Abstract
Despite effective therapies for smoking cessation, most smokers find quitting difficult and most successful quitters relapse. Considerable evidence supports a genetic risk for nicotine dependence; however, less is known about the pharmacogenetics of smoking cessation. In the first pharmacogenetic investigation of the efficacy of varenicline and bupropion, we examined whether genes important in the pharmacodynamics and pharmacokinetics of these drugs and nicotine predict medication efficacy and adverse events. Subjects participated in randomized, double-blind, placebo-controlled smoking cessation clinical trials, comparing varenicline, a nicotinic acetylcholine receptor (nAChR) partial agonist, with bupropion, a norepinephrine/dopamine reuptake inhibitor, and placebo. Primary analysis included 1175 smokers of European ancestry, and 785 single nucleotide polymorphisms from 24 genes, representing 254 linkage disequilibrium (LD) bins (genes included nAChR subunits, additional varenicline-specific genes, and genes involved in nicotine or bupropion metabolism). For varenicline, continuous abstinence (weeks 9–12) was associated with multiple nAChR subunit genes (including CHRNB2, CHRNA5, and CHRNA4) (OR=1.76; 95% CI: 1.23–2.52) (p<0.005); for bupropion, abstinence was associated with CYP2B6 (OR=1.78; 95% CI: 1.27–2.50) (p<0.001). Incidence of nausea was associated with several nAChR subunit genes (OR=0.50; 95% CI: 0.36–0.70) (p<0.0001) and time to relapse after quitting was associated with HTR3B (HR=1.97; 95% CI: 1.45–2.68) (p<0.0001). These data provide evidence for multiple genetic loci contributing to smoking cessation and therapeutic response. Different loci are associated with varenicline vs bupropion response, suggesting that additional research may identify clinically useful markers to guide treatment decisions.
Keywords: varenicline, bupropion, pharmacogenetics, nicotine, nicotinic receptor, CYP2B6
INTRODUCTION
Nicotine dependence is a chronic, relapsing addiction (Lerman et al, 2007), that afflicts >20% of the population worldwide (Fiore et al, 2008). Nicotine exerts its effect primarily at heterogeneous acetylcholine receptors (nAChRs) in the brain. This activity stimulates dopaminergic and other pathways and this increase in dopamine contributes to the rewarding effects of nicotine (Dani and Heinemann, 1996). The principal subtypes of these pentameric receptors include α4 and β2 subunits, sometimes complimented by additional subunits of a different type (eg, α5) (Mineur and Picciotto, 2008; Ray et al, 2010). Nicotine shows high affinity for α4β2-containing nicotinic receptors, with Ki values in the low nanomolar range (Gotti et al, 2006).
The persistence of smoking can be attributed to multiple diverse causes. Chief among these are genetic risk factors contributing to smoking behavior (Li et al, 2003; Maes et al, 2004; Sullivan and Kendler, 1999; The Tobacco and Genetics Consortium, 2010). Genome-wide association studies (GWAS) have identified a primary genetic locus on chromosome 15q25 that increases the likelihood of nicotine dependence by 30–40% in individuals who carry common risk alleles (Amos et al, 2008; Hung et al, 2008; Thorgeirsson et al, 2008), as well as increasing the risk for several smoking-related diseases (Broderick et al, 2009; Landi et al, 2009; Pillai et al, 2009; Thorgeirsson et al, 2008). This locus includes three nicotinic receptor subunit genes (CHRNA5, CHRNB4, and CHRNA3), and a gene expressed in the lungs (IREB2), any (or several) of which may contain variants that contribute to nicotine dependence risk (DeMeo et al, 2009). Indeed, evidence points to the presence of multiple independent polymorphisms associated with nicotine dependence (Saccone et al, 2010a, 2010b). GWAS meta-analyses have identified four additional loci associated with nicotine dependence (The Tobacco and Genetics Consortium, 2010; Thorgeirsson et al, 2010). Two of these loci map close to additional nicotinic receptor subunits (CHRNA6 and CHRNB3) and to enzymes important for the metabolism of nicotine (CYP2A6 and CYP2B6).
An additional factor in the persistence of smoking behaviors is the difficulty in quitting. Although many methods have been developed that improve quit rates, none is effective in all smokers (Lerman et al, 2007). Among these methods are several pharmacological agents, including nicotine replacement therapy (NRT), bupropion, and varenicline. Bupropion is a dopamine/norepinephrine reuptake inhibitor that also acts as a nicotinic receptor antagonist (Warner and Shoaib, 2005); varenicline is a partial agonist of the α4β2 nAChR subtype (Coe et al, 2005). The determinants of successful smoking cessation, like nicotine dependence itself, are likely to be diverse. The genetic components of successful smoking cessation are less well understood than nicotine dependence itself, although, like nicotine dependence, a significant proportion (∼50%) of the likelihood of quitting is genetic in origin (Broms et al, 2006; Lessov et al, 2004; Xian et al, 2003), suggesting that specific genetic risk factors could be identified. Indeed, the chromosome 15q25 locus described above has been associated with successful quitting in pregnant women (Freathy et al, 2009). However, many of the genetic loci affecting quitting are likely to be distinct from genetic determinants of nicotine dependence (Maes et al, 2004; The Tobacco and Genetics Consortium, 2010; Thorgeirsson et al, 2010).
In order to better understand the genetic determinants of smoking cessation, recent pharmacogenetic studies have investigated genes that may impact nicotine or bupropion activity and metabolism, and also components of the dopaminergic system related to addiction (Conti et al, 2008; Kortmann et al, 2010; Lee et al, 2007; Ray et al, 2010). Although replications are needed, variants identified in CYP2B6 and CHRNB2 may influence cessation rates for bupropion (Conti et al, 2008; Lee et al, 2007), and variants in the choline acetyltransferase (CHAT) gene may influence the success of NRT (Ray et al, 2010). In addition, several pharmacogenomics studies have investigated the effect of nicotine metabolism rates directly, through analysis of nicotine metabolites (Benowitz, 2009) with reproducible associations with smoking cessation (Ray et al, 2009).
