Abstract
Methamphetamine is a highly addictive psychomotor stimulant yet the neurobiological consequences of methamphetamine self-administration remain under-characterized. Thus, we employed microdialysis in rats trained to self-administer intravenous (IV) infusions of methamphetamine (METH-SA) or saline (SAL) and a group of rats receiving non-contingent IV infusions of methamphetamine (METH-NC) at 1 or 21 days withdrawal to determine the dopamine and glutamate responses in the nucleus accumbens (NAC) to a 2 mg/kg methamphetamine intraperitoneal challenge. Furthermore, basal NAC extracellular glutamate content was assessed employing no net-flux procedures in these three groups at both time points. At both 1- and 21-day withdrawal points, methamphetamine elicited a rise in extracellular dopamine in SAL animals and this effect was sensitized in METH-NC rats. However, METH-SA animals showed a much greater sensitized dopamine response to the drug challenge compared with the other groups. Additionally, acute methamphetamine decreased extracellular glutamate in both SAL and METH-NC animals at both time-points. In contrast, METH-SA rats exhibited a modest and delayed rise in glutamate at 1-day withdrawal and this rise was sensitized at 21 days withdrawal. Finally, no net-flux microdialysis revealed elevated basal glutamate and increased extraction fraction at both withdrawal time-points in METH-SA rats. Although METH-NC rats exhibited no change in the glutamate extraction fraction, they exhibited a time-dependent elevation in basal glutamate levels. These data illustrate for the first time that a history of methamphetamine self-administration produces enduring changes in NAC neurotransmission and that non-pharmacological factors have a critical role in the expression of these methamphetamine-induced neurochemical adaptations.
Keywords: methamphetamine, addiction, nucleus accumbens, dopamine, glutamate, self-administration
INTRODUCTION
Methamphetamine is a highly addictive, potent amphetamine derivative and the increasing prevalence of methamphetamine addiction poses serious social and health concerns world-wide (SAMHSA, 2009). Long-term methamphetamine abuse produces pronounced cognitive, behavioral, and emotional deficits that are associated with a high potential for relapse following periods of abstinence (Berman et al, 2009; Elkashef et al., 2008), yet current therapeutic interventions are extremely limited. Thus, a deeper understanding of the neurobiological consequences associated with the development and maintenance of methamphetamine self-administration in animal models will facilitate the design of rationale pharmaco-therapeutics for the management of methamphetamine addiction.
A circuit of interconnected brain structures mediating cognitive, motivational, and emotional processing has been characterized, which is critical for stimulant-related behaviors and addictive processes. Specifically, the activation of goal-directed behavior, including drug-seeking, requires intact dopamine projections from the ventral tegmental area (VTA) to several forebrain limbic structures, including the nucleus accumbens (NAC), prefrontal cortex (PFC), ventral pallidum, and the basolateral amygdala, whereas intact glutamate signaling within projections from the PFC, amygdala, and hippocampus to the NAC is critical for the manifestation of various forms of addiction-related behavioral plasticity (eg, Everitt et al, 2008; Kalivas, 2009; Wolf, 2010). Thus, the NAC is a focal point for both dopamine and glutamate systems in addiction-related processes. Methamphetamine is a highly potent releaser of monoamines throughout the mesocorticolimbic circuit (eg, Abekawa et al, 1994; Akimoto et al, 1990; Kashihara et al, 1991; Kazahaya et al, 1989; Stephans and Yamamoto, 1995; Weihmuller et al, 1991; Bustamante et al, 2002; Zhang et al, 2001). In animal models, repeated, non-contingent methamphetamine injections elicit robust sensitization of methamphetamine-elicited dopamine release (ie, dopamine sensitization) within striatal regions (eg, Arai et al, 1996; Camp et al, 1994; Shimada et al, 1996; Suzuki et al, 1997) that is associated with sensitization of methamphetamine-elicited locomotor activity (ie, behavioral sensitization; eg, Akiyama et al, 1994; Camp et al, 1994; Vanderschuren and Kalivas, 2000) and appears to be critical for drug-related learning and drug-craving during withdrawal (cf, Belin et al, 2009; Bradberry 2007; Di Chiara and Bassareo, 2007; Robinson and Berridge, 2008; Thomas et al, 2008).
Limited attention has been paid to the effects of methamphetamine on glutamate neurotransmission, however, a critical role of glutamate signaling in the ‘addictive' properties of methamphetamine is supported by findings that both intravenous (IV) methamphetamine self-administration and cue-induced reinstatement of methamphetamine-seeking are blocked by mGluR5 antagonism in rats (Osborne and Olive, 2008). Moreover, an active role for glutamate in methamphetamine-induced behavioral plasticity has been implicated by two lines of research. First, pharmacological blockade of NMDA, AMPA, or mGluR5 antagonists, or deletion of the NMDA episilon 1 subunit, attenuates the conditioned rewarding and behavioral sensitizing effects of methamphetamine (Kim and Jang, 1997; Miyamoto et al, 2004; Miyatake et al, 2005). Similarly, enhancement and inhibition of glutamate transporters attenuates and facilitates, respectively, methamphetamine-induced place-preference and behavioral sensitization (Fujio et al, 2005a, 2005b; Nakagawa et al, 2005). Second, low-dose (ie, <4 mg/kg intraperitoneal (IP)) experimenter-administered/non-contingent methamphetamine produces changes in forebrain glutamate transmission in some, but not all, studies (Fang et al, 2005; Ohmori et al, 1996; Shoblock et al, 2003; Xue et al, 1996; Zhang et al, 2001); but, in contrast to other stimulants (Vanderschuren and Kalivas, 2000; Wolf, 2010), the glutamate-altering effects of repeated methamphetamine injections are only slightly augmented by withdrawal (Fang et al, 2005; Ohmori et al, 1996; Shoblock et al, 2003; Xue et al, 1996; Zhang et al, 2001). However, there exists a large literature indicating methamphetamine–glutamate interactions in mediating high-dose methamphetamine-induced neuronal toxicity within the forebrain (Abekawa et al, 1994; Battaglia et al, 2002; Burrows et al, 2000; Davidson et al, 2007; Golembiowska et al, 2003; Marshall et al, 1993; Simões et al, 2007, 2008; Sonsalla et al, 1989; Tata and Yamamoto, 2007). Although these latter studies are relevant to cellular processes induced by very heavy methamphetamine use, they fail to assess the potential role for glutamate in the establishment of methamphetamine self-administration when drug intake is relatively low (eg, Gass et al, 2009; Schwendt et al, 2009; Shepard et al, 2006, Stefanski et al, 1999, 2002, 2004).
Given the above issue and considering that the manifestation of drug-induced cellular and molecular adaptations, including neurochemical sensitization, can depend on the behavioral contingency of drug delivery (eg, Lecca et al, 2007a, 2007b; McFarland et al, 2003; Stefanski et al, 1999, 2002), this study sought to extend the aforementioned research by examining the short- and longer-term consequences of a history of IV methamphetamine self-administration on NAC extracellular dopamine and glutamate levels. It was hypothesized that a history of contingent vs non-contingent methamphetamine exposure would exert differential effects on basal and/or methamphetamine-stimulated neurotransmitter release. On the basis of the existing literature for cocaine (eg, McFarland et al, 2003), we predicted that methamphetamine self-administration would lower basal NAC glutamate content and elicit greater dopamine and glutamate sensitization in animals with contingent methamphetamine exposure. The present findings demonstrate that methamphetamine self-administration produces neurochemical adaptations within the NAC that are distinct from those produced by non-contingent methamphetamine treatment, indicating that non-pharmacological factors critically regulate methamphetamine-induced neuroplasticity within NAC dopamine and glutamate systems.
SUBJECTS AND METHODS
Subjects
Male Sprague–Dawley rats (300–325 g) from Charles-River (Wilmington, MA) were individually housed in a temperature- and humidity-controlled vivarium on a 12-h light–dark cycle (lights off: 0700 hours). Rats were allowed ad libitum access to rat chow and water. All procedures followed the ‘Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research' (National Research Council 2003) and were approved by our Institutional Animal Care and Use Committee.
Surgery
The surgical procedures for implanting chronic indwelling IV catheters and bilateral guide cannulae into the NAC of rats were identical to those described previously by our group (eg, Kerstetter et al, 2008; Kippin et al, 2008; Zayara et al, 2011). In brief, rats were anesthetized by inhalation with 4% isoflurane. Chronic indwelling catheters were constructed using a bent steel cannula with a screw-type connector (Plastics One, Roanoke, VA), silastic tubing (10 cm, i.d. 0.64 mm, o.d. 1.19 mm; Dow Corning, Midland, MI), prolite polypropylene monofilament mesh (Atrium Medical, Hudson, NH), and cranio-plastic cement. One end of the catheter was inserted into the right jugular vein and secured to surrounding tissue with suture and the other end ran subcutaneously to the cannula port, which was placed posterior to the rat's shoulder blades. Immediately following jugular surgery, rats were placed into a Kopf stereotaxic device and implanted with bilateral cannulae (20 gauge; 20 mm long; Plastics One) directed 2 mm above the interface between the shell and core subdivisions of the NAC using the following coordinates (in mm; derived from Paxinos and Watson, 1998): AP: +1.2 mm; ML: ±2.5 mm, DV: −4.6 mm. Guide cannulae were secured with jeweler screws and dental acrylic and were occluded by a 24-gauge dummy cannula (Plastics One).
For 7 days following surgery, the animals were monitored and the catheters were flushed once daily with 0.1 ml of Timentin antibiotic solution (GlaxoSmithKline, Research Triangle Park, NC) and then 0.1 ml of heparinized solution (100 U/ml; APP Pharmaceuticals, LCC, Schaumburg, IL). Catheter patency was verified periodically by infusing 0.1 ml of methohexital sodium (10 mg/ml IV; Eli Lilly, Indianapolis, IN) and assessing loss of muscle tone.
Methamphetamine Self-Administration
Behavioral tests were conducted during the rats' dark cycle in sound-attenuated operant conditioning chambers (30 × 20 × 24 cm high; Med Associates, St Albans, VT) equipped with two retractable levers, stimulus lights above each lever and a speaker connected to a tone generator (78 dB, 2 kHz; ANL-926, Med Associates). At the start of each session, the rat's catheter was flushed with 0.1 ml of heparin solution and then connected to a liquid swivel (Instech, Plymouth Meeting, PA) via polyethylene 20 tubing that was encased in steel spring leashes (Plastics One) with the swivel suspended above the chamber and connected to an infusion pump (Model PHM-100, Med Associates). Following each session, the rat's catheter was flushed with 0.1 ml each of the Timentin and heparin solutions.
