fits with evidence of increased impulsivity in the 5-CSRTT when the ITI is lengthened from 5 to 7+ seconds (see for example, Harrison et al, 1997; Fletcher et al, 2007). Indeed, lengthening the premature delay in this task is used to screen for individual differences in trait impulsivity (Dalley et al, 2007; Belin et al, 2008).
Unlike the Fixed 4-s condition, subjects in the Fixed 60-s condition showed a consistent likelihood of responding throughout the premature phase. That is, even though the length of the delay interval was predictable, subjects did not exhibit anticipatory responding toward the end of the interval. It is unlikely that the difference in these two patterns reflects an ability to time short (4 s) but not long (60 s) intervals as rats responding under DRL schedules can accurately assess delays of the longer duration (Seiden et al, 1979; Lobarinas and Falk, 1999; Bizot 1998; Fowler et al, 2009). The DRL task differs from the fixed delay version of the RI task in that the delay period in the latter is signaled. Perhaps this presence of a clear signal reduces the need to time an interval, which would be particularly taxing at the long delay. In other words, rats may rely on different cognitive mechanisms to inhibit responding for long and short delays: timing the former and relying on the visual stimulus in the latter condition.
In contrast to the Fixed 4-s and 60-s conditions, subjects in the Variable condition showed a greater likelihood of responding at the beginning of the premature phase. This highlights the usefulness of breaking down the probability of responding across time in that a cursory examination of responses at each delay shows more premature responding on trials with longer delay intervals; this may have led to the erroneous conclusion that animals are more likely to respond at the end of the premature phase. Responses that occurred early in the delay interval may have been elicited by the lever insertion, although it is not clear why this would occur in the Variable, but not in the Fixed 60-s, condition. Another possibility is that the increased responding in the initial time bins is a rapid adaptation to one or more short delays presented in the random sequence. That is, the alternation of delay intervals occasionally produced several trials in a row with the 1-s or 4-s delay, which could cause subjects to anticipate another short delay and respond accordingly.
Amphetamine-Induced Changes in Impulsive Action May Interact with Underlying Cognitive Processes
One of the most important findings in our study is that amphetamine has opposite effects on impulsive action when the delay interval is predictable or unpredictable. Amphetamine increased premature responding in fixed conditions of the RI task, regardless of whether the delay interval was short (4 s) or long (60 s). In contrast, if subjects were unable to predict the duration of the delay interval (Variable condition), then amphetamine decreased premature responses. Thus, the effect of amphetamine on impulsive action (at least in the RI rat model) depends on whether animals can time the delay interval. This suggests that amphetamine is altering some cognitive process (eg, timing, attention, or conditioned avoidance), which has differential effects on premature responding in the three conditions.
The simplest explanation for an amphetamine-induced increase in premature responding in the fixed conditions of the RI task is that the drug altered timing abilities. This fits with evidence that amphetamine disrupts the ability to discriminate cues of different durations (Meck, 1996; Bizot, 1997) and produces a leftward shift of peak intervals in DRL tasks (Taylor et al, 2007; Eckerman et al, 1987). The latter finding suggests that amphetamine accelerates the perception of time. If this were true, increased premature responding could simply be an exacerbation of the normal, anticipatory responses we observed in the Fixed 4-s condition. In agreement with this idea, amphetamine increases premature responses in other tasks with consistent delays, such as the 5-CSRTT (Cole and Robbins, 1987; Harrison et al, 1997; van Gaalen et al, 2006) and DRL tasks (Seiden et al, 1979; Lobarinas and Falk, 1999; Bizot 1998; Fowler et al, 2009), but not in the stop task which uses a variable delay to the signal for RI (Feola et al, 2000; Eagle and Robbins, 2003). Disruptions in timing abilities would not affect performance in the Variable condition, so it is not surprising that we failed to observe an amphetamine-induced increase in impulsive action in this version of the task.
Although amphetamine increased impulsive action in both Fixed conditions of the RI task, the drug had different effects on the distribution of errors at longer and shorter delay intervals. More specifically, unlike the Fixed 4-s condition, amphetamine did not produce time-sensitive errors in the probability of responding at the longer (60-s) delay interval, although a strong trend toward time-dependent errors late in the delay was present. These data argue against the idea that amphetamine is promoting impulsive actions through an accelerated perception of time, at least when the interval to be timed is relatively long. As noted previously, however, rats may successfully inhibit responding at these long delays by attending to the sensory cue that signals the end of the interval, rather than timing the interval itself. Performance improves (ie, impulsivity decreases) in a 15-s DRL task when a cue is presented at the end of the delay (Carey and Kritkausky, 1972), suggesting that attending to an external sensory cue improves performance at intermediate delays. More importantly, amphetamine does not produce a leftward shift in IRTs when a signal is present (Wiley et al, 2000), although it does increase the response rate and decrease the number of reinforcers obtained, a pattern of deficits that points to elevated impulsivity but no alteration in timing abilities. The findings also fit with our idea that the effect of a drug on impulsive action (or any other response) depends on the cognitive process that is controlling behavior. If animals are timing delay intervals, then amphetamine speeds up this mechanism; if they are relying on external sensory cues, then amphetamine may impact performance through another process.