Here, we describe the first pharmacogenetic analysis of smoking cessation in a large population of smokers derived from placebo-controlled clinical trials testing the efficacy of varenicline and bupropion. In addition to drug metabolizing and nicotinic receptor genes, we investigated the primary varenicline transporter (SLC22A2), additional genes in the chromosome 15q25 locus (IREB2, LOC123688, and PSMA4), and two serotonin receptors (HTR3A and HTR3B) whose expression in the gut may contribute to nausea while on varenicline treatment (Gershon, 2004). We also examined whether variation in these candidate genes is associated with time to relapse to smoking or to nausea while on treatment.
PATIENTS AND METHODS
Study Population
Subjects included in this study had participated in one of three randomized, double-blind, placebo-controlled smoking cessation clinical trials comparing varenicline, a nAChR partial agonist, with bupropion, a norepinephrine and dopamine reuptake inhibitor, and placebo (Box 1). The three trials have been described in detail previously (Gonzales et al, 2006; Jorenby et al, 2006; Oncken et al, 2006). In brief, all of the studies included a 12-week treatment period and 40-week non-treatment follow-up. Participants were healthy men and women; all had smoked an average of at least 10 cigarettes per day (CPD) during the past year, with no period of abstinence longer than 3 months, and were motivated to quit. For these clinical trials, patients were excluded if they had a history of alcohol or other drug abuse or dependence in the previous 12 months. Clinical trial endpoints included carbon monoxide-confirmed continuous abstinence from weeks 9 to 12 and weeks 9 to 52 (Gonzales et al, 2006; Jorenby et al, 2006; Oncken et al, 2006), and time to smoking relapse. Relapse to smoking after abstinence from weeks 9 to 12 was indicated either by patient-reported cigarette (as little as a single puff) or other nicotine use, or by an expiratory carbon monoxide measurement exceeding 10 p.p.m.
Box 1 Varenicline Clinical Studies Included in the Pharmacogenetics Analysis.
An optional blood sample was collected from clinical trial subjects for pharmacogenetic analysis to investigate potential associations between genetic variants and varenicline response and general characteristics of smoking cessation. Sample collection was not required for participation in the original clinical trials; however, >50% of clinical trial subjects across all treatment arms volunteered to participate in the pharmacogenetic analysis.
Genotyping
Candidate genes of interest included the genes identified on Chr15q25 (including the CHRNA5, CHRNA3, and CHRNB4 gene cluster), the remaining nAChR subunit genes, genes encoding the varenicline transporter (SLC22A2) and serotonergic targets hypothesized to be involved in varenicline-induced nausea (HTR3A, HTR3B), as well as cytochrome P450 (CYP) genes involved in nicotine and bupropion metabolism (Box 2). Varenicline is highly selective for the α4β2 nicotinic receptor (Ki=0.17 nM) (Pfizer, 2010), but also shows moderate affinity (Ki=350 nM) for the 5-HT3 receptor and other common nicotinic receptors.
Box 2 Candidate Gene List.

Primary genotyping was performed on the Illumina GoldenGate platform, with 975 candidate gene single-nucleotide polymorphisms (SNPs) and 216 ancestry informative marker SNPs. In addition, 89 complementary SNPs were genotyped using ABI Taqman and SNPlex methods. Genotyping call rates for all samples were ⩾94% (mean=99.8% median=99.8%). Genotyping call rates for all SNPs were ⩾97% (mean=99.8%, median=99.9%). SNPs were tested for Hardy–Weinberg equilibrium. In total, 12 SNPs were significantly out of HWE (p<0.0001). None of these were associated with any phenotypes tested here. Multidimensional scaling analysis of the ancestry informative markers identified four subjects that did not cluster with the subjects of European ancestry. These subjects were excluded from the analysis.
Statistical Analysis
Genetic associations for continuous abstinence rates and nausea incidence were assessed using logistic regression, assuming an additive genetic model. A survival model was used to assess the relapse data; specifically, a proportional hazards model was fitted to determine whether the time to relapse was affected by genotype. The proportional hazards assumption was checked using plots of the log-log survival (relapse) curves. The relapse analysis also assumed an additive genetic model. Our analysis was limited to SNPs whose minor allele frequency was >5% in our overall genotyped population, reducing the total number of candidate gene SNPs analyzed to 785. For each analysis, a treatment × genotype interaction term was initially included in the model. For markers with at least marginally significant interaction with treatment (p<0.20), the analysis was performed for each treatment group separately, as well as for all subjects together and adjusted for treatment. When the interaction was not significant, the data were analyzed as a single pooled sample, adjusted for treatment. To correct for multiple testing in which many markers were in strong linkage disequilibrium (LD), we selected individual SNPs representing bins of highly linked SNPs (r2>0.8), with a single SNP for each bin included in a false discovery rate, multiple test correction approach (bins were derived from the Caucasian population described here). This reduced the total estimated number of independent tests from 785 to 254. All of the p-values reported here fell below a q-value of 0.2, using this approach.
RESULTS
Study Population
Across the three trials, 2699 patients were randomized to treatment (including 826 to varenicline 1.0 mg twice per day titrated dose, 671 to bupropion, and 814 to placebo). DNA was extracted from blood samples of 1476 consenting individuals (524 varenicline; 440 bupropion; 512 placebo), and primary genetic analysis was performed on 1175 smokers of European ancestry. The genotyped subset was comparable to the overall clinical data set for baseline characteristics and outcomes, including age, gender, race, weight, smoking history, Fagerström Test for Nicotine Dependence, therapeutic smoking cessation response (continuous abstinence from weeks 9 to 12), and adverse event profile (including nausea incidence with varenicline).
Baseline Characteristics
The demographic and smoking history characteristics of genotyped participants from the three clinical trials are shown in Table 1. Patients in the analysis had a mean age of approximately 43 years, and nearly 50% of the population was female. They smoked on average 22 CPD (SD 9.4), and an average score of 5.3 on the Fagerström Test for Nicotine Dependence (SD 2.1) indicated a moderate level of addiction. There were no significant differences in demographic variables or baseline characteristics across the three treatment groups.
