Abstract
Background
The clinical diagnosis of acute appendicitis (AA) in children is still problematic in status.
Objectives
To investigate the diagnostic value of mean platelet volume (MPV) in acute AA at childhood.
Methods
One hundred patients diagnosed as AA patients and 100 healthy individuals. Laboratory tests were studied in the hematology laboratory of the hospital.
Results
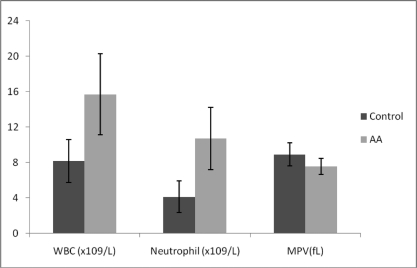
The MPV was found to be lower than normal in 48 cases in the AA group, and it was normal or higher than normal in 52 cases. In the control group, while MPV was found to be lower than normal in 13 cases, it was normal or higher than normal in 87 cases. The MPV was significantly lower in the AA group compared to the control group (p<0.001).
Conclusion
Our study indicated that MPV significantly decreased in pediatric AA patients. Hence, we believe that taking the MPV decrease into consideration along with the White Blood Cell Count elevation would be beneficial in patients with suspicion of AA.
Keywords: Mean platelet volume, diagnosis, acute appendicitis, Children
Introduction
The clinical diagnosis of acute appendicitis (AA) in children is still problematic. Delay in the diagnosis of AA leads to an increase in the risk of severe and advanced complications such as perforation. Furthermore, negative exploration rates still are 20–30%1–3. Unfortunately, clinical history, physical examination and routine laboratory tests do not provide sufficient data to confirm the diagnosis of AA in the early period. Imaging methods such as ultrasonography and computerized tomography are promising for the early and accurate diagnosis, however, they are not sufficient4–5. Thus, determining new methods to distinguish non-specific abdominal pain from AA and decreasing the rate of negative laparatomy is still required. New markers are needed that would decrease the rate of negative laparatomies in cases with the prediagnosis of AA and that would not lead to a delay in the diagnosis, that would be easily applicable everywhere, be cheap, non-invasive, and that would not cause a loss in time. Thus, many studies investigating the diagnostic value of laboratory inflammatory markers have been carried out in recent years. However, the results of these studies have been found distinct and conflicting3.
The mean platelet volume (MPV) is a parameter detected during routine blood count and to which clinicians do not usually pay attention. Platelet volume is known to be a marker determined from megakaryocytes during platelet production, which is associated with platelet function and activation6–10. There are available studies performed on adults available indicating that MPV values may be a valuable method in the diagnosis of acute appendicitis11. In this study, we aimed to investigate the diagnostic value of MPV in acute appendicitis at childhood.
Method
Current study was carried out with 100 patients diagnosed as acute appendicitis chosen retrospectively among patients admitted to the Pediatrics and Pediatric surgery clinics of Diyarbakir Children's Hospital between 2009 and 2010, and 100 healthy individuals. The study design was approved by the Ethics Committee of Yuzuncu Yil University. Patients who had been operated on with the preliminary diagnosis of AA in the light of clinical, laboratory findings and imaging methods, and in whom the definite diagnoses were confirmed with post-operative pathological examination were enrolled in the study. The healthy control group was chosen among children aged 1 to 15 years, who had been admitted to the pediatrics clinic, who did not have heart failure, peripheral vascular disease, hematologic disease, acute or chronic infections, liver disease, and who did not use anticoagulant or steroids.
Laboratory tests were studied in the hematology laboratory of the hospital. In all the cases, complete blood count was studied using horiba ABX Pentra DX 120 automatic blood count device in two hours after 4.5 ml of blood had been drawn from the antecubital vein into tubes containing 15% ethylene diamin tetra acetic acid (K3EDTA). White blood cell (WBC), neutrophil, platelet counts and MPV values of the patients were recorded. Normal WBC, neutrophil and platelet counts were accepted as 4–11×109/L, 2–8×109/L and 150–400×109/L, respectively, according to reference values of the hematology laboratory. The normal MPV reference value was accepted as 8.9±1.29 fL (12)12.
Statistical Analysis: Descriptive statistics for the studied variables (characteristics) were presented as mean, standard deviation, minimum and maximum values. The Student t test was used to compare the means of the control and the patient groups for the studied variables. The Pearson correlation analysis was carried out to examine the linear relationships among the variables. The cut-off values of parameters for discrimination of the control and patient groups were determined using the ROC analysis. Statistical significance levels were considered as 5%. The SPSS (ver. 13) statistical program was used for all the statistical computations.