When relying on external signals, particularly in the Fixed 60-s condition, rats may successfully inhibit responding by actively avoiding the lever. In DRL tasks (24 or 72 s), rats position themselves away from the operant manipulandum until a few seconds before responding (Fowler et al, 2009), reminiscent of a pre-commitment strategy in pigeons (Rachlin and Green, 1972) that reduces impulsivity. Just as a dieter avoids temptation by throwing out the junk food in their house, rodents may avoid approaching the lever to prevent premature responding. If rats are actively avoiding the lever in the Fixed 60-s condition, then the locomotor-activating effects of amphetamine (Kalivas and Stewart, 1991) may disrupt this strategy: promoting movement toward the lever, thereby increasing premature responding. Lever avoidance would be a disadvantageous strategy in the Variable condition, as it would slow the latency to respond at short intervals, potentially delaying reward presentation. In fact, the latency to respond was reduced at short (1 and 4 s) intervals in the Variable condition, which suggests that subjects were not actively avoiding the lever. Conditioned avoidance, therefore, may reduce the likelihood of making an error at long, but not at short, delays. This highlights, once again, that rats may employ different cognitive strategies to inhibit responding, and helps to explain the differential effects of amphetamine on action impulsivity when delays to respond are predictable or unpredictable.
The RI task does not place strong attentional demands on the subject: Rats have up to 10 s to respond during the correct phase and the correct and premature phases have distinct signals that can be detected from any place in the chamber. Nonetheless, enhancing attention could improve performance, particularly in the Variable condition because subjects must constantly monitor the environment as they wait for the signal to respond. Amphetamine improves attention (Bizarro et al, 2004; Grilly, 2000) and decreases distractibility (Agmo et al, 1997a, 1997b), which may explain why it improves performance (ie, decreases impulsive action) in the Variable condition, but not in the two Fixed conditions. On the other hand, it may be that amphetamine improves attention in all three conditions, but the effects on impulsive action are masked in the Fixed conditions by drug-induced disruptions in other processes, such as timing or conditioned avoidance.
We have identified three cognitive processes (timing, conditioned avoidance, and attention) that may explain the differential effects of amphetamine in fixed and variable delay versions of the RI task. This list, however, is not exhaustive: other cognitive processes may contribute to successful performance in the RI task. For example, amphetamine has well-established effects on motivation, increasing responding for sucrose on break-point schedules of reinforcement (Mayorga et al, 2000; Poncelet et al, 1983). In the RI task, differences in the length of time between reward delivery (ie, under different delay conditions) could impact the motivation to respond. In addition, external signals, such as the houselight, that explicitly signaled the premature phase may interact with the pharmacological effects of amphetamine (Wiley et al, 2000). The latter possibility is particularly intriguing in that amphetamine produced opposing effects on impulsive choice when a houselight was present or absent during the delay to reward delivery (Cardinal et al, 2000). External cues, therefore, may alter the behavioral effects of pharmacological manipulations. Previously, we emphasized that each behavioral paradigm relies on a unique combination of cognitive processes, and that these should be carefully considered in designing rodent tests of impulsive action (Hayton and Olmstead, 2009).
Neurobiology of Impulsive Actions
Amphetamine's distinct effects on the Fixed and Variable conditions of the RI task may reflect the drug's effects on various neurotransmitter systems. Amphetamine is not a selective drug: it acts preferentially on the dopamine transporter, but also has an action on serotonin and noradrenaline transporter systems (Sulzer et al, 2005). Increasing noradrenergic tone, through selective reuptake inhibitors, decreases impulsive action in the 5-CSRTT (Navarra et al, 2008; Robinson et al, 2008) and the stop task (Robinson et al, 2008), and improves the ability to correctly time intervals (Balci et al, 2008). Increases in serotonergic tone also decrease impulsive action on DRL schedules (Richards et al, 1993; Sokolowski and Seiden, 1999), whereas serotonin depletion increases this measure in the 5-CSRTT (Harrison et al, 1997) and on DRL schedules (Jolly et al, 1999). Serotonergic mechanisms interact with the effect of amphetamine in the 5-CSRTT in that blockade of the 5-HT2A receptor prevents amphetamine-induced increases in premature responding (Fletcher et al, 2011), although this drug also reduces impulsive actions when administered alone (Fletcher et al, 2007; Higgins et al, 2003). Amphetamine, therefore, may affect performance in the RI task by interacting with the multiple neurotransmitter systems that directly or indirectly (ie, via timing, avoidance, or attention) alter impulsive action.