Table 1. Characteristics of Genotyped Patients from the Clinical Trials.
| Varenicline | Bupropion | Placebo | |
|---|---|---|---|
| Age (SD) | 43.8 (11.1) | 42.7 (12.0) | 42.7 (11.9) |
| Gender, % male | 52 | 56 | 57 |
| Race, % Caucasian | 82 | 82 | 79 |
| Weight, kg (SD) | 79.7 (16.2) | 79.0 (15.9) | 79.3 (15.6) |
| Smoking history, CPD (SD) | 22.5 (9.9) | 21.9 (8.7) | 22.3 (9.7) |
| FTND total score (SD) | 5.34 (2.2) | 5.27 (2.1) | 5.28 (2.0) |
Abbreviations: CPD, cigarettes per day; FTND, Fagerström Test for Nicotine Dependence.
Analysis of baseline smoking behavior among all genotyped subjects, as measured by the number of CPD, revealed a genetic association with the primary nicotine dependence locus on chromosome 15q25, indicating that the population studied here is consistent with previous populations evaluated for nicotine dependence (Thorgeirsson et al, 2008). The most significant SNP detected in this region was rs4275821 (p<0.003), although we also detected nominal associations with other SNPs, including rs16969968 (p<0.03). The CPD association with the 15q25 region was detected in this population despite the absence of a full range of smoking phenotypes (individuals smoking <10 CPD were excluded from the clinical trials) (Gonzales et al, 2006; Jorenby et al, 2006; Oncken et al, 2006). We also detected an association between the 15q25 locus and scores from the Fagerström Test for Nicotine Dependence (p<0.004, rs12443170).
Continuous Abstinence
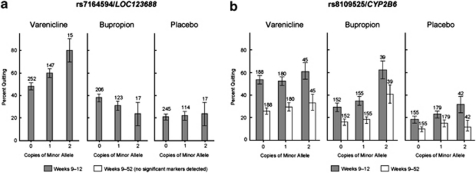
SNPs associated with continuous abstinence during weeks 9–12 of the treatment period are shown in Table 2. Among the subjects randomized to varenicline treatment, two polymorphisms in CHRNB2 (rs3811450 and rs4262952) were associated with the largest increased odds of continuous smoking abstinence from weeks 9 to 12 (OR=2.52 (CI: 1.32–4.78); OR=2.44 (CI: 1.28–4.63)). Additionally, there were significant associations of SNPs within the chromosome 15q25 locus, as well as the CHRNA4 and CHRNA7 gene loci. The SNP most significantly associated with continuous abstinence from weeks 9 to 12 in the varenicline-treated smokers, rs7164594, is within the 15q25 locus and is in strong LD with rs2036534 (r2=0.98), a SNP previously determined to be significantly associated with lung cancer (Amos et al, 2008) (Figure 1a).
Table 2. Pharmacogenomics of Continuous Abstinence at Weeks 9–12.
| SNP | MAF | Gene | Region | Varenicline p-value | OR | 95% CI | q-value |
|---|---|---|---|---|---|---|---|
| rs7164594 | 0.21 | LOC123688 | 15q25 | 0.0019 | 1.76 | 1.23–2.52 | 0.181 |
| rs3787138 | 0.13 | CHRNA4 | 20q13 | 0.0030 | 1.89 | 1.24–2.88 | 0.181 |
| rs6494212 | 0.30 | CHRNA7 | 15q13 | 0.0038 | 1.58 | 1.16–2.15 | 0.181 |
| rs3811450 | 0.07 | CHRNB2 | 1q21 | 0.0048 | 2.52 | 1.32–4.78 | 0.181 |
| rs2236196 | 0.27 | CHRNA4 | 20q13 | 0.0063 | 1.54 | 1.13–2.09 | 0.181 |
| rs4292956 | 0.07 | CHRNB2 | 1q21 | 0.0067 | 2.44 | 1.28–4.63 | 0.181 |
| rs518425 | 0.28 | CHRNA5 | 15q25 | 0.0071 | 1.62 | 1.14–2.31 | 0.181 |
| rs6062899 | 0.18 | CHRNA4 | 20q13 | 0.0071 | 1.63 | 1.14–2.33 | 0.181 |
| rs2938674 | 0.21 | IREB2 | 15q25 | 0.0095 | 1.60 | 1.12–2.27 | 0.196 |
| Bupropion p-value | |||||||
| rs8109525 | 0.34 | CYP2B6 | 19q13 | 0.0008 | 1.78 | 1.27–2.50 | 0.120 |
| rs3762528 | 0.07 | CHRND | 2q37 | 0.0012 | 2.98 | 1.54–5.77 | 0.120 |
| rs1808682 | 0.25 | CYP2B6 | 19q13 | 0.0030 | 1.70 | 1.20–2.41 | 0.154 |
| rs6725786 | 0.10 | CHRND | 2q37 | 0.0036 | 2.23 | 1.30–3.84 | 0.154 |
| rs1042389 | 0.20 | CYP2B6 | 19q13 | 0.0038 | 1.90 | 1.23–2.92 | 0.154 |
| rs2113103 | 0.16 | CYP2B6 | 19q13 | 0.0049 | 1.82 | 1.20–2.76 | 0.167 |
| Overall p-value | |||||||
| rs8109525 | 0.34 | CYP2B6 | 19q13 | 0.0011 | 1.37 | 1.13–1.66 | 0.111 |
| rs1808682 | 0.25 | CYP2B6 | 19q13 | 0.0013 | 1.40 | 1.14–1.72 | 0.111 |
| rs2014141 | 0.40 | CYP2B6 | 19q13 | 0.0020 | 0.75 | 0.62–0.90 | 0.115 |
| rs2113103 | 0.16 | CYP2B6 | 19q13 | 0.0030 | 1.43 | 1.13–1.82 | 0.129 |
| rs6010918 | 0.05 | CHRNA4 | 20q13 | 0.0048 | 1.75 | 1.19–2.59 | 0.167 |
Abbreviations: CI, confidence interval; MAF, minor allele frequency; OR, odds ratio; SNP, single-nucleotide polymorphism.
Figure 1.
Genetic markers most significantly associated with continuous abstinence: (a) varenicline, (b) bupropion.