For rats in the methamphetamine self-administration condition (METH-SA; n=7), rats were trained to press a lever according to a FR 1 schedule of methamphetamine reinforcement (Sigma-Aldrich, St Louis, MO). Lever presses on the ‘active' lever resulted in a 3.7-s activation of the infusion pump, a 0.05 ml infusion and a 5-s presentation of a tone-light stimulus complex above the active lever. This was followed by a 20-s time-out period. Responses on the ‘inactive' lever were recorded but had no programmed consequences. METH-SA rats were initially trained to self-administer methamphetamine at a dose of 0.01 mg/kg per infusion during 5 daily 1-h sessions. In order to progressively increase methamphetamine intake, rats were then allowed to self-administer methamphetamine at the same dose for 2 daily 2-h sessions and next the dose was increased to 0.05 mg/kg per infusion for 10 daily 2-h sessions. For rats in the non-contingent methamphetamine condition (METH-NC; n=10), each rat was placed in identical chambers with their catheter connected to the infusion line and both levers extended. Lever presses were recorded but had no programmed consequences. METH-NC rats received methamphetamine infusions in a passive fashion with the amount matching that received by METH-SA rats (ie, same number and dose of infusions accompanied by the light+tone stimulus complex with the frequency of infusions distributed evenly throughout a session)—although this pattern of administration does not perfectly match the self-administration pattern (ie, absence of loading phase; see Figure 2d), given the long half-life of methamphetamine in rats (60–70 min; Melega et al, 1995; Rivie‘re et al, 1999), the levels of intoxication are expected to be similar in the METH-SA and METH-NC groups (see below). For rats in the saline self-administration condition (SAL; n=9), each rat was tested in the identical fashion as the METH-SA rats above except that each active lever response resulted in an infusion of saline accompanied by the light+tone stimulus complex and followed by a 20-s time-out.
In Vivo Microdialysis
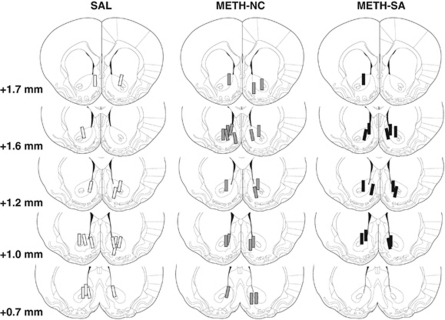
Each rat underwent two in vivo microdialysis sessions occurring 1 day and 21 days following the last self-administration session using previously described conventional and no net-flux procedures (eg, Kapasova and Szumlinski, 2008; Kippin et al, 2008; Zayara et al, 2011). In order to minimize the impact of methamphetamine-paired cues on neurochemical measures, microdialysis procedures were conducted in a different chamber within a room separate from those in which IV administration took place and by a separate experimenter. On the day before the microdialysis session, a dialysis probe (24 gauge, 20 mm in length with 1.6–1.8 mm of active membrane) was inserted unilaterally and counter-balanced by hemisphere across animals. The animals were then hooked up to a liquid swivel, placed in a holding cage and microdialysis buffer (NaCl, 147.2 mM; CaCl2, 1.53 mM; KCl, 2.7 mM; MgCl2, 2.1 mM; adjusted to pH=7.4) was perfused overnight at a rate of 0.25 μl/min. Sample collection began the next day, following a 1-h equilibration period with the flow rate at 2 μl/min and was conducted in 20-min intervals into collection vials containing 10 μl of preservative (10% methanol (v/v), 15% acetonitrile (v/v), 150 mM NaPO4, 4.76 mM citric acid, 3 mM SDS, 50 μM EDTA, pH=5.6). For no net-flux studies, glutamate was dissolved in microdialysis buffer and, following 1 h of baseline sample collection, glutamate concentrations (2.5, 5, and 10 μM) were perfused through the microdialysis probe via a liquid switch (CMA Microdialysis; Acton, MA) in ascending order for 60-min intervals. All baseline samples and the last two samples of each drug concentration were averaged to calculate the net-flux of glutamate and used in the statistical analysis of the data. Following 1 h of baseline re-establishment, a challenge injection of 2 mg/kg methamphetamine was administered IP—this dose and route of administration was selected because it elicits robust increases in dopamine and decreases in glutamate in the NAC (eg, Shoblock et al, 2003) and minimizes subject attrition because of loss of catheter patency during protracted withdrawal from methamphetamine self-administration. Following the first microdialysis session, the probe was removed and the dummy cannula was replaced. The second microdialysis session occurred 3 weeks later and employed identical procedures to the first, using the opposite hemisphere. Microdialysis probe placements were verified using standard histochemical methods (Figure 1).
Figure 1.
Verification of microdialysis probe membrane location. Post-mortem examination revealed that the probe membranes were located within the nucleus accumbens in all animals tested. Probe placements were consistent between all treatment groups. METH-NC, non-contingent IV methamphetamine; METH-SA, methamphetamine self-administering; SAL, saline self-administering; numbers in figure indicate distance from Bregma based on Paxinos and Watson (1998).
HPLC Analysis of Glutamate and Dopamine
The high-pressure liquid chromatography (HPLC) procedures for the sequential detection of dopamine and glutamate within a dialysate sample were identical to those described previously by our group (eg, Kapasova and Szumlinski, 2008; Kippin et al, 2008; Zayara et al, 2011). In brief, glutamate and dopamine were measured using electrochemical detection on an ESA Coularray HPLC system (ESA, Bedford, MA). For HPLC analysis of dopamine, the MD-TM mobile phase was employed (ESA), and neurotransmitters in 30 μl from each 50 μl sample were separated using a MD-150 × 3.2 mm column (ESA). An ESA 5014B analytical cell was used for the detection of monoamines (oxidation and reduction electrode potentials of +220 and −150 mV, respectively). For glutamate, the mobile phase consisted of 3.5% acetonitrile (v/v), 22% methanol (v/v), 100 mM NaPO4, pH=6.75. A reversed phase column (50 × 3.0 mm capcell PAK; Shiseido, Tokyo, Japan) was used to separate the amino acids, and precolumn derivatization with o-phthaladehyde (2.7 mg/ml) of the 20 μl from each 50 μl sample was performed using an ESA Model 540 autosampler (ESA). Glutamate was detected using an electrochemical analytical cell with an oxidizing potential of +550 mV. Glutamate and dopamine content in each sample were analyzed by peak height and compared with an external standard curve for quantification (glutamate standards: 2.5, 5.0, 10 μM; dopamine standards: 1.25, 2.5, 5 nM).
Statistical Analyses
Behavioral and neurochemical data were analyzed using analyses of variance (ANOVAs). The self-administration data were analyzed using a Treatment (SAL, METH-NC, and METH-SA) × Lever (active vs inactive) × Session ANOVA, with repeated measures on the Session factor (17 days). For analyses of cumulative dosing and estimated intoxication, data for the last day of methamphetamine administration were analyzed in real-time and then blocked into 10-min intervals; estimated intoxication was calculated from cumulative dosing subtracting estimated metabolism based on a half-life of 70 min. Cumulative dose and estimated intoxication levels were analyzed using a Treatment × Time ANOVA with repeated measures on the Time factor (12, 10-min bins). The neurochemical data collected under conventional microdialysis procedures were analyzed using a Treatment × Withdrawal × Time ANOVA, with repeated measures on the Time factor (12, 20-min bins). The average basal glutamate content (x-intercept) and extraction fraction (slope) of the linear regression analyses from the glutamate no net-flux study were analyzed using a Treatment × Withdrawal ANOVA. In order to include the data from all the animals that underwent microdialysis procedures, the Withdrawal factor was treated as a between-subjects factor for the neurochemical analyses. As in previous studies (eg, Kapasova and Szumlinski, 2008; Zayara et al, 2011), this statistical approach was used to minimize subject attrition as data could not be obtained for both microdialysis sessions for all animals because of nonsystematic microdialysis probe failure and technical issues with the HPLC. Significant interactions were deconstructed for main effects and post-hoc comparisons were conducted using Least Significant Difference tests when appropriate, α=0.05.
RESULTS
Effect of Contingent vs Non-Contingent Methamphetamine on Operant Lever Responding Behavior
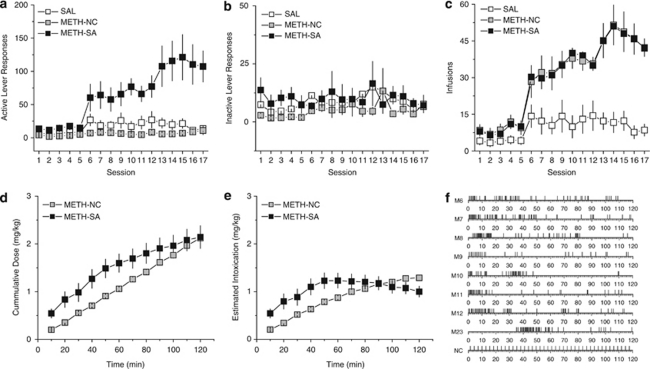
Methamphetamine infusions delivered in a contingent fashion under an escalating dose and access schedule (see above) produced a selective increase in responding on the active lever in METH-SA rats, relative to both that on the inactive lever and that exhibited by SAL rats. In contrast, non-contingent methamphetamine infusions failed to elevate responding on either lever in METH-NC rats (Figures 2a and b) (Treatment × Lever × Session: F(32, 768)=2.95, p<0.0001). For the METH-SA rats, there was a significant session by lever interaction (F(16, 224)=2.61, p<0.001) with the number of active lever responses exceeding the number of inactive lever responses during sessions 6–17 (all p's<0.05), but not during sessions 1–5. Conversely, for the METH-NC rats, there were no significant main effects or interaction between sessions or levers (all p's>0.05). For the SAL rats, there was a significant main effect of session (F(16, 256)=3.77, p<0.001), with transiently elevated responding on both levers during sessions 6 and 12, but there was no significant main effect of lever, nor was there a significant interaction between session and lever. In addition, an analysis of active lever responses revealed a significant interaction (Treatment × Session: F(32, 384)=2.84, p<0.0001), with the METH-SA rats exhibiting higher responding than the other groups during sessions 6–17 (all p's<0.05), but no significant differences were found between the SAL and METH-NC groups during any session. In contrast, the same analysis of inactive lever responses failed to reveal significant main effects of either Treatment or Session nor a significant interaction between these factors (all p's>0.05). Together, these data indicate that the 0.01 and 0.05 mg/kg methamphetamine infusions delivered in a contingent fashion were reinforcing during 2-h sessions, whereas equivalent levels of non-contingent methamphetamine exposure fails to increase lever responses and contingent saline paired with the light-tone complex failed to be reinforcing.