Results
While the AA group included 44 girls and 56 boys, the control group included 46 girls and 54 boys. The mean age was 8.18±3.48 years (1–15) in the AA group and 8.75±3.63 years (1–14) in the control group. The groups were similar in terms of age and gender (p=0.258). The mean ± standard deviation, minimum and maximum white blood cell, neutrophil and platelet counts and MPV, and the p values of both groups have been presented in table 1.
Table 1.
Data of MPV, Neutrophil and WBC count according to groups
| Control Group | Acute Appendicitis Group | ||||||||
| Mean | SD | Min | Max | Mean | SD | Min | Max | p | |
| WBC (×109/L) | 8.162 | .41 | 3.70 | 14.70 | 15.69 | 4.59 | 6.50 | 33.40 | 0.001 |
| Neutrophil (×109/L) | 4.11 | 1.78 | 1.04 | 8.77 | 10.68 | 3.51 | 4.18 | 20.33 | 0.001 |
| PLT (×109/L) | 380.76 | 87.07 | 172.00 | 663.00 | 303.24 | 85.97 | 88.00 | 551.00 | 0.001 |
| MPV(fL) | 8.90 | 1.29 | 6.10 | 11.90 | 7.55 | .89 | 6.00 | 10.40 | 0.001 |
WBC: White Blood Cell Count; PLT: Platelet; MPV: Mean Platelet Volume
While WBC and neutrophil counts were found to be higher than normal in 84 and 77 subjects, respectively, in the AA group, they were normal or lower than normal in 16 and 23 cases, respectively. In the control group, while the WBC and the neutrophil counts were found to be higher than normal in 11 and 9 cases, respectively, they were normal or lower than normal in 89 and 91 subjects, respectively. The elevations in WBC and neutrophil counts were found to be statistically significant in the AA group compared to the control group (p<0.001).
When the platelet counts were analyzed according to the groups, they were found to be statistically significantly lower in the AA group (p<0.001). When the Pearson correlation coefficient was analyzed, there was a linear relationship between MPV and the platelet count in the AA group. In other words, MPV decreased as the platelet count decreased [Correlation is significant at the 0.01 level (0,372)]. No such relationship was present in the control group.
While MPV was found to be lower than normal in 48 cases in the AA group, it was normal or higher than normal in 52 cases. In the control group, while MPV was found to be lower than normal in 13 cases, it was normal or higher than normal in 87 cases. The MPV was significantly lower in the AA group compared to the control group (p<0.001). The WBC, neutrophil count and MPV values have been graphically displayed in figure 1.
Figure 1.
Mean Platelet Volume, Neutrophil and White Blood Cell Count
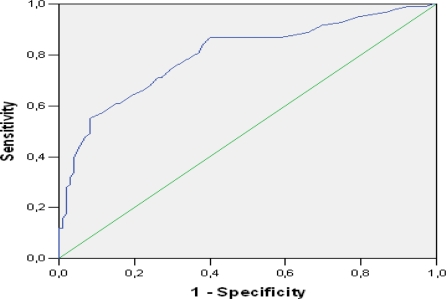
While specificity was found as 89% and 91%, respectively for elevations in the WBC (>11 ×109 / L) and neutrophil (>8 × 109 /L) counts in the AA diagnosis, sensitivity was found as 84% and 77%. Specificity was determined as 54% and sensitivity was found as 87% for the decrease in MPV (<7.4 fL). The cut-off value that had the best specificity-sensitivity values according to ROC curve analysis was determined as 11.050 × 109 /L (89%–84%) for WBC, 6.425 × 109 /L (90%–93%) for the neutrophil count, and 7.55 fL (60%–87%) for MVP. The ROC curve for MPV has been presented in figure 2. The area under the curve (AUC) was determined as 80% for MPV, 94.5% for WBC, and 96.8% for the neutrophil count.
Figure 2.
ROC curve of mean platelet volume
Discussion
Alterations in MPV values have been studied recently, especially in some inflammatory diseases. To the best of our knowledge, MPV alterations in acute appendicitis have been studied only in adults11, but not in children.
The MPV is a marker that is easily studied with complete blood count and which is an indicator of platelet function and activation. The platelet volume was found to be associated with platelet function and activation7–10. In general, platelet production increases as the platelet count decreases, and young platelets become larger and more reactive, and therefore, the MPV values are higher13–17. In recent years, in some studies in which MPV was tested as a simple inflammatory marker, MPV was reported to have been affected by inflammation, and that it increases significantly in myocardial infarction, sepsis, cerebrovascular diseases, respiratory distress syndrome and chronic pulmonary diseases7,9,18,19. Kisacik et al. found the platelet volume to be low in active ankylosing spondylitis and rheumatoid arthritis, and that MPV levels increased and normalized with treatment20,21.