Among the bupropion-treated subjects and in all three treatment groups combined, continuous abstinence at weeks 9–12 was strongly associated with several SNPs in CYP2B6 as well as two SNPs in CHRND, which encodes the nAChR delta subunit. Interestingly, one of the CYP2B6 SNPs (rs8109525; Figure 1b) is contained within a LD bin that includes rs7260329, a SNP recently identified to be significantly associated with the number of cigarettes smoked per day (Thorgeirsson et al, 2010). rs7260329 itself is nominally associated with continuous abstinence in the bupropion and combined populations, although to a lesser degree (p<0.008, p<0.02, respectively; q values >0.2). No SNP tested was significantly associated with abstinence on placebo treatment.
During longer-term follow-up, through 52 weeks, no polymorphism was significantly associated with continuous abstinence in the varenicline or placebo treatment groups. However, in the bupropion group, as well as in the three treatment groups combined, continuous abstinence was again associated with several SNPs within CYP2B6 (Table 3), including rs8109525 (p=0.0028, Figure 1b). Several of the polymorphisms tested in CYP2B6 were in LD with this SNP (including rs7260329, described above); however, none of these linked SNPs correspond to functionally described CYP2B6 alleles (Anon, 2008). In the combined treatment group, variants in CYP2A6 were also significantly associated with continued abstinence.
Table 3. Pharmacogenomics of Continuous Abstinence at Weeks 9–52.
| SNP | MAF | Gene | Region | Bupropion p-value | OR | 95% CI | q-value |
|---|---|---|---|---|---|---|---|
| rs1808682 | 0.251 | CYP2B6 | 19q13 | 0.00008 | 2.27 | 1.51–3.40 | 0.019 |
| rs1042389 | 0.201 | CYP2B6 | 19q13 | 0.0003 | 2.49 | 1.52–4.09 | 0.036 |
| rs2113103 | 0.156 | CYP2B6 | 19q13 | 0.0010 | 2.16 | 1.36–3.43 | 0.087 |
| rs8100458 | 0.339 | CYP2B6 | 19q13 | 0.0028 | 1.82 | 1.23–2.70 | 0.180 |
| Overall p-value | |||||||
| rs1808682 | 0.251 | CYP2B6 | 19q13 | 0.0001 | 1.58 | 1.25–1.99 | 0.019 |
| rs11606194 | 0.076 | HTR3B | 11q23 | 0.0023 | 0.47 | 0.29–0.76 | 0.197 |
| rs892216 | 0.348 | CYP2B6 | 19q13 | 0.0042 | 1.37 | 1.11–1.70 | 0.197 |
| rs7123164 | 0.115 | CHRNA10 | 11p15 | 0.0050 | 1.55 | 1.14–2.11 | 0.197 |
| rs7255616 | 0.067 | CYP2A6 | 19q13 | 0.0057 | 1.69 | 1.16–2.45 | 0.197 |
Abbreviations: CI, confidence interval; MAF, minor allele frequency; OR, odds ratio; SNP, single-nucleotide polymorphism.
rs8109525, shown in Figure 1, is in strong LD with both rs8100458 and rs892216 (r2>0.95).
No SNPs were associated with continuous abstinence in patients receiving varenicline.
Relapse to Smoking, Following Successful Quitting, All Treatments
For all treatment groups combined, time to smoking relapse following initial abstinence from weeks 9–12 was associated with SNPs in HTR3B (rs11606194, rs3758987) and HTR3A (rs11607240) (Table 4). Figure 2 shows the time to relapse associated with the two most predictive SNPs, rs11606194 and rs3758987.
Table 4. Loci Associated With Relapse During Weeks 12–52, Among Those who had Successfully Quit at Weeks 9–12, All Groups Combined (n=420).
| SNP | MAF | Gene | Region | p-value | HR | 95% CI | q-value |
|---|---|---|---|---|---|---|---|
| rs11606194 | 0.076 | HTR3B | 11q23 | 1.53E–05 | 1.97 | 1.45–2.68 | 0.003 |
| rs3758987 | 0.271 | HTR3B | 11q23 | 0.0006 | 1.46 | 1.18–1.81 | 0.049 |
| rs11607240 | 0.074 | HTR3A | 11q23 | 0.0015 | 1.65 | 1.21–2.25 | 0.084 |
Abbreviations: CI, confidence interval; HR, hazard ratio; MAF, minor allele frequency; SNP, single-nucleotide polymorphism.
Figure 2.
Time to relapse among those who had successfully quit at weeks 9–12, all groups combined: (a) rs11606194, (b) rs3758987.
Nausea
In these clinical trials, the adverse event most commonly reported in patients receiving varenicline (1 mg twice per day) was nausea (Gonzales et al, 2006; Jorenby et al, 2006; Oncken et al, 2006). Nausea was reported by 30% of individuals in the varenicline group (ranging from 28% to 35% across the three clinical trials) vs 10% in the bupropion group, and 9% in the placebo group. The incidence of nausea in the varenicline treatment group, and in all three treatment groups combined, was associated primarily with SNPs in the chromosome 15q25 locus (Table 5 and Supplementary Table 1). Among varenicline-treated patients, nausea was most significantly associated with rs555018 in the CHRNA5 gene (OR=1.62; 95% CI: 1.19–2.20) and rs1190449 in the CHRNG gene (OR=1.57; 95% CI: 1.18–2.08), while in the three treatment groups combined, the most significant association was with rs6495309 in the CHRNB4 gene (OR=0.50; 95% CI: 0.36–0.70; p=4.04E–05).