Figure 2.
Escalating access to IV methamphetamine elicits increased lever-pressing behavior and intake across training days. Summary of the number of active (a) and inactive (b) lever responses and infusions received (c) exhibited by rats self-administering IV methamphetamine (METH-SA) or saline (SAL), as well as rats receiving non-contingent IV infusions of methamphetamine (METH-NC). During operant training, METH-SA animals had access to 0.01 mg/kg per infusion for 60 min on days 1–5, then 0.01 mg/kg per infusion for 120 min on days 6–7, followed by 0.05 mg/kg per infusion for 120 min on days 8–17. This access resulted in a progressive increase in both the number of lever-presses and drug infusions exhibited by METH-SA animals across days. The cumulative dosing (d) and estimated intoxication (e) levels for the METH-SA and METH-NC groups during the final day of treatment indicate a faster dosing in the METH-SA rats but nearly identical total dosing and peak intoxication levels. Time course of methamphetamine infusions for each of the METH-SA rats (f, top 8 graphs) and METH-NC rats (f, bottom graph) during the last day of treatment.
Similarly, the METH-SA and METH-NC groups received more infusions than did the saline groups during most of the sessions (Figure 2c) (Treatment × Session: F(32, 384)=7.37, p<0.0001), with the METH-SA and METH-NC rats receiving more infusions than the SAL rats during sessions 4–17 (all p's<0.05). The methamphetamine exposure for the METH-SA and METH-NC rats during the last three sessions averaged 2.27 mg/kg per session and the total exposure was 21.61 mg/kg for the entire experiment. Notably, the cumulative dose and rate of intoxication differed between the METH-SA and METH-NC groups (Figures 2d and e) (for cumulative dose, Treatment × Time: F(1, 11)=4.21, p<0.0001; for estimated intoxication, Treatment × Time: F(1, 11)=11.69, p<0.0001); METH-SA rats exhibited significantly higher cumulative dosing than METH-NC rats for intervals 0–10 min through 60–70 m and higher estimated intoxication for intervals 0–10 min through 50–60 min, whereas estimated intoxication was significantly higher in the METH-NC rats than METH-SA rats for the interval 110–120 min. Nevertheless, the average peak intoxication levels were almost identical for METH-SA and METH-NC groups at 1.30±0.16 and 1.27±0.06 mg/kg, respectively. Further, qualitative analyses of the time-course of infusions during the last self-administration session revealed a high degree of variability between rats (Figure 2f). Although all METH-SA rats exhibited a loading phase, it could be delayed a few (M8) or many (M23) min and the number of infusions during the loading phase varied dramatically between rats (eg, M10 vs M12). Further, rats often exhibited long periods without infusions (eg, >60 min by M10). Additionally, the pattern varied across days substantially; for instance, M23 exhibited a delay of 34.9 min before the first self-administered infusion on the final day of treatment but only a delay of 11 s the preceding day. Conversely, the METH-NC received a consistent pattern of infusions in a predictable fashion (Figure 2f).
Differential Effects of Self-Administered vs Non-Contingent Methamphetamine on NAC Dopamine Release
Neither a history of methamphetamine administration nor the duration of withdrawal significantly affected basal extracellular dopamine levels in the NAC (Table 1). The lack of group and withdrawal effects was confirmed by the results of a Treatment × Withdrawal ANOVA (all p's>0.05).
Table 1. Comparison of Average Baseline Glutamate and Dopamine Levels Before a 2 mg/kg METH Challenge Conducted at 24 h and 3 Weeks Withdrawal from Self-Administration, as Assessed Using a Conventional In Vivo Microdialysis Approach.
| SAL | NC | METH | |
|---|---|---|---|
| Glutamate (ng per sample) | |||
| 24 h | 10.0±3.4 (9) | 7.5±1.6 (10) | 3.7±0.4 (7) |
| 3 Weeks | 7.9±3.7 (9) | 7.3±1.4 (8) | 4.3±1.0 (5) |
| Dopamine (pg per sample) | |||
| 24 h | 4.2±0.8 (8) | 6.3±1.1 (10) | 4.5±0.6 (7) |
| 3 Weeks | 4.5±0.7 (9) | 3.5±0.6 (8) | 5.2±0.9 (5) |
Abbreviations: METH, methamphetamine self-administering rats; NC, non-contingent METH rats; SAL, saline self-administering rats.
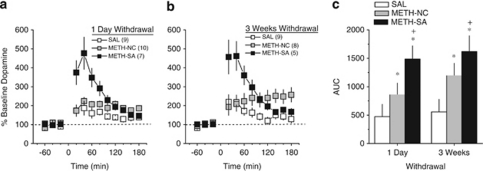
As there were no significant differences in basal extracellular dopamine levels, the data for the dopamine response to an IP challenge injection of 2 mg/kg methamphetamine obtained at 1 day and 3 weeks withdrawal was expressed as a percent change from the average baseline levels (from Table 1) and presented in Figures 3a and b, respectively. As can be observed from Figure 3, both the magnitude and the time-course of the NAC dopamine response to the methamphetamine challenge injection were unaffected by the duration of withdrawal (no main effects of, or interactions with the Withdrawal factor, p>0.05). During both microdialysis sessions, METH-SA animals exhibited the most robust methamphetamine-elicited rise in dopamine, which peaked between 400 and 500% above baseline levels during early sampling and declined progressively to levels approximately 140% above baseline by the end of the 3-h microdialysis session (Figure 3). While the general shape of the time-course of methamphetamine-induced dopamine release was similar between METH-SA and SAL animals (ie, early peak, followed by a progressive decline in levels), the magnitude of the early peak was considerably lower (175–200% above baseline) in SAL rats, indicating that a history of methamphetamine self-administration elicits dopamine sensitization in the NAC. Intriguingly, the time-course of methamphetamine-induced dopamine release exhibited by METH-NC rats was essentially flat, with dopamine levels between 175 and 250% above baseline for the duration of the 3-h microdialysis session (Treatment × Time: F(22, 418)=14.03, p<0.0001).
Figure 3.
The magnitude of methamphetamine-induced accumbens dopamine sensitization is greatest in rats with contingent methamphetamine exposure. At 24 h (a) and 3 weeks (b) following the last operant session, conventional microdialysis was employed to assay the effects of a 2 mg/kg IP methamphetamine challenge injection on accumbens dopamine of rats with a history of methamphetamine self-administration (METH-SA), rats with a history of saline self-administration (SAL) and rats with a history of non-contingent IV methamphetamine exposure (METH-NC). To facilitate visualization of group differences in the magnitude of methamphetamine-induced dopamine release, the area under the time-course curves (AUC) from panels (a, b) were calculated and are presented in panel (c). *p<0.05 vs SAL; +p<0.05 vs METH-NC (LSD post-hoc tests).
As it was not entirely clear from the time-course data whether or not METH-NC rats exhibited dopamine sensitization (ie, had a greater dopamine response vs SAL controls), we calculated and then compared the area under the curve (AUC) for the rise in dopamine post-injection (Figure 3c). Although METH-SA animals exhibited the greatest rise in dopamine, the dopamine rise in METH-NC rats was also significantly greater than that of their SAL counterparts and the group differences did not vary with the duration of withdrawal (Treatment effect: F(2, 44)=9.54, p<0.0001; LSD post-hoc tests). Together, these data provide novel evidence that a history of methamphetamine self-administration elicits dopamine sensitization in the NAC and, moreover, that the magnitude of this dopamine sensitization reflects more than the pharmacological consequences of IV exposure to the drug.
Differential Effects of Self-Administered vs Non-Contingent Methamphetamine on NAC Glutamate Sensitization
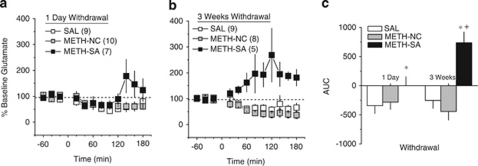
An analysis of the average basal levels of glutamate obtained by conventional in vivo microdialysis procedures before methamphetamine challenge suggested lower glutamate levels in METH-SA animals, relative to the other treatment groups (Table 1). However, the results of the statistical analysis failed to indicate group differences at either withdrawal time-point (Treatment × Withdrawal ANOVA, all p's>0.05). Thus, as per dopamine, the data for the glutamate response to the 2 mg/kg challenge injection was expressed as a percent change from the average baseline values to better illustrate group differences in methamphetamine's effects on glutamate levels and these data are presented in Figures 4a and b.
Figure 4.
Withdrawal from methamphetamine self-administration augments the glutamate response to challenge methamphetamine. (a) When examined 24 h following the final self-administration session, an acute methamphetamine injection (2 mg/kg, IP) produced a mild reduction in the levels of extra-cellular glutamate in saline self-administering animals (SAL) and this effect was not altered in animals receiving non-contingent methamphetamine (METH-NC) exposure. In contrast, the challenge injection of methamphetamine produced a latent, modest rise in extra-cellular glutamate in methamphetamine self-administering rats (METH-SA) and (b) extended withdrawal produced an increase in the magnitude of this effect as well as a more rapid onset. (c) Analysis of the area under the time-course curves (AUC) revealed no differences in the glutamate response to challenge methamphetamine between saline self-administering rats and those receiving non-contingent methamphetamine infusions. However, in methamphetamine self-administering animals, the challenge injection of methamphetamine produced a significant elevation in extra-cellular glutamate, the magnitude of which was significantly greater following 3 weeks withdrawal. *p<0.05 vs SAL; +p<0.05 vs METH-NC (LSD post-hoc tests).