Impulsive actions are a feature of several psychiatric disorders, including ADHD (Kollins, 2008). In this investigation, we examined amphetamine's effect on impulsive action, but our analysis was on the entire population, instead of subjects with elevated impulsivity. Interestingly, individual differences in trait impulsivity are strongly correlated with changes in dopamine receptor binding in the striatum (Dalley et al, 2007), which suggests a possible mechanism for amphetamine's effects in the clinical population.
This investigation aimed to reconcile how stimulants, such as amphetamine, can have distinct effects on impulsivity, depending on the design of the behavioral paradigm. Our findings further emphasize that the effects of a drug on any behavioral measure must be interpreted in the context of the cognitive processes that are controlling responding.
Acknowledgments
This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to MCO, and by NSERC postgraduate scholarships to SJH and ACM.
The authors declare no conflict of interest.
References
- Agmo A, Belzung C, Rodriguez C. A rat model of distractibility: effects of drugs modifying dopaminergic, noradrenergic and GABAergic neurotransmission. J Neural Transm. 1997a;104:11–29. doi: 10.1007/BF01271291. [DOI] [PubMed] [Google Scholar]
- Agmo A, Medrano A, Garrido N, Alonso P. GABAergic drugs inhibit amphetamine-induced distractibility in the rat. Pharmacol Biochem Behav. 1997b;58:119–126. doi: 10.1016/s0091-3057(96)00380-2. [DOI] [PubMed] [Google Scholar]
- Balci F, Ludvig EA, Gibson JM, Allen BD, Frank KM, Kapustinski BJ, et al. Pharmacological manipulations of interval timing using the peak procedure in male C3H mice. Psychopharmacology (Berl) 2008;201:67–80. doi: 10.1007/s00213-008-1248-y. [DOI] [PubMed] [Google Scholar]
- Befort K, Mahoney MK, Chow C, Hayton SJ, Kieffer BL, Olmstead MC. Effects of delta opioid receptors activation on a response inhibition task in rats. Psychopharmacology (Berl) 2011;214:967–976. doi: 10.1007/s00213-010-2108-0. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizarro L, Patel S, Murtagh C, Stolerman IP. Differential effects of psychomotor stimulants on attentional performance in rats: nicotine, amphetamine, caffeine and methylphenidate. Behav Pharmacol. 2004;15:195–206. [PubMed] [Google Scholar]
- Bizot JC. Effects of psychoactive drugs on temporal discrimination in rats. Behav Pharmacol. 1997;8:293–308. doi: 10.1097/00008877-199708000-00003. [DOI] [PubMed] [Google Scholar]
- Bizot JC. Effects of various drugs including organophosphorus compounds (OPC) and therapeutic compounds against OPC on DRL responding. Pharmacol Biochem Behav. 1998;59:1069–1080. doi: 10.1016/s0091-3057(97)00519-4. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology. 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Kritkausky RP. Absence of a response-rate-dependent effect of d-amphetamine on a DRL schedule when reinforcement is signaled. Psychon Sci. 1972;26:285–286. [Google Scholar]
- Cole BJ, Robbins TW. Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic-noradrenergic interactions. Psychopharmacology (Berl) 1987;91:458–466. doi: 10.1007/BF00216011. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci. 2003;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- Eckerman DA, Segbefia D, Manning S, Breese GS. Effects of methylphenidate and d-amphetamine on timing in the rat. Pharmacol Biochem Behav. 1987;27:513–515. doi: 10.1016/0091-3057(87)90356-x. [DOI] [PubMed] [Google Scholar]
- Feola TW, de Wit H, Richards JB. Effects of d-amphetamine and alcohol on a measure of behavioral inhibition in rats. Behav Neurosci. 2000;114:838–848. doi: 10.1037/0735-7044.114.4.838. [DOI] [PubMed] [Google Scholar]
- Fester CB, Skinner BF. Schedules of Reinforcement. Appleton-Century-Crofts: New York; 1957. [Google Scholar]
- Fletcher PJ, Rizos Z, Noble K, Higgins GA. Impulsive action induced by amphetamine, cocaine and MK801 is reduced by 5-HT(2C) receptor stimulation and 5-HT(2A) receptor blockage. Neuropharmacology. 2011;61:468–477. doi: 10.1016/j.neuropharm.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA. Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 2007;195:223–234. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Pinkston J, Vorontsova E. Timing and space usage are disrupted by amphetamine in rats maintained on DRL 24-s and DRL 72-s schedules of reinforcement. Psychopharmacology (Berl) 2009;204:213–225. doi: 10.1007/s00213-008-1451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse S, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Grilly DM. A verification of psychostimulant-induced improvement in sustained attention in rats: effects of d-amphetamine, nicotine, and pemoline. Exp Clin Psychopharmacol. 2000;8:14–21. doi: 10.1037//1064-1297.8.1.14. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology (Berl) 1997;133:329–342. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- Hayton SJ, Olmstead MC.2009Fractionating animal models of motor impulsivity: reconciling the neurochemistry of disinhibitionIn: Granon S (ed).Endophenotypes of Psychiatric and Neurodegenerative Disorders in Rodent Models Transworld Research Network: Kerala, India; 135–158. [Google Scholar]