Table 5. Nausea Pharmacogenomics, Overall.
| SNP | MAF | Gene | Region | p-value | OR | 95% CI | q-value |
|---|---|---|---|---|---|---|---|
| rs6495309 | 0.205 | CHRNB4 | 15q25 | 4.04E–05 | 0.50 | 0.36–0.70 | 0.007 |
| rs4887072 | 0.216 | CHRNB4 | 15q25 | 0.0002 | 0.55 | 0.40–0.75 | 0.015 |
| rs1878399 | 0.424 | CHRNA3 | 15q25 | 0.0012 | 1.48 | 1.17–1.87 | 0.066 |
| rs1190449 | 0.447 | CHRNG | 2q37 | 0.0021 | 1.42 | 1.13–1.77 | 0.092 |
| rs6741278 | 0.369 | CHRNG | 2q37 | 0.0033 | 0.69 | 0.54–0.88 | 0.099 |
| rs578776 | 0.273 | CHRNA3 | 15q25 | 0.0039 | 0.67 | 0.51–0.88 | 0.099 |
| rs595374 | 0.215 | SLC22A2 | 6q25 | 0.0040 | 0.64 | 0.48–0.87 | 0.099 |
| rs4243083 | 0.416 | PSMA4 | 15q25 | 0.0049 | 1.40 | 1.11–1.77 | 0.105 |
| rs684513 | 0.197 | CHRNA5 | 15q25 | 0.0076 | 0.65 | 0.48–0.89 | 0.138 |
| rs12899425 | 0.274 | IREB2 | 15q25 | 0.0087 | 1.39 | 1.09–1.77 | 0.138 |
| rs2869546 | 0.370 | CHRNA3 | 15q25 | 0.0088 | 1.37 | 1.08–1.73 | 0.138 |
| rs11899983 | 0.437 | CHRNG | 2q37 | 0.0113 | 1.34 | 1.07–1.68 | 0.157 |
| rs17406522 | 0.072 | IREB2 | 15q25 | 0.0125 | 1.67 | 1.12–2.50 | 0.157 |
| rs12443170 | 0.126 | CHRNA3 | 15q25 | 0.0128 | 0.62 | 0.42–0.90 | 0.157 |
| rs12441998 | 0.199 | CHRNB4 | 15q25 | 0.0151 | 0.68 | 0.50–0.93 | 0.173 |
Abbreviations: CI, confidence interval; OR, odds ratio; SNP, single-nucleotide polymorphism.
DISCUSSION
This study represents the first genetic association study of varenicline for smoking cessation. The pharmacogenetic analysis, focusing largely on gene loci encoding nicotinic cholinergic receptor subunits and drug metabolizing enzymes, offers preliminary evidence that variants in CHRNA4, CHRNB2, and CHRNA7 as well as in the 15q25 LD chromosomal region, including the nAChR genes CHRNA3, CHRNA5, and CHRNB4, influence the outcome (success or failure) of smoking cessation attempts with varenicline. In contrast, genetic analysis of response to bupropion suggests that the success of smoking cessation with this drug is determined in part by variation in CYP2B6, the gene encoding the primary enzyme responsible for the metabolism of bupropion (Faucette et al, 2000), rather than by genetic variation in nicotinic cholinergic receptor pathways. In addition to smoking cessation pharmacogenetics, we also evaluated pharmacogenetic associations with the presence of nausea while attempting to quit smoking, as well as relapse to smoking following a successful period of continuous abstinence. As with response to varenicline, genetic variation in the nicotinic cholinergic receptor genes appears to contribute to the risk of experiencing nausea during smoking cessation, and interestingly, relapse to smoking was significantly associated with polymorphisms in the serotonergic receptor genes, HTR3A and HTR3B.
Varenicline
Response to varenicline treatment is associated here with polymorphisms in multiple loci encoding nicotinic receptor subunits. Chief among these are the CHRNA4 and CHRNB2 loci, encoding the α4 and β2 nicotinic receptor subunits. Receptors comprised of these subunits are the specific targets of varenicline's activity, thus it is perhaps not surprising that these loci would be associated with varenicline response. In addition to these loci, there is also an association to varenicline response with polymorphisms in the chr15q25 locus. This, too, is consistent with varenicline activity, particularly given recent evidence demonstrating the importance of α5 subunits in predominantly α4β2 nicotinic receptors and the emergence of risk alleles in this locus as a primary factor in nicotine dependence (Fowler et al, 2011; Kuryatov et al, 2011; Zheng et al, 2011). An association between the 15q25 locus and response to varenicline also supports an argument that response to varenicline is related to nicotine dependence itself. Due to the high degree of LD among loci in this region, it is difficult to discriminate causative loci. The positive markers for varenicline response are in LD (>0.6) with nicotine dependence markers (as well as markers for nausea; see below), thus these associations may be reflecting common functional alleles. In fact, the alleles that are associated with increased odds of quitting with varenicline are in LD with alleles associated with a decreased risk of nicotine dependence. This may be true of bupropion and placebo responders as well, although there are not sufficient subjects in these groups to detect such an effect. The relevance of the association with the CHRNA7 locus is less clear, although varenicline does bind to homopentameric α7 nicotinic receptors (Coe et al, 2005), however with reduced affinity in comparison with α4β2 receptors (Amos et al, 2008; Hung et al, 2008; Thorgeirsson et al, 2008).
Previous pharmacogenetic studies of smoking cessation, focusing on bupropion treatment, also identified polymorphisms in CHRNB2 associated with treatment response. In each of these studies, there is evidence to suggest that response to treatment in the placebo group may also be associated with CHRNB2, albeit to a lesser degree (Conti et al, 2008; Heitjan et al, 2008; Perkins et al, 2009). In our study, we detect a similar, nominal association in our placebo group with a CHRNB2 SNP (rs2072659, p=0.003, q=0.38). Thus, the genetic association of CHRNB2 with response to varenicline might be explained in part by an increased likelihood to quit, regardless of treatment.
Bupropion
In our study, we observed an association between CYP2B6 polymorphisms and response to bupropion treatment. CYP2B6 is the primary metabolizing enzyme for bupropion (as well as a secondary enzyme for nicotine metabolism) thus the effectiveness of bupropion treatment in smoking cessation may be determined by the rate of its metabolism, as indicated by the CYP2B6 genotype. This is consistent with previous studies, in which CYP2B6 polymorphisms have been shown to affect the pharmacokinetics of bupropion (Kirchheiner et al, 2003) and the likelihood of achieving abstinence with bupropion in smoking cessation trials (Lee et al, 2007). However, the polymorphisms associated with response to bupropion in our study differed from those identified in previous studies (which were not successfully genotyped in our study), thus this does not represent a true replication. A better understanding of these associations may come from the identification and characterization of additional functional alleles affecting CYP2B6 activity or expression.