As illustrated in Figure 4, group differences were observed regarding the time-course of methamphetamine-induced glutamate release in the NAC and these group differences depended on the duration of withdrawal (Treatment × Withdrawal × Time: F(22, 462)=2.27, p=0.001). Both SAL and METH-NC rats exhibited an approximately 50% reduction in glutamate relative to baseline levels that (1) persisted throughout sampling and (2) did not change as a function of the withdrawal period (SAL: Time effect: F(11, 176)=4.12, p<0.0001; Withdrawal × Time: p=0.70; METH-NC: F(11, 176)=10.95, p<0.0001; Withdrawal × Time: p=0.53). In contrast, the NAC glutamate response to methamphetamine in METH-SA animals varied as a function of withdrawal (Time effect: F(11, 110)=2.33, p=0.01; Withdrawal × Time: F(11, 110)=1.91, p=0.04). At 1-day withdrawal, the glutamate response to methamphetamine in METH-SA animals was biphasic with respect to time; animals exhibited a drop in glutamate levels followed by a modest, nonsignificant, rise during the final hour of sampling (Figure 4a) (Time effect: p=0.06). However, at 3 weeks withdrawal, the methamphetamine injection elevated glutamate significantly above baseline levels and this effect persisted for the duration of sampling (Time effect: F(11, 44)=2.10, p=0.04). A comparison of the cumulative change in glutamate during the 3-h sampling periods (AUC) better illustrates the time-dependency of group differences in the effects of the methamphetamine challenge injection on NAC glutamate (Figure 4c) (Treatment × Withdrawal: F(2, 42)=4.39, p=0.02). Taken together, these data provide novel evidence that a history of methamphetamine self-administration elicits sensitization of methamphetamine-elicited glutamate release (ie, glutamate sensitization) in the NAC and, perhaps more importantly, that this glutamate sensitization is not a direct pharmacological consequence of IV exposure to the methamphetamine.
Contingent and Non-Contingent Methamphetamine Produce Distinct Effects on NAC Basal Glutamate
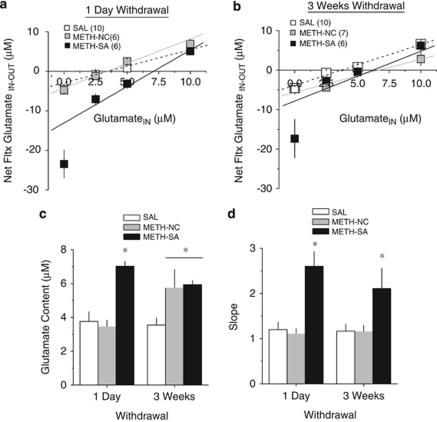
A history of cocaine produces anomalies in basal NAC glutamate content that are associated with greater behavioral sensitivity and relapse vulnerability during withdrawal (cf, Kalivas, 2009). Thus, we employed a no net-flux approach to quantify group differences in glutamate content, as, unlike conventional in vivo microdialysis approaches, this method yields estimates of neurotransmitter content that are independent of potential individual differences in probe recovery (Parsons and Justice 1994). The plots of the net-flux of glutamate vs glutamate infused through the microdialysis probe for the 1-day and 3-week withdrawal periods are presented in Figures 5a and b, respectively. As is apparent in Figures 5a and b, the relation between the net-flux of glutamate vs the amount of glutamate infused through the probe was linear at both withdrawal time-points in SAL and METH-NC animals and these two groups did not appear to differ in this regard. In contrast, the 0 μM data point for the METH-SA animals appeared to deviate from the line of best-fit at both withdrawal time-points, with the relatively large negative glutamate net-flux values indicative of greater gain of glutamate in the perfusate (ie, higher basal extracellular glutamate levels). To confirm this observation, the point at which no glutamate is gained or lost from the perfusate (ie, y=0) was calculated from a linear regression analyses of the plots to yield an estimate of basal glutamate content, independent of probe recovery (Bungay et al, 2003; Chefer et al, 2006; Parsons and Justice, 1994). An analysis of the point of no net-flux (y=0) revealed significant group differences that depended on the duration of withdrawal (Treatment × Withdrawal, F(2, 43)=3.79, p=0.03). Relative to SAL animals, METH-SA rats exhibited elevated NAC extracellular glutamate content at both 1-day and 3 weeks withdrawal, while elevated glutamate content was observed in the METH-NC group only at the 3-week withdrawal time-point (Figure 5c). An analysis of the slopes of the linear regressions (ie, the extraction fraction) also revealed an influence of prior methamphetamine history on this variable, but this was independent of withdrawal (Treatment effect: F(2, 43)=15.80, p<0.0001; no main effect of, or interaction with, the Withdrawal factor, p>0.05). The net-flux data points are theorized to reflect both release (at data points below y=0; particularly, the 0 μM concentration) and uptake (data points near and above y=0) mechanisms with slope or ‘extraction fraction' largely reflecting uptake mechanisms (see Bungay et al, 2003; Chefer et al, 2006; Parsons and Justice, 1994). Relative to SAL animals, only METH-SA rats exhibited increased slope and this group difference was apparent at both withdrawal time-points (Figure 5d). Given concern that the estimates of basal glutamate levels and extraction fraction derived from the linear regression analyses of the plots for the METH-SA animals might be heavily influenced by the large gain of glutamate in the perfusate at the 0 μM concentration (Figures 5a and b), we reanalyzed the data without the 0 μM data point. The results of this follow-up analysis revealed comparable statistical results for both y=0 (Treatment × Withdrawal: F(2, 43)=4.67, p=0.01) and for the slope of the linear regressions (Treatment effect: F(1, 43)=8.48, p=0.001; no main effect of, or interaction with, the Withdrawal factor, p>0.05). These data, in conjunction with the data for methamphetamine-induced changes in NAC glutamate (Figure 3) demonstrate that (1) a history of methamphetamine self-administration produces pronounced increases in both basal and methamphetamine-stimulated glutamate release, as well as glutamate uptake, within the NAC; (2) perturbations in NAC glutamate produced by a history of methamphetamine self-administration are present early in withdrawal and persist for at least several weeks; and (3) the glutamate perturbations produced by a history of methamphetamine self-administration are distinct from those produced by non-contingent methamphetamine administration despite very similar levels of intoxication. These data indicate a critical role for non-pharmacological factors in the development of glutamate plasticity of potential relevance for methamphetamine addiction.
Figure 5.
A history of methamphetamine exposure elevates basal levels of extra-cellular glutamate in the nucleus accumbens. The data from panels (a, b) are summarized in panel (c). (a) When assessed using no net-flux microdialysis, methamphetamine self-administering animals (METH-SA) exhibited a significant elevation in basal glutamate concentrations of approximately 75% compared with saline self-administering animals (SAL) when tested 24 h after the final operant session (a). The elevation in glutamate observed in methamphetamine self-administering animals persisted into extended withdrawal at which point a similar elevation was observed in non-contingent methamphetamine-infused animals (METH-NC) (b). Analysis of the slope of the plots indicated a significant change in the methamphetamine self-administering animals at both of the tested time-points, likely indicating a change in reuptake/release mechanisms (d). *p<0.05 vs SAL.
DISCUSSION
This study examined both the short and enduring effects of a history of escalating IV methamphetamine self-administration (∼0.1 mg/kg/day to ∼2.5 mg/kg/day) on NAC levels of two neurotransmitters—dopamine and glutamate—highly implicated in mediating the psychomotor-activating and addictive properties of psychomotor stimulant drugs (cf, Di Chiara and Bassareo, 2007; Everitt et al, 2008; Kalivas, 2009; Wolf, 2010). Consistent, in part, with our hypothesis, the present results indicate that a history of methamphetamine self-administration elicits an enduring dopamine and glutamate sensitization in the NAC that is distinct from that exhibited by animals infused non-contingently with the same amount of drug. However, contrary to our hypothesis that a history of IV methamphetamine infusions would reduce basal NAC extracellular glutamate levels as reported following IV cocaine self-administration (cf, Kalivas, 2009), a history of IV methamphetamine infusions elevated, rather than lowered, basal NAC glutamate content—although this neurochemical adaptation was apparent during early withdrawal only in animals with methamphetamine self-administration experience. Importantly, the employed method of non-contingent methamphetamine exposure produced nearly identical peak intoxication levels as those produced under self-administration (Figure 2e), albeit, the cumulative dosing occurred at a slower rate (Figure 2d). However, the employment of a predictable pattern of non-contingent exposure is likely to have minimized distress (Mutschler and Miczek, 1998), lethality (Dworkin et al, 1995), and differential HPA activation (Mantsch and Goeders, 2000; Palamarchouk et al, 2009; see, however, Galici et al, 2000) that have been observed with cocaine yoking procedures, but have not been well reported for non-contingent methamphetamine exposure. Thus, the data indicate a critical role for behavioral contingency associated with operant responding for methamphetamine in the manifestation of neurochemical adaptations of relevance for our understanding of the neurobiology and treatment of methamphetamine addiction.
Effects of Methamphetamine on Basal and Evoked Neurotransmission Involve Psychological and Pharmacological Factors
Relative to other drugs of abuse, the role for behavioral contingency of drug self-administration in the neurobiological consequences of repeated methamphetamine exposure is poorly characterized. Although several studies have examined extracellular neurotransmitter levels in animals with a history of d-amphetamine self-administration (eg, Di Ciano et al, 1995, 1996, 2001; Ranaldi et al, 1999; Vezina et al, 2002), the present report is the first to assess the effects of a history of IV methamphetamine self-administration on extracellular neurotransmitter levels, as well as, the role for the behavioral contingency of methamphetamine delivery in these effects. In fact, only three reports by Stefanski et al (1999, 2002, 2004) have examined the issue of behavioral contingency of IV methamphetamine delivery on its neurobiological consequences and revealed that methamphetamine-induced reductions in cell body and terminal dopamine receptor expression, as well as increases in NAC sigma-1 receptor expression, occur during short-term (24 h) withdrawal only in animals self-administering methamphetamine. Moreover, a comparison of results between studies using experimenter-injected animals and those employing methamphetamine self-administration models reveals a number of discrepancies with respect to dopamine receptor/transporter expression, as well as neurotoxicity; typically, IV methamphetamine self-administering animals exhibit either no or very transient changes in the expression of dopamine-related proteins, without obvious signs of terminal toxicity (Schwendt et al, 2009; Shepard et al, 2006; Stefanski et al, 2002; Volz et al, 2007). Consistent with these latter findings, we failed to observe significant effects of methamphetamine self-administration on basal NAC extracellular dopamine levels (Table 1), while reductions in NAC extracellular dopamine content are typically reported following repeated, high dose, neurotoxic methamphetamine injections (eg, Broom and Yamamoto, 2005).