- Hayton SJ, Lovett-Barron M, Dumont EC, Olmstead MC. Target-specific encoding of response inhibition: increased contribution of AMPA to NMDA receptors at excitatory synapses in the prefrontal cortex. J Neurosci. 2010;30:11493–11500. doi: 10.1523/JNEUROSCI.1550-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Enderlin M, Haman M, Fletcher PJ. The 5-HT2A receptor antagonist M100,907 attenuates motor and ‘impulsive-type' behaviours produced by NMDA receptor antagonism. Psychopharmacology (Berl) 2003;170:309–319. doi: 10.1007/s00213-003-1549-0. [DOI] [PubMed] [Google Scholar]
- Jolly DC, Richards JB, Seiden LS. Serotonergic mediation of DRL 72s behavior: Receptor subtype involvement in a behavioral screen for antidepressant drugs. Biol Psychiatry. 1999;45:1151–1162. doi: 10.1016/s0006-3223(98)00014-6. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kollins SH. ADHD, substance use disorders, and psychostimulant treatment: current literature and treatment guidelines. J Atten Disord. 2008;12:115–125. doi: 10.1177/1087054707311654. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Falk JL. Dose-dependent effects but not sensitization of DRL 45-s performance by oral d-amphetamine with cumulative- and repeated-dosing regimens. Behav Pharmacol. 1999;10:739–746. doi: 10.1097/00008877-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Popke EJ, Fogle CM, Paule MG. Similar effects of amphetamine and methylphenidate on performance of complex operant tasks in rats. Behav Brain Res. 2000;109:59–68. doi: 10.1016/s0166-4328(99)00165-5. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Brain Res Cogn Brain Res. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Meck WH, Penney TB, Pouthas V. Cortico-striatal representation of time in animals and humans. Curr Opin Neurobiol. 2008;18:145–152. doi: 10.1016/j.conb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, et al. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Poncelet M, Chermat R, Soubrie P, Simon P. The progressive ratio schedule as a model for studying the psychomotor stimulant activity of drugs in the rat. Psychopharmacology (Berl) 1983;80:184–189. doi: 10.1007/BF00427967. [DOI] [PubMed] [Google Scholar]
- Rachlin HC, Green L. Commitment, choice and self-control. J Exp Anal Behav. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, Seiden LS. Fluoxetine prevents the disruptive effects of fenfluramine on differential-reinforcement-of-low-rate 72-second schedule performance. J Pharmacol Exp Ther. 1993;267:1256–1263. [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, et al. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Andresen J, MacPhail RC. Methylphenidate and d-amphetamine: effects and interactions with alphamethyltyrosine and tetrabenazine on DRL performance in rats. Pharmacol Biochem Behav. 1979;10:577–584. doi: 10.1016/0091-3057(79)90236-3. [DOI] [PubMed] [Google Scholar]
- Sokolowski JD, Seiden LS. The behavioral effects of sertraline, fluoxetine, and paroxetine differ on the differential-reinforcement-of-low-rate 72-second operant schedule in the rat. Psychopharmacology (Berl) 1999;147:153–161. doi: 10.1007/s002130051155. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Taylor KM, Horvitz JC, Balsam PD. Amphetamine affects the start of responding in the peak interval timing task. Behav Processes. 2007;74:168–175. doi: 10.1016/j.beproc.2006.11.005. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Brueggeman RJ, Bronius PF, Schoffelmeer AN, Vanderschuren LJ. Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology (Berl) 2006;187:73–85. doi: 10.1007/s00213-006-0396-1. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Compton AD, Golden KM. Separation of drug effects on timing and behavioral inhibition by increased stimulus control. Exp Clin Psychopharmacol. 2000;8:451–461. doi: 10.1037//1064-1297.8.4.451. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]