Overall Quit Success at 1 Year
The nicotinic receptor polymorphisms associated with response to varenicline measured from weeks 9 to 12 were not associated with continuous abstinence through the non-treatment follow-up period (including all weeks 9–52). During this period, no markers were significantly associated with continuous abstinence in the varenicline-treated smokers alone. In contrast, among the smokers treated with bupropion, CYP2B6 polymorphisms were associated with continuous abstinence from weeks 9 to 52, as well as weeks 9 to 12. In fact, this association with CYP2B6 polymorphisms extended to all smokers, regardless of treatment during the first 12 weeks. This may be due to a broader effect of CYP2B6 on nicotine dependence, independent of its role in bupropion metabolism. This interpretation is consistent with a prior study identifying an association of a functional CYP2B6 SNP with placebo response in a bupropion clinical trial (Lee et al, 2007). Alternatively, this genetic signal, although more proximate to CYP2B6, may have a more direct effect on the adjacent CYP2A6 locus, which is itself associated with continuous abstinence from weeks 9 to 52. In addition to (or perhaps because of) its role in nicotine metabolism, the CYP2A6 locus has also recently been found to be genetically associated with nicotine dependence (The Tobacco and Genetics Consortium, 2010; Thorgeirsson et al, 2010), and phenotypic markers of nicotine metabolism rate have reproducible associations with prospective smoking cessation (Ray et al, 2009). Continuous abstinence from weeks 9 to 52 also showed weaker associations with HTR3B and CHRNA10 in the entire cohort. The association with HTR3B may be explained relative to its association with relapse to smoking (described below). The potential role of CHRN10A in smoking cessation is less clear, although it has been shown recently to be associated with nicotine dependence in an African-American population (Saccone et al, 2010a, 2010b).
Relapse
We analyzed time to relapse to smoking in the subset of subjects who successfully quit smoking while on treatment during weeks 9–12, but relapsed during weeks 13–52. Unexpectedly, the strongest associations with this phenotype were with polymorphisms in genes encoding the serotonin receptors HTR3A and HTR3B. These genes were originally included in our study to test whether they might be related to nausea associated with varenicline treatment, although they are expressed widely in the brain as well as the gut (Niesler et al, 2008). Previous investigations using an HTR3 antagonist, ondansetron, provide some insights into why these loci may be important for relapse to smoking. This medicine, originally developed to prevent nausea (Cubeddu et al, 1990), has been shown to lower cravings for alcohol and to ease the withdrawal symptoms of opioid addictions (Chu et al, 2009; Johnson et al, 2002). These results and our observations raise the intriguing possibility that this serotonin receptor family may be mediating its effects on relapse by impacting nicotine withdrawal symptoms, suggesting a potential role for HTR3 inhibition in reducing such withdrawal symptoms, regardless of the initial treatment method.
Nausea
In this study, the primary locus associated with nausea while attempting to quit smoking was the chr15q25 locus. This locus, which includes genes encoding the β4, α5, and α3 nicotinic receptor subunits (CHRNB4, CHRNA5, and CHRNA3), is the predominant locus associated with nicotine dependence (Amos et al, 2008; Hung et al, 2008; Thorgeirsson et al, 2008). Carriers of risk alleles at this locus are 30–40% more likely to become nicotine dependent. As with the response to varenicline, the significant LD in this region suggests that these associations are likely to overlap with those for nicotine dependence, and thus nausea experienced while quitting may be directly related to nicotine dependence. Smokers who are more dependent smoke more cigarettes per day and have a higher daily intake of nicotine. The link between the chr15q25 locus genes and nausea may be explained by tolerance, such that those with a greater daily intake of nicotine are more tolerant and therefore experience less nausea in response to a nicotinic partial agonist such as varenicline. Alternatively, incidence of nausea may be elevated in subjects with greater risk for nicotine dependence because their nicotine intake is greater before quitting, therefore exacerbating the symptoms of withdrawal on quitting. SNPs associated with nausea are also linked to SNPs associated with the expression of CHRNA5, suggesting another mechanistic link between nicotine dependence and nausea on quitting. For subjects treated with varenicline, CHRNG, which encodes the nAChRγ subunit, is also associated with nausea. The importance of this locus for nausea in the varenicline-treated subjects is not clear, although recent studies have identified associations with this region, which also includes the CHRND gene, and nicotine dependence (Saccone et al, 2009), suggesting that the association with nausea may also be driven by nicotine dependence.
Strengths and Limitations of This Study
The recent successes of genome-wide genetic association studies have highlighted the need for large study populations in order to detect the small effect sizes often associated with common genetic polymorphisms. However, most pharmacogenomic studies conducted to date have involved relatively small sample populations (Holmes et al, 2009). Thus, the strengths of this analysis include: (a) its large sample population—the largest such study to date of bupropion for smoking cessation, the first (and therefore also largest) pharmacogenetic analysis of varenicline, and the first head-to-head analysis of these treatments; and (b) the availability of robust phenotypic data from three rigorously controlled clinical trials, providing a database of patient data, adverse events, and, importantly, carbon monoxide-confirmed smoking cessation outcomes. Limitations of this analysis include: (a) a lack of generalizability of the study findings to non-treatment-seeking smokers—although the highly motivated (to quit smoking) population recruited to these trials is the subset of the broader smoking population to which pharmacogenetic tailoring approaches would be provided, efforts to encourage non-motivated smokers to quit may have different pharmacogenetic characteristics; and (b) a lack of ethnic diversity among the sample population—the candidate gene analysis was limited to individuals of European ancestry in order to avoid the effects of population stratification, and the relatively small number of subjects of non-European descent in our population prevented meaningful analysis of this latter group. For this reason, the study findings may not apply to populations of non-European descent. These results will also require replication in independent populations for validation. Ideally, larger populations will also permit a genome-wide analysis of smoking cessation pharmacogenetics, which could identify additional novel loci affecting these phenotypes.