However, the behavioral contingency of IV methamphetamine delivery dramatically impacted the magnitude of dopamine sensitization (Figure 3), the development of glutamate sensitization (Figure 4), the time of onset of elevated basal glutamate levels during withdrawal and changes in indicators of basal glutamate release and uptake (Figure 5), with methamphetamine producing greater, earlier or selective effects in animals with self-administration history, compared with animals infused passively with drug but with similar levels of intoxication. The present in vivo microdialysis data are consistent not only with earlier reports for methamphetamine (Stefanski et al, 1999, 2004; but see Stefanski et al, 2002), but also with reports of active–passive distinctions in the development of NAC neurochemical plasticity as derived from studies using animal models of heroin, cocaine or MDMA self-administration (eg, Lecca et al, 2007a 2007b; McFarland et al, 2003; Orejarena et al, 2009).
Given the purported role for the NAC in anticipation of reward delivery, reward-related learning and goal-directed behavior (for recent reviews, Heinz and Schlagenhauf, 2010; Kehagia et al, 2010; Roesch et al, 2010; Schultz, 2010), it was not entirely surprising that methamphetamine affected NAC glutamate selectively within self-administering animals or that the effects of methamphetamine on NAC dopamine were greater in self-administering vs non-contingently infused animals. However, it is interesting to note that, in this study, methamphetamine increased dopamine and glutamate responsiveness in animals with a prior history of methamphetamine self-administration compared with those receiving passive IV infusions, while studies of MDMA and d-amphetamine self-administration indicated significantly reduced basal and/or drug-stimulated dopamine release in animals infused contingently vs non-contingently with these amphetamine compounds (Orejarena et al, 2009; Ranaldi et al, 1999). Whether or not the self-administration of these three structurally related stimulants also produces divergent effects on NAC glutamate (or other neurotransmitters) is not known; however, non-contingent administration studies indicate that this might be the case (eg, Nash and Yamamoto, 1992; Shoblock et al, 2003; Yeh et al, 2002). Moreover, it is also unclear whether or not the divergent dopamine effects produced by the self-administration of different amphetamine derivatives relate to distinctions in their affinities for the different monoamine transporters, issues related to drug dose or other procedural differences. Nevertheless, while drug dose, route of administration and temporal patterning of drug delivery may certainly contribute to the differential effects of injected vs self-infused methamphetamine on neurochemical measures and neurotoxicity within the cell body and terminal regions of the mesolimbic and nigrostriatal dopamine systems (see Schwendt et al, 2009; Shepard et al, 2006 for discussion), it is clear from the microdialysis studies conducted to date, in which total daily drug dose/intoxication levels, route of administration, and/or temporal patterning of drug delivery were similar between groups (Figure 5), that the behavioral contingency of drug delivery is a critical factor influencing the magnitude, time-course and duration of dopamine and glutamate sensitization within the NAC (this study; LaLumiere and Kalivas, 2008; Lecca et al, 2007a, 2007b; McFarland et al, 2003; Orejarena et al, 2009; Ranaldi et al, 1999).
The question then arises as to the precise psychological processes engaged during a history of drug self-administration that regulate the development of NAC glutamate sensitization and the elevated magnitude of dopamine sensitization in the NAC of methamphetamine self-administering animals. Distinct from the majority of earlier microdialysis studies in which dialysate was collected within the same environment (ie, the operant chamber) as that experienced by the animals during self-administration training (eg, LaLumiere and Kalivas, 2008; Lecca et al, 2007a, 2007b; McFarland et al, 2003; Ranaldi et al, 1999;Suto et al, 2010), our microdialysis procedures and those in some other studies (eg, Kippin et al, 2008; Xi et al, 2006) were conducted in an experimental room and in microdialysis chambers distinct from those employed during the self-administration phase of the experiment. Moreover, our microdialysis procedures were conducted by personnel distinct from those involved in self-administration training to further reduce the influence of conditioned factors associated with drug self-administration on neurotransmitter levels. The MDMA self-administration study conducted by Orejarena et al (2009) employed a similar experimental design as that used in this study and also observed active–passive distinctions in basal, as well as, MDMA-induced dopamine release. Thus, while the presence of discrete or contextual cues associated with drug delivery can influence the extent to which a challenge injection of drug elicits neurotransmitter release within the NAC following a history of drug self-administration (eg, Di Ciano et al, 2002), drug-associated conditioning is not likely a major contributing factor to our observed neurochemical differences between METH-SA and METH-NC rats. Further, given that the METH-SA and METH-NC groups received methamphetamine exposure in the same context (including the opportunity to engage in lever-pressing behavior) and methamphetamine exposure was associated with the same predictive discrete cues, the observed differences in neurochemistry were likely related to the behavioral history associated with methamphetamine exposure (ie, the act of self-administration).
Conversely, physical and psychological stressors elicit dopamine release within forebrain regions, including the NAC (cf, Cabib and Puglisi-Allegra, 1996; Marinelli and Piazza, 2002; Piazza and Le Moal, 1996; Pezze and Feldon, 2004; Yap and Miczek, 2008) and stressors elicit behavioral and neurochemical cross-sensitization with psychomotor stimulant drugs (cf, Piazza and Le Moal, 1996; Robinson and Becker, 1986; Vanderschuren and Kalivas, 2000). As METH-SA rats were accustomed to self-regulating their drug intake, while METH-NC rats were not, it is possible that the stress associated with the experimenter-administered IP methamphetamine injection/lack of control over the amount of methamphetamine present in their system, heightened the mesocorticolimbic neurochemical responsiveness of METH-SA animals. For practical reasons, a non-contingent saline challenge was not included in this study and so it is not possible to discern from its design whether or not interactions between ‘injection stress' and methamphetamine history contributed to the differential neurotransmitter responses to the methamphetamine challenge. However, arguing against this potential interpretation, greater drug-induced dopamine sensitization was reported in the NAC of rats during IV heroin and cocaine self-administration, compared with yoked, drug-infused controls (Lecca et al, 2007a, 2007b; Suto et al, 2010) and similarly, priming produced greater dopamine increases in rats with a history of cocaine self-administration than cocaine-yoked controls (McFarland et al, 2003). Thus, there clearly exist other factors associated with the act of drug-taking that are augmenting NAC neurochemical sensitivity to subsequent exposures to addictive drugs and impacting basal NAC glutamate levels.
Possible Mediators of Changes in NAC Dopamine Produced by Contingent and Non-Contingent Methamphetamine
The molecular mechanisms through which a history of IV methamphetamine self-administration augments the magnitude of NAC dopamine sensitization in methamphetamine self-administering animals are unclear. Methamphetamine increases extracellular levels of monoamines via interactions with plasma membrane- and vesicular-monamine transporters, as well as the inhibition of monoamine oxidase. Together, these mechanisms result in a dramatic elevation of cytoplasmic monoamine levels, which alters the membrane concentration gradient of these neurotransmitters and promotes their ‘reverse transport' out of neurons (eg, Sulzer et al, 2005). Moreover, amphetamines prevent the clearance of excess extracellular monoamines from the synapse by competing with monoamine neurotransmitters for shared binding sites on plasma membrane transporters (eg, Sulzer et al, 2005). It has been previously shown that methamphetamine self-administration produces a reduction in the expression of VTA D2 autoreceptors and terminal D1 receptors, which does not occur in animals passively infused with methamphetamine (Stefanski et al, 1999). Moreover, methamphetamine self-administration can reduce corticostriatal DAT expression (Schwendt et al, 2009), as well as enhance VTA tyrosine hydroxylase levels (Shepard et al, 2006). Although changes in D2 receptors and DAT could contribute to an elevated magnitude of dopamine sensitization, our dopamine effects either persist into protracted withdrawal or grow with the passage of time (Figure 3), while changes in D1/D2 receptor and tyrosine hydroxylase expression are not observed at withdrawal time-points beyond 1 day, even in animals with extended access to the drug (Shepard et al, 2006; Stefanski et al, 2002). Moreover, pretreatment with either D1 or D2 antagonists reduce, rather than augment, IP methamphetamine-induced dopamine and glutamate release within striatal structures (Ito et al, 2006; Mark et al, 2004; Stephans and Yamamoto 1995) and enduring changes in corticostriatal DAT expression are observed in animals with extended access to IV methamphetamine (intakes ∼7 mg/kg/day; ∼3 times that observed in this study), but not in animals self-administering under shorter periods of methamphetmine access as in this study (Schwendt et al, 2009). Such data argue against a direct role for drug-induced changes in D1/D2 receptors or DAT function in the augmented neurochemical sensitization observed following a history of methamphetamine self-administration.
Possible Mediators of Changes in NAC Glutamate Produced by Contingent and Non-Contingent Methamphetamine
While less well-investigated than the dopamine system, evidence also exists supporting persistent abnormalities in glutamate turnover within frontal cortex of abstinent methamphetamine addicts (Chang et al, 2007; Sailasuta et al, 2010). The results of preclinical studies employing antagonists for different glutamate receptors or transporters also support a necessary role for intact glutamate signaling in the manifestation of methamphetamine-seeking behavior (Fujio et al, 2005a; Gass et al, 2009; Kim and Jang, 1997; Miyatake et al, 2005; Nakagawa et al, 2005; Osborne and Olive, 2008). Further, systemic pretreatment with glutamate antagonists also blunts the capacity of non-contingent methamphetamine injections to elevate or sensitize striatal dopamine and/or protects against methamphetamine-induced dopamine toxicity, indicating an important role for glutamate transmission in regulating methamphetamine-induced neurochemical plasticity (Arai et al, 1996, 1997; Battaglia et al, 2002; Golembiowska et al, 2003; Ohmori et al, 1995, 1996; Shimazoe et al, 2002). As reported by Shoblock et al (2003), an acute challenge injection of 2 mg/kg methamphetamine lowered NAC glutamate levels below baseline in SAL animals; this effect was indistinguishable from that observed in METH-NC rats, despite their prior methamphetamine experience (Figure 4c). However, consistent with earlier reports of active–passive distinctions for cocaine- and heroin-induced NAC glutamate release following a history of drug self-administration (LaLumiere and Kalivas, 2008; McFarland et al, 2003), not only was the methamphetamine-induced reduction in glutamate absent at 24-h withdrawal in METH-SA rats (Figure 4c), METH-SA rats exhibited a trend toward an increase in extracellular glutamate levels during the last hour of dialysate collection (Figure 4a). Thus, a history of methamphetamine self-administration produced tolerance to the glutamate-reducing effects of acute methamphetamine.