Future Perspectives
The goal of pharmacogenetic studies of smoking cessation therapy is to help increase the likelihood of an individual quitting smoking, and reduce the likelihood of adverse effects of smoking cessation therapy by individualizing treatment strategies according to genetic profile. Although pharmacogenetic tests for some conditions have had successful application in clinical practice (Mallal et al, 2008; Relling et al, 1999), this has not been without challenges (Higgs et al, 2010; Ikediobi et al, 2009). Even leaving aside issues of cost-effectiveness and concerns around tests that could result in effective therapies being withheld (Epstein et al, 2009), several important issues need to be considered before pharmacogenetic testing for selection of smoking cessation treatment becomes standard clinical practice. Multiple genes and environmental factors probably combine to influence the response to smoking cessation therapies, yet most pharmacogenetic studies currently focus on a limited set of alleles and/or single genes and have limited power to account for gene–gene and gene–environment interactions. The development of effective individualized treatments for smoking cessation will likely require that future pharmacogenetic studies evaluate these multifactorial interactions. However, despite such challenges, access to larger populations and more detailed information regarding the molecular genetics of nicotine dependence and smoking cessation should lead to better optimization of cessation approaches, and to a reduction in overall smoking prevalence.
CONCLUSIONS
These data provide both novel and supporting evidence for genetic loci contributing to smoking cessation and therapeutic response. Importantly, different genetic signals are associated with varenicline vs bupropion treatment response, suggesting that future research may lead to clinically useful markers to guide treatment decisions, resulting in improved smoking cessation rates overall, and a reduction in smoking prevalence.
Acknowledgments
Editorial support, in the form of collating review comments, proofreading, formatting of references, tables and figures, and styling the paper for submission, was provided by Geraldine Thompson, Abegale Templar, and Fiona Nitsche of UBC Scientific Solutions and was funded by Pfizer. Laura Bierut's research was supported by the National Institute of Health grant K02DA021237 (LJB) from the National Institute on Drug Abuse.
This study was supported by Pfizer. David King, Sara Paciga, Eve Pickering, and Peter Park are or were employees of Pfizer. Laura Bierut is listed as an inventor on a patent ‘Markers for Addiction' (US 20070258898), covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction, and has acted as a consultant for Pfizer in 2008. David Conti acted as a consultant for Pfizer in 2008. Caryn Lerman has served as a consultant and/or has received research funding from GlaxoSmith-Kline, AstraZeneca, Pfizer, Targacept, and Novartis in the past 3 years. Neal Benowitz serves on Pfizer advisory boards related to varenicline and smoking cessation, as a paid consultant to other pharmaceutical companies developing smoking cessation medications, and has served as a paid expert witness in litigation against tobacco companies. Jaakko Kaprio has acted as a consultant for Pfizer in 2008, and has received funding for genetic analyses of nicotine dependence by a GRAND award, funded by Pfizer.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anon 2008CYP2B6 allele nomenclatureAvailable from: http://www.cypalleles.ki.se/cyp2b6.htm .
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick P, Wang Y, Vijayakrishnan J, Matakidou A, Spitz MR, Eisen T, et al. Deciphering the impact of common genetic variation on lung cancer risk: a genome-wide association study. Cancer Res. 2009;69:6633–6641. doi: 10.1158/0008-5472.CAN-09-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broms U, Silventoinen K, Madden PA, Heath AC, Kaprio J. Genetic architecture of smoking behavior: a study of Finnish adult twins. Twin Res Hum Genet. 2006;9:64–72. doi: 10.1375/183242706776403046. [DOI] [PubMed] [Google Scholar]
- Chu LF, Liang DY, Li X, Sahbaie P, D'Arcy N, Liao G, et al. From mouse to man: the 5-HT3 receptor modulates physical dependence on opioid narcotics. Pharmacogenet Genomics. 2009;19:193–205. doi: 10.1097/FPC.0b013e328322e73d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Conti DV, Lee W, Li D, Liu J, Van Den Berg D, Thomas PD, et al. Nicotinic acetylcholine receptor beta2 subunit gene implicated in a systems-based candidate gene study of smoking cessation. Hum Mol Genet. 2008;17:2834–2848. doi: 10.1093/hmg/ddn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubeddu LX, Hoffmann IS, Fuenmayor NT, Finn AL. Efficacy of ondansetron (GR 38032F) and the role of serotonin in cisplatin-induced nausea and vomiting. N Engl J Med. 1990;322:810–816. doi: 10.1056/NEJM199003223221204. [DOI] [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- DeMeo DL, Mariani T, Bhattacharya S, Srisuma S, Lange C, Litonjua A, et al. Integration of genomic and genetic approaches implicates IREB2 as a COPD susceptibility gene. Am J Hum Genet. 2009;85:493–502. doi: 10.1016/j.ajhg.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RS, Frueh FW, Geren D, Hummer D, McKibbin S, O'Connor S, et al. Payer perspectives on pharmacogenomics testing and drug development. Pharmacogenomics. 2009;10:149–151. doi: 10.2217/14622416.10.1.149. [DOI] [PubMed] [Google Scholar]
- Faucette SR, Hawke RL, Lecluyse EL, Shord SS, Yan B, Laethem RM, et al. Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos. 2000;28:1222–1230. [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, et al. 2008Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Executive Summary US Department of Health and Human Services. Public Health Service: Rockville MD; Available from: http://www.ahrq.gov/path/tobacco.htm#clinic . [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freathy RM, Ring SM, Shields B, Galobardes B, Knight B, Weedon MN, et al. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet. 2009;18:2922–2927. doi: 10.1093/hmg/ddp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD. Review article: serotonin receptors and transporters—roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther. 2004;20 (Suppl 7:3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Heitjan DF, Guo M, Ray R, Wileyto EP, Epstein LH, Lerman C. Identification of pharmacogenetic markers in smoking cessation therapy. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:712–719. doi: 10.1002/ajmg.b.30669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs J, Gambhir N, Ramsden SC, Poulton K, Newman WG. Pharmacogenetic testing in the United Kingdom genetics and immunogenetics laboratories. Genet Test Mol Biomarkers. 2010;14:121–125. doi: 10.1089/gtmb.2009.0156. [DOI] [PubMed] [Google Scholar]