Furthermore, METH-SA rats expressed a sensitized glutamate response to the methamphetamine challenge injection when assayed at 3 weeks withdrawal (Figure 4), indicating time-dependent changes in the molecular mechanisms governing glutamate release within this region. Further support in favor of enhanced glutamate release in animals with a history of methamphetamine self-administration is derived from the results of the no net-flux study (Figure 5), in which METH-SA animals exhibited increases in the net-flux at the 0 μM glutamate concentration, which is largely dependent on neurotransmitter release (for detailed discussion, see Bungay et al, 2003) and this result was apparent at both the 1- and 21-day withdrawal periods. Although the magnitude of the increased extraction fraction exhibited by METH-SA animals during no net-flux procedures diminished to a certain extent during protracted withdrawal (Figure 5d), the methamphetamine-induced glutamate release exhibited by these same animals was not only earlier in onset, but also greater in magnitude and longer in duration, following protracted withdrawal, compared with the 1-day withdrawal time-point (Figures 4a vs b). Thus, the present data indicate persistent enhancement of basal release and, possibly ‘incubating' enhanced methamphetamine-induced glutamate release within the NAC during the course of withdrawal from methamphetamine self-administration. Although not yet assayed in an animal model of drug-taking or drug-seeking behavior, elevating extracellular glutamate levels via intra-cerebroventricular infusion of a non-selective glutamate reuptake inhibitor facilitates the development of methamphetamine-induced behavioral sensitization, without influencing the acute locomotor response to methamphetamine (Fujio et al, 2005b). Such data support an active role for methamphetamine-induced glutamate sensitization in regulating at least certain forms of methamphetamine-induced behavioral plasticity, which, based on a rapidly growing body of literature derived from both human and animal studies (eg, Nakagawa and Kaneko, 2008; Vanderschuren and Kalivas, 2000; Wolf, 1998), may extend to methamphetamine-taking and/or -seeking behavior.
The molecular mechanisms through which self-administered, but not non-contingent, methamphetamine induces glutamate sensitization are unclear at this time. The temporal profile of the methamphetamine-sensitized glutamate rise observed within the NAC of METH-SA animals is delayed, peaking approximately 2-h post-injection (Figure 4b). The temporal profile of methamphetamine-sensitized glutamate release in METH-SA rats is similar to that observed within the NAC on amphetamine challenge in amphetamine-experienced rats (Xue et al, 1996), but is distinct from the reported temporal profiles of cocaine-sensitized glutamate release within the NAC. In studies of experimenter-administered cocaine (eg, Baker et al, 2003; Pierce et al, 1996; Zayara et al, 2011), the cocaine-sensitized glutamate response is often bimodal and characterized by a TTX-sensitive rise during the first 20- to 40-min post-injection (Pierce et al, 1996), which is followed by a larger and more persistent rise that is TTX-insensitive (Pierce et al, 1996), but N-acetyl-cystine-sensitive (Baker et al, 2003). Such observations indicate that, within the confines of non-contingent drug exposure, the majority of the cocaine-sensitized NAC glutamate response is derived from non-vesicular glutamate sources (Pierce et al, 1996) that have been related to dysregulated cystine-glutamate exchange and/or GLT-1 glutamate transporter function (eg, Baker et al, 2003; Knackstedt et al, 2010). Interestingly, akin to the present results for METH-NC animals, an IP cocaine challenge injection fails to elevate NAC glutamate levels above baseline in rats with a history of yoked cocaine exposure (McFarland et al, 2003). However, in the majority of studies of rats with a history of self-administered cocaine, the cocaine-induced rise in NAC glutamate is very rapid in onset whether micordialysis is performed during subsequent IV self-administration (Miguens et al, 2008; Suto et al, 2010) or before an IP cocaine challenge, following some form of extinction training regardless of testing environment (in the self-administration context: Baker et al, 2003; Li et al, 2010, McFarland et al, 2003; Madayag et al, 2010; Miguens et al, 2008; Xi et al, 2010; or in a neutral context: Kippin et al, 2008; Xi et al, 2006). In studies where the cocaine is self-administered (Miguens et al, 2008; Suto et al, 2010), the cocaine-induced rise in NAC glutamate is maintained, whereas in studies where the cocaine challenge was administered IP to animals with a cocaine self-administration and extinction history, the glutamate rise is transient (Baker et al, 2003; Kippin et al, 2008; Li et al, 2010; McFarland et al, 2003; Xi et al, 2006, 2010; but see also Madayag et al, 2010) and can be blocked completely by N-acetyl-cystine pretreatment (Baker et al, 2003), suggesting a critical role for non-neuronal glutamate sources in this regard (Baker et al, 2002). Although the source of glutamate undergoing methamphetamine-induced plasticity on self-administration remains to be determined, it is clear from the present data that this source is heavily influenced by factors related to the act of drug-taking.
Consistent with the notion that basal extracellular glutamate within the NAC is derived primarily from non-neuronal sources (Baker et al, 2002), drug-induced anomalies in Xct and GLT-1 function within the NAC are theorized to underpin the marked reduction in basal extracellular glutamate content reported following a period of cocaine withdrawal in animals with histories of either contingent or non-contingent cocaine experience (eg, Baker et al, 2003; Kippin et al, 2008; McFarland et al, 2003; Zayara et al, 2011; but see Madayag et al, 2010). Although the results of our injection study indicated equivocal effects of IV methamphetamine self-administration on baseline extracellular glutamate (Table 1), a marked increase in basal extracellular glutamate was observed in METH-SA rats at the 0 μM glutamate dose in the no net-flux study (equivalent to conventional procedures) and confirmed through determinations of the point of no net-flux on the addition of glutamate to the perfusate (Figure 5). Interestingly, the methamphetamine-induced increase in NAC glutamate was apparent at 24-h withdrawal in self-administering animals and persisted into protracted withdrawal, while that observed in non-contingently exposed animals appeared to require the passage of time. Also in contrast to earlier reports for at least non-contingent cocaine (Baker et al, 2003), a history of methamphetamine self-administration, but not non-contingent IV methamphetamine administration, elevated the glutamate extraction fraction (ie, slope) in the no net-flux study (Figure 5d). Interestingly, the greater glutamate extraction fraction exhibited by METH-SA animals primarily reflected greater gains in perfusate glutamate at concentrations below, rather than above, y=0. Although a body of data derived from studies of non-contingent methamphetamine models have indicated elevated striatal glutamate clearance following neurotoxic dosing regiments (Mark et al, 2007; Nishino et al, 1996; Shirai et al, 1996), our in vivo data suggest enhanced basal glutamate release, most probably from extra-synaptic sources given the nature of microdialysis, as at least one mechanism to account for the elevated basal NAC glutamate levels observed in METH-SA animals (see Bungay et al, 2003; Chefer et al, 2006; Parsons and Justice, 1994 for detailed discussion). This being said, METH-NC animals also exhibited elevated basal glutamate but failed to exhibit drug-induced changes in the extraction fraction. Such discrepancies provide further evidence that the molecular mechanisms underpinning drug-induced neurochemical adaptations are very much dependent on the behavioral contingency of drug delivery and highlight the role had by the self-administration context in regulating extracellular glutamate levels within the NAC (see Suto et al, 2010).
Conclusions
Regardless of the precise neural substrates involved in mediating the observed neurochemical adaptations produced by a history of repeated methamphetamine exposure, it is clear that the magnitude, time-course or manifestation of methamphetamine-induced changes in basal and/or drug-stimulated dopamine and glutamate release depend strongly on the behavioral contingency of methamphetamine delivery. Accordingly, the present and similar findings reiterate the importance of studying drug-induced neuro-adaptations in animal models of addiction, which employ behavioral contingencies in order to elucidate the mechanisms of drug-seeking behavior.
Acknowledgments
This work was supported by NIDA Grants DA-024038 (KKS) and DA-027525 (TEK), as well as NARSAD Young Investigator Awards to KKS and TEK. We would like to thank Dr Larry Parsons (Scripps Research Institute) for his expert assistance in the preparation of this paper.
The authors declare no conflict of interest.
References
- Abekawa T, Ohmori T, Koyama T. Effects of repeated administration of a high dose of methamphetamine on dopamine and glutamate release in rat striatum and nucleus accumbens. Brain Res. 1994;643:276–281. doi: 10.1016/0006-8993(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Akimoto K, Hamamura T, Kazahaya Y, Akiyama K, Otsuki S. Enhanced extracellular dopamine level may be the fundamental neuropharmacological basis of cross-behavioral sensitization between methamphetamine and cocaine--an in vivo dialysis study in freely moving rats. Brain Res. 1990;507:344–346. doi: 10.1016/0006-8993(90)90295-m. [DOI] [PubMed] [Google Scholar]
- Akiyama K, Kanzaki A, Tsuchida K, Ujike H. Methamphetamine-induced behavioral sensitization and its implications for relapse of schizophrenia. Schizophr Res. 1994;12:251–257. doi: 10.1016/0920-9964(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Arai I, Shimazoe T, Shibata S, Inoue H, Yoshimatsu A, Watanabe S. Enhancement of dopamine release from the striatum through metabotropic glutamate receptor activation in methamphetamine sensitized rats. Brain Res. 1996;729:277–280. [PubMed] [Google Scholar]
- Arai I, Shimazoe T, Shibata S, Inoue H, Yoshimatsu A, Watanabe S. Methamphetamine-induced sensitization of dopamine release via a metabotropic glutamate receptor mediated pathway in rat striatal slices. Jpn J Pharmacol. 1997;73:243–246. doi: 10.1254/jjp.73.243. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynatpic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G, Fornai F, Busceti CL, Aloisi G, Cerrito F, De Blasi A. Selective blockade of mGlu5 metabotropic glutamate receptors is protective against methamphetamine neurotoxicity. J Neurosci. 2002;22:2135–2141. doi: 10.1523/JNEUROSCI.22-06-02135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Berman SM, Kuczenski R, McCracken JT, London ED. Potential adverse effects of amphetamine treatment on brain and behavior: a review. Mol Psychiatry. 2009;14:123–142. doi: 10.1038/mp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW. Cocaine sensitization and dopamine mediation of cue effects in rodents, monkeys, and humans: areas of agreement, disagreement, and implications for addiction. Psychopharmacology. 2007;191:705–717. doi: 10.1007/s00213-006-0561-6. [DOI] [PubMed] [Google Scholar]
- Broom SL, Yamamoto BK. Effects of subchronic methamphetamine exposure on basal dopamine and stress-induced dopamine release in the nucleus accumbens shell of rats. Psychopharmacology. 2005;181:467–476. doi: 10.1007/s00213-005-0007-6. [DOI] [PubMed] [Google Scholar]
- Bungay PM, Newton-Vinson P, Isele W, Garris PA, Justice JB., Jr Microdialysis of dopamine interpreted with quantitative model incorporating probe implantation trauma. J Neurochem. 2003;86:932–946. doi: 10.1046/j.1471-4159.2003.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows KB, Nixdorf WL, Yamamoto BK. Central administration of methamphetamine synergizes with metabolic inhibition to deplete striatal monoamines. Pharmacol Exp Ther. 2000;292:853–860. [PubMed] [Google Scholar]
- Bustamante D, You ZB, Castel MN, Johansson S, Goiny M, Terenius L, et al. Effect of single and repeated methamphetamine treatment on neurotransmitter release in substantia nigra and neostriatum of the rat. J Neurochem. 2002;83:645–654. doi: 10.1046/j.1471-4159.2002.01171.x. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. Stress, depression and the mesolimbic dopamine system. Psychopharmacology. 1996;128:331–342. doi: 10.1007/s002130050142. [DOI] [PubMed] [Google Scholar]
- Camp DM, Browman KE, Robinson TE. The effects of methamphetamine and cocaine on motor behavior and extracellular dopamine in the ventral striatum of Lewis vs Fischer 344 rats. Brain Res. 1994;668:180–193. doi: 10.1016/0006-8993(94)90523-1. [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102 (Suppl 1:16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Zapata A, Shippenberg TS, Bungay PM. Quantitative no-net-flux microdialysis permits detection of increases and decreases in dopamine uptake in mouse nucleus accumbens. J Neurosci Methods. 2006;155:187–193. doi: 10.1016/j.jneumeth.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Davidson C, Chen Q, Zhang X, Xiong X, Lazarus C, Lee TH, et al. Deprenyl treatment attenuates long-term pre- and post-synaptic changes evoked by chronic methamphetamine. Eur J Pharmacol. 2007;583:100–110. doi: 10.1016/j.ejphar.2007.06.046. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn't do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. Changes in dopamine oxidation currents in the nucleus accumbens during unlimited-access self-administration of d-amphetamine by rats. Behav Pharmacol. 1996;7:714–729. doi: 10.1097/00008877-199611000-00016. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. Inhibition of dopamine efflux in the rat nucleus accumbens during abstinence after free access to d-amphetamine. Behav Brain Res. 2002;128:1–12. doi: 10.1016/s0166-4328(01)00265-0. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Coury A, Depoortere RY, Egilmez Y, Lane JD, Emmett-Oglesby MW, et al. Comparison of changes in extracellular dopamine concentrations in the nucleus accumbens during intravenous self-administration of cocaine or d-amphetamine. Behav Pharmacol. 1995;6:311–322. [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent vs response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology. 1995;117:262–266. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Elkashef A, Vocci F, Hanson G, White J, Wickes W, Tiihonen J. Pharmacotherapy of methamphetamine addiction: an update. Subst Abus. 2008;29:31–49. doi: 10.1080/08897070802218554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang YR, Abekawa T, Li XB, Wang ZC, Inoue T, Koyama T. Effect of the protein kinase C inhibitor, staurosporine, on the high dose of methamphetamine-induced behavioral sensitization to diziclopine (MK-801) Psychopharmacology. 2005;180:100–106. doi: 10.1007/s00213-005-2145-2. [DOI] [PubMed] [Google Scholar]
- Fujio M, Nakagawa T, Sekiya Y, Ozawa T, Suzuki Y, Minami M, et al. Gene transfer of GLT-1, a glutamate transporter, into the nucleus accumbens shell attenuates methamphetamine- and morphine-induced conditioned place preference in rats. Eur J Neurosci. 2005a;22:2744–2754. doi: 10.1111/j.1460-9568.2005.04467.x. [DOI] [PubMed] [Google Scholar]
- Fujio M, Nakagawa T, Suzuki Y, Satoh M, Kaneko S. Facilitative effect of a glutamate transporter inhibitor (2S,3S)-3-{3-[4-(trifluoromethyl)benzoylamino]benzyloxy}aspartate on the expression of methamphetamine-induce. J Pharmacol Sci. 2005b;99:415–418. doi: 10.1254/jphs.sc0050144. [DOI] [PubMed] [Google Scholar]
- Galici R, Pechnick RN, Poland RE, France CP. Comparison of noncontingent vs contingent cocaine administration on plasma corticosterone levels in rats. Eur J Pharmacol. 2000;387:59–62. doi: 10.1016/s0014-2999(99)00780-3. [DOI] [PubMed] [Google Scholar]
- Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology. 2009;34:820–833. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golembiowska K, Konieczny J, Wolfarth S, Ossowska K. Neuroprotective action of MPEP, a selective mGluR5 antagonist, in methamphetamine-induced dopaminergic neurotoxicity is associated with a decrease in dopamine outflow and inhibition of hyperthermia in rats. Neuropharmacology. 2003;45:484–492. doi: 10.1016/s0028-3908(03)00209-0. [DOI] [PubMed] [Google Scholar]
- Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 2010;36:472–485. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Abekawa T, Koyama T. Relationship between development of cross-sensitization to MK-801 and delayed increases in glutamate levels in the nucleus accumbens induced by a high dose of methamphetamine. Psychopharmacology. 2006;187:293–302. doi: 10.1007/s00213-006-0423-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32:617–631. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Kashihara K, Okumura K, Onishi M, Otsuki S. MK-801 fails to modify the effect of methamphetamine on dopamine release in the rat striatum. Neuroreport. 1991;2:236–238. doi: 10.1097/00001756-199105000-00005. [DOI] [PubMed] [Google Scholar]
- Kazahaya Y, Akimoto K, Otsuki S. Subchronic methamphetamine treatment enhances methamphetamine- or cocaine-induced dopamine efflux in vivo. Biol Psychiatry. 1989;25:903–912. doi: 10.1016/0006-3223(89)90270-9. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol. 2010;20:199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology. 2008;198:63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Kim HS, Jang CG. MK-801 inhibits methamphetamine-induced conditioned place preference and behavioral sensitization to apomorphine in mice. Brain Res Bull. 1997;44:221–227. doi: 10.1016/s0361-9230(97)00093-2. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Szumlinski KK, Kapasova Z, Rezner B, See RE. Prenatal stress enhances responsiveness to cocaine. Neuropsychopharmacology. 2008;33:769–782. doi: 10.1038/sj.npp.1301447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G. Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology. 2007a;191:653–667. doi: 10.1007/s00213-006-0496-y. [DOI] [PubMed] [Google Scholar]
- Lecca D, Valentini V, Cacciapaglia F, Acquas E, Di Chiara G. Reciprocal effects of response contingent and noncontingent intravenous heroin on in vivo nucleus accumbens shell vs core dopamine in the rat: a repeated sampling microdialysis study. Psychopharmacology. 2007b;194:103–116. doi: 10.1007/s00213-007-0815-y. [DOI] [PubMed] [Google Scholar]
- Li Z, Jie L, Gardner EL, Xi ZX. Activation of mGluR7s inhibits cocaine-induced reinstatement of drug-seeking behavior by a nucleus accumbens glutamate-mGluR2/3 mechanism in rats. J Neurochem. 2010;114:1368–1380. doi: 10.1111/j.1471-4159.2010.06851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madayag A, Kau KS, Lobner D, Mantsch JR, Wisniewski S, Baker DA. Drug-induced plasticity contributing to heightened relapse susceptibility: Neurochemical changes and augmented reinstatement in high-intake rats. J Neurosci. 2010;30:210–217. doi: 10.1523/JNEUROSCI.1342-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE. Effects of cocaine self-administration on plasma corticosterone in rats: relationship to hippocampal type II glucocorticoid receptors. Prog Neuro-Psychopharmacol Biol Psychiat. 2000;24:633–646. doi: 10.1016/s0278-5846(00)00098-1. [DOI] [PubMed] [Google Scholar]
- Mark KA, Quinton MS, Russek SJ, Yamamoto BK. Dynamic changes in vesicular glutamate transporter 1 function and expression related to methamphetamine-induced glutamate release. J Neurosci. 2007;27:6823–6831. doi: 10.1523/JNEUROSCI.0013-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark KA, Soghomonian JJ, Yamamoto BK. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J Neurosci. 2004;24:11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Marshall JF, O'Dell SJ, Weihmuller FB. Dopamine–glutamate interactions in methamphetamine-induced neurotoxicity. J Neural Transm Gen Sect. 1993;91:241–254. doi: 10.1007/BF01245234. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega WP, Williams AE, Schmitz DA, DiStefano EW, Cho AK. Pharmacokinetic and pharmacodynamic analysis of the actions of D-amphetamine and D-methamphetamine on the dopamine terminal. J Pharmacol Exp Ther. 1995;274:90–96. [PubMed] [Google Scholar]