- Holmes MV, Shah T, Vickery C, Smeeth L, Hingorani AD, Casas JP. Fulfilling the promise of personalized medicine? Systematic review and field synopsis of pharmacogenetic studies. PLoS One. 2009;4:e7960. doi: 10.1371/journal.pone.0007960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- Ikediobi ON, Shin J, Nussbaum RL, Phillips KA, Walsh JM, Ladabaum U, et al. Addressing the challenges of the clinical application of pharmacogenetic testing. Clin Pharmacol Ther. 2009;86:28–31. doi: 10.1038/clpt.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Ait-Daoud N, Zanca NA, Velazquez M. Ondansetron reduces the craving of biologically predisposed alcoholics. Psychopharmacology (Berl) 2002;160:408–413. doi: 10.1007/s00213-002-1002-9. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kirchheiner J, Klein C, Meineke I, Sasse J, Zanger UM, Murdter TE, et al. Bupropion and 4-OH-bupropion pharmacokinetics in relation to genetic polymorphisms in CYP2B6. Pharmacogenetics. 2003;13:619–626. doi: 10.1097/00008571-200310000-00005. [DOI] [PubMed] [Google Scholar]
- Kortmann GL, Dobler CJ, Bizarro L, Bau CH. Pharmacogenetics of smoking cessation therapy. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:17–28. doi: 10.1002/ajmg.b.30978. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Berrettini W, Lindstrom J. Acetylcholine receptor (AChR) alpha5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (alpha4beta2)alpha5 AChR function. Mol Pharmacol. 2011;79:119–125. doi: 10.1124/mol.110.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi MT, Chatterjee N, Yu K, Goldin LR, Goldstein AM, Rotunno M, et al. A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am J Hum Genet. 2009;85:679–691. doi: 10.1016/j.ajhg.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Jepson C, Hoffmann E, Epstein L, Hawk LW, Lerman C, et al. CYP2B6 genotype alters abstinence rates in a bupropion smoking cessation trial. Biol Psychiatry. 2007;62:635–641. doi: 10.1016/j.biopsych.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Lerman C, LeSage MG, Perkins KA, O'Malley SS, Siegel SJ, Benowitz NL, et al. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov. 2007;6:746–762. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Martin NG, Statham DJ, Todorov AA, Slutske WS, Bucholz KK, et al. Defining nicotine dependence for genetic research: evidence from Australian twins. Psychol Med. 2004;34:865–879. doi: 10.1017/s0033291703001582. [DOI] [PubMed] [Google Scholar]
- Li MD, Ma JZ, Cheng R, Dupont RT, Williams NJ, Crews KM, et al. A genome-wide scan to identify loci for smoking rate in the Framingham Heart Study population. BMC Genet. 2003;4 (Suppl 1:S103. doi: 10.1186/1471-2156-4-S1-S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes HH, Sullivan PF, Bulik CM, Neale MC, Prescott CA, Eaves LJ, et al. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychol Med. 2004;34:1251–1261. doi: 10.1017/s0033291704002405. [DOI] [PubMed] [Google Scholar]
- Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568–579. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR. Genetics of nicotinic acetylcholine receptors: relevance to nicotine addiction. Biochem Pharmacol. 2008;75:323–333. doi: 10.1016/j.bcp.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesler B, Kapeller J, Hammer C, Rappold G. Serotonin type 3 receptor genes: HTR3A, B, C, D, E. Pharmacogenomics. 2008;9:501–504. doi: 10.2217/14622416.9.5.501. [DOI] [PubMed] [Google Scholar]
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166:1571–1577. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Mercincavage M, Fonte CA, Briski JL. Nicotinic acetylcholine receptor beta2 subunit (CHRNB2) gene and short-term ability to quit smoking in response to nicotine patch. Cancer Epidemiol Biomarkers Prev. 2009;18:2608–2612. doi: 10.1158/1055-9965.EPI-09-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizer 2010Chantix (Varenicline) Tablets. Prescribing Information Pfizer: New York, NY; Available from: http://www.pfizer.com/files/products/uspi_chantix.pdf . [Google Scholar]
- Pillai SG, Ge D, Zhu G, Kong X, Shianna KV, Need AC, et al. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet. 2009;5:e1000421. doi: 10.1371/journal.pgen.1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R, Mitra N, Baldwin D, Guo M, Patterson F, Heitjan DF, et al. Convergent evidence that choline acetyltransferase gene variation is associated with prospective smoking cessation and nicotine dependence. Neuropsychopharmacology. 2010;35:1374–1382. doi: 10.1038/npp.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R, Tyndale RF, Lerman C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J Neurogenet. 2009;23:252–261. doi: 10.1080/01677060802572887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relling MV, Hancock ML, Rivera GK, Sandlund JT, Ribeiro RC, Krynetski EY, et al. Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst. 1999;91:2001–2008. doi: 10.1093/jnci/91.23.2001. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, Cichon S, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010a;6 doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, et al. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:453–466. doi: 10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, Schwantes-An TH, Wang JC, Grucza RA, Breslau N, Hatsukami D, et al. Multiple cholinergic nicotinic receptor genes affect nicotine dependence risk in African and European Americans. Genes Brain Behav. 2010b;9:741–750. doi: 10.1111/j.1601-183X.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS.1999The genetic epidemiology of smoking Nicotine Tob Res 1(Suppl 2S51–S57.discussion S69–70. [DOI] [PubMed] [Google Scholar]
- The Tobacco and Genetics Consortium Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–453. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner C, Shoaib M. How does bupropion work as a smoking cessation aid. Addict Biol. 2005;10:219–231. doi: 10.1080/13556210500222670. [DOI] [PubMed] [Google Scholar]
- Xian H, Scherrer JF, Madden PA, Lyons MJ, Tsuang M, True WR, et al. The heritability of failed smoking cessation and nicotine withdrawal in twins who smoked and attempted to quit. Nicotine Tob Res. 2003;5:245–254. [PubMed] [Google Scholar]
- Zheng X, Duan W, Xu J, Nie C, Yang Z, Wang H, et al. 2011Functionally significant nicotine acetylcholine receptor subunit alpha5 promoter haplotypes are associated with susceptibility to lung cancer in Chinese Cancere-pub ahead of print 29 March 2011). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.