- Miguens M, Del Olmo N, Higuera-Matas A, Torres I, Garcia-Lecumberri, Ambrosio E. Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacology. 2008;196:303–313. doi: 10.1007/s00213-007-0958-x. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Yamada K, Nagai T, Mori H, Mishina M, Furukawa H, et al. Behavioural adaptations to addictive drugs in mice lacking the NMDA receptor epsilon1 subunit. Eur J Neurosci. 2004;19:151–158. doi: 10.1111/j.1460-9568.2004.03086.x. [DOI] [PubMed] [Google Scholar]
- Miyatake M, Narita M, Shibasaki M, Nakamura A, Suzuki T. Glutamatergic neurotransmission and protein kinase C play a role in neuron-glia communication during the development of methamphetamine-induced psychological dependence. Eur J Neurosci. 2005;22:1476–1488. doi: 10.1111/j.1460-9568.2005.04325.x. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from a self-administered or non-contingent cocaine binge: differences in ultrasonic distress vocalizations in rats. Psychopharmacology. 1998;135:161–168. doi: 10.1007/s002130050584. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Fujio M, Ozawa T, Minami M, Satoh M. Effect of MS-153, a glutamate transporter activator, on the conditioned rewarding effects of morphine, methamphetamine and cocaine in mice. Behav Brain Res. 2005;156:233–239. doi: 10.1016/j.bbr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kaneko S. Neuropsychotoxicity of abused drugs: molecular and neural mechanisms of neuropsychotoxicity induced by methamphetamine, 3,4-methylenedioxymethamphetamine (ecstasy), and 5-methoxy-N,N-diisopropyltryptamine (foxy) J Pharmacol Sci. 2008;106:2–8. doi: 10.1254/jphs.fm0070141. [DOI] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxymethamphetamine. Brain Res. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- Nishino N, Shirai Y, Kajimoto Y, Kitamura N, Yamamoto H, Yang CQ, et al. Increased glutamate transporter (GLT-1) immunoreactivity in the rat striatum after repeated intermittent administration of methamphetamine. Ann NY Acad Sci. 1996;801:310–314. doi: 10.1111/j.1749-6632.1996.tb17451.x. [DOI] [PubMed] [Google Scholar]
- Ohmori T, Abekawa T, Koyama T. Environment modifies the expression of behavioral sensitization produced by methamphetamine: behavioral and neurochemical studies. Behav Pharmacol. 1995;6:133–142. [PubMed] [Google Scholar]
- Ohmori T, Abekawa T, Koyama T. The role of glutamate in behavioral and neurotoxic effects of methamphetamine. Neurochem Int. 1996;29:301–307. doi: 10.1016/0197-0186(95)00152-2. [DOI] [PubMed] [Google Scholar]
- Orejarena MJ, Berrendero F, Maldonado R, Robledo P. Differential changes in mesolimbic dopamine following contingent and non-contingent MDMA self-administration in mice. Psychopharmacology. 2009;205:457–466. doi: 10.1007/s00213-009-1554-z. [DOI] [PubMed] [Google Scholar]
- Osborne MP, Olive MF. A role for mGluR5 receptors in intravenous methamphetamine self-administration. Ann NY Acad Sci. 2008;1139:206–211. doi: 10.1196/annals.1432.034. [DOI] [PubMed] [Google Scholar]
- Palamarchouk V, Smagin G, Goeders NE. Self-administered and passive cocaine infusions produce different effects on corticosterone concentrations in the medial prefrontal cortex (MPC) of rats. Pharm Biochem Behav. 2009;94:163–168. doi: 10.1016/j.pbb.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Justice JB., Jr Quantitative approaches to in vivo brain microdialysis. Crit Rev Neurobiol. 1994;8:189–220. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, Fourth Edition. Academic Press: New York; 1998. [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1660. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaldi R, Pocock D, Zereik R, Wise RA. Dopamine fluctuations in the nucleus accumbens during maintenance, extinction, and reinstatement of intravenous D-amphetamine self-administration. J Neurosci. 1999;19:4102–4109. doi: 10.1523/JNEUROSCI.19-10-04102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivie‘re GJ, Byrnes KA, Gentry WB, Owens SM. Spontaneous locomotor activity and pharmacokinetics of intravenous methamphetamine and its metabolite amphetamine in the rat. J Pharmacol Exp Ther. 1999;291:1220–1226. [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Calu DJ, Esber GR, Schoenbaum G. All that glitters … dissociating attention and outcome expectancy from prediction errors signals. J Neurophysiol. 2010;104:587–595. doi: 10.1152/jn.00173.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailasuta N, Abulseoud O, Harris KC, Ross BD. Glial dysfunction in abstinent methamphetamine abusers. J Cereb Blood Flow Metab. 2010;30:950–960. doi: 10.1038/jcbfm.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331:555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Chuang DT, Shaham Y, Morales M. Effect of methamphetamine self-administration on tyrosine hydroxylase and dopamine transporter levels in mesolimbic and nigrostriatal dopamine pathways of the rat. Psychopharmacology. 2006;185:505–513. doi: 10.1007/s00213-006-0316-4. [DOI] [PubMed] [Google Scholar]
- Shimada A, Yamaguchi K, Yanagita T. Neurochemical analysis of the psychotoxicity of methamphetamine and cocaine by microdialysis in the rat brain. Ann NY Acad Sci. 1996;801:361–370. doi: 10.1111/j.1749-6632.1996.tb17456.x. [DOI] [PubMed] [Google Scholar]
- Shimazoe T, Doi Y, Arai I, Yoshimatsu A, Fukumoto T, Watanabe S. Both metabotropic glutamate I and II receptors mediate augmentation of dopamine release from the striatum in methamphetamine-sensitized rats. Jpn J Pharmacol. 2002;89:85–88. doi: 10.1254/jjp.89.85. [DOI] [PubMed] [Google Scholar]
- Shirai Y, Shirakawa O, Nishino N, Saito N, Nakai H. Increased striatal glutamate transporter by repeated intermittent administration of methamphetamine. Psychiatry Clin Neurosci. 1996;50:161–164. doi: 10.1111/j.1440-1819.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology. 2003;165:359–369. doi: 10.1007/s00213-002-1288-7. [DOI] [PubMed] [Google Scholar]
- Simões PF, Silva AP, Pereira FC, Marques E, Grade S, Milhazes N, et al. Methamphetamine induces alterations on hippocampal NMDA and AMPA receptor subunit levels and impairs spatial working memory. Neuroscience. 2007;150:433–441. doi: 10.1016/j.neuroscience.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Simões PF, Silva AP, Pereira FC, Marques E, Milhazes N, Borges F, et al. Methamphetamine changes NMDA and AMPA glutamate receptor subunit levels in the rat striatum and frontal cortex. Ann NY Acad Sci. 2008;1139:232–241. doi: 10.1196/annals.1432.028. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Nicklas WJ, Heikkila RE. Role for excitatory amino acids in methamphetamine-induced nigrostriatal dopaminergic toxicity. Science. 1989;243:398–400. doi: 10.1126/science.2563176. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Justinova Z, Hayashi T, Takebayashi M, Goldberg SR, Su TP. Sigma1 receptor upregulation after chronic methamphetamine self-administration in rats: a study with yoked controls. Psychopharmacology. 2004;175:68–75. doi: 10.1007/s00213-004-1779-9. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur J Pharmacol. 1999;371:123–135. doi: 10.1016/s0014-2999(99)00094-1. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Lee SH, Yasar S, Cadet JL, Goldberg SR. Lack of persistent changes in the dopaminergic system of rats withdrawn from methamphetamine self-administration. Eur J Pharmacol. 2002;439:59–68. doi: 10.1016/s0014-2999(02)01301-8. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BY. Effect of repeated methamphetamine administrations on dopamine and glutamate efflux in rat prefrontal cortex. Brain Res. 1995;700:99–106. doi: 10.1016/0006-8993(95)00938-m. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), Office of Applied Studies . The TEDS Report: Trends in Methamphetamine Admissions to Treatment: 1997-2007. Rockville, MD; 2009. [Google Scholar]
- Suto N, Ecke LE, You ZB, Wise RA. Extracellular fluctuations of dopamine and glutamate in the nucleus accumbens core and shell associated with lever-pressing during cocaine self-administration, extinction, and yoked cocaine administration. Psychopharmacology. 2010;211:267–275. doi: 10.1007/s00213-010-1890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Shishido T, Watanabe Y, Abe H, Shiragata M, Honda K, et al. Changes of behavior and monoamine metabolites in the rat brain after repeated methamphetamine administration: effects of duration of repeated administration. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:359–369. doi: 10.1016/s0278-5846(97)00006-7. [DOI] [PubMed] [Google Scholar]
- Tata DA, Yamamoto BK. Interactions between methamphetamine and environmental stress: role of oxidative stress, glutamate and mitochondrial dysfunction. Addiction. 2007;102 (Supplement 1:49–60. doi: 10.1111/j.1360-0443.2007.01770.x. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22:4654–4662. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz TJ, Fleckenstein AE, Hanson GR. Methamphetamine-induced alterations in monoamine transport: implications for neurotoxicity, neuroprotection and treatment. Addiction. 2007;102 (Suppl 1:44–48. doi: 10.1111/j.1360-0443.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- Weihmuller FB, O'Dell SJ, Cole BN, Marshall JF. MK-801 attenuates the dopamine-releasing but not the behavioral effects of methamphetamine: an in vivo microdialysis study. Brain Res. 1991;549:230–235. doi: 10.1016/0006-8993(91)90462-5. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–8536. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Li X, Peng XQ, Li J, Chun L, Gardner EL, et al. Inhibition of n-acetylated-alpa-linked-acidic dipeptidase (NAALADase) by 2-PMPA attenuates cocaine-induced relapse in rats: a NAAG-mGluR2/3-mediated mechanism. J Neurochem. 2010;112:564–576. doi: 10.1111/j.1471-4159.2009.06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue CJ, Ng JP, Li Y, Wolf ME. Acute and repeated systemic amphetamine administration: effects on extracellular glutamate, aspartate and serine levels in rat ventral tegmental area and nucleus accumbens. J Neurochem. 1996;67:352–363. doi: 10.1046/j.1471-4159.1996.67010352.x. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Miczek KA. Stress and rodent odels of drug addiction: role of VTA-accumbens-PFC-amygdala circuit. Drug Discov Today Dis Models. 2008;5:259–270. doi: 10.1016/j.ddmod.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh GC, Chen JC, Tsai HC, Wu HH, Lin CY, Hsu PC, et al. Amphetamine inhibits the N-methyl-D-aspartate receptor-mediated responses by directly interacting with the receptor/channel complex. J Pharmacol Ther. 2002;300:1008–1016. doi: 10.1124/jpet.300.3.1008. [DOI] [PubMed] [Google Scholar]
- Zayara AE, McIver G, Valdivia PN, Lominac KD, McCreary AC, Szumlinski KK. Blockade of nucleus accumbens 5-HT2A and 5-HT2C receptors prevents the expression of cocaine-induced behavioral and neurochemical sensitization in rats. Psychopharmacology. 2011;213:321–335. doi: 10.1007/s00213-010-1996-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Loonam TM, Noailles PA, Angulo JA. Comparison of cocaine- and methamphetamine-evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions. Ann NY Acad Sci. 2001;937:93–120. doi: 10.1111/j.1749-6632.2001.tb03560.x. [DOI] [PubMed] [Google Scholar]