In the literature, MPV has been reported to decrease in some inflammatory bowel diseases such as ulcerative colitis, especially in the active period, and that it could be used for determination of the disease activity10,21–24. Similarly, Makay et al. reported that while MPV was not different in FMF between attacks compared to the control group, it decreased significantly during the attacks, and that this decrease was related to morbidity25. This condition is thought to have been related to the release of bioactive molecules of pro-inflammatory active platelets in the presence of inflammation. Danese et al. speculated that the MPV decrease in inflammatory bowel diseases could be related to the consumption and sequestration of large active platelets in the vascular segments of the inflamed bowel26.
In the current study, the MPV values were found to be significantly lower in the AA group compared to the control group (p<0.001). This finding is similar to the result of the study carried out by Albayrak et al. in an adult AA group11. A comparison could not be made as no study in a children group is yet available in the literature. Although the pathogenesis of this decrease in MPV has not been fully explained, it seems reasonable to explain this with the consumption and sequestration of large active platelets in the vascular segments of the inflamed bowel as Danese et al. claimed26. Furthermore, the fact that Albayrak et al. found MPV values to be statistically significantly lower in the group who presented late while there was no difference in terms of WBC and neutrophil counts in patients who had presented within 24 hours and 24 hours after onset of symptoms, may support this pathogenesis11.
While the sensitivity of WBC elevation for the AA diagnosis was reported as 85.8%, 97.8%, 67%, and 76.5%, and the specifity as 31.9%, 55.6%, 80%, and 90.8% 2,11,27,28, the sensitivity of neutrophil count elevation was reported as 87.2%, 98.9% and 68.6%, and the specificity was reported as 33.1%, 38.9% and 86.4%2,11,27. In the current study, while the sensitivity was found as 84% and 77%, respectively, for WBC (>11×109/L) and neutrophil (>8×109/L) count elevations in the AA diagnosis, the specificity was found as 89% and 91%, respectively. These results were found to be consistent with that in the literature.
In the presented study, specificity was found as 54% and sensitivity was found as 87% for the MPV decrease (<7.4 fL). The sensitivity and specificity values for MPV were lower than those of WBC and neutrophil count elevations. Although we could not make a comparison due to absence of studies in the children group in the literature, it was lower in terms of sensitivity and similar in terms of specificity when compared to results of the study reporting MPV sensitivity as 65.5% and specificity as 87.9% for the AA diagnosis conducted in the adult age group by Albayrak et al11.
In the current study, the cut-off value that had the best sensitivity-specificity values (87%–60%) according to the ROC curve analysis for MPV, was determined as 7.55 fL. This result was similar to the 7.6fL value found by Albayrak et al11. However, our sensitivity value was higher and the specificity value was found to be lower compared to the sensitivity-specificity values reported for this value (73%–84%). Additionally, while the area under the curve (AUC) was found as 0.80 for MPV, the AUC was higher in the study of Albayrak et al. (0.86)11.
Conclusion
Detection of an elevation in WBC and neutrophil counts is significant in the AA diagnosis when there is suspicion of AA. Furthermore, paying attention to the decrease in MPV values that is routinely studied in complete blood counting and causing no extra cost and time loss seems to be a method that reinforces the AA diagnosis. The current study indicated that MPV significantly decreased in pediatric AA patients. Hence, we believe that taking the MPV decrease into consideration along with the WBC elevation would be beneficial in patients with suspicion of AA. However, the fact that these studies should be carried out with higher patient numbers and prospectively should not be ignored.
Acknowledgement
We would like to express our gratitude to Assoc. Prof. Siddik Keskin who performed the statistical analysis for the study.
References
- 1.Andersson RE, Hugander A, Thulin AJ. Diagnostic accuracy and perforation rate in appendicitis: association with age and sex of the patient and with appendicectomy rate. Eur J Surg. 1992;158:37–41. [PubMed] [Google Scholar]
- 2.Yang HR, Wang YC, Chung PK, Chen WK, Jeng LB, Chen RJ. Laboratory tests in patients with acute appendicitis. ANZ J Surg. 2006;76:71–74. doi: 10.1111/j.1445-2197.2006.03645.x. [DOI] [PubMed] [Google Scholar]
- 3.Sack U, Biereder B, Elouahidi T, Bauer K, Keller T, Tröbs RB. Diagnostic value of blood inflammatory markers for detection of acute appendicitis in children. BMC Surg. 2006;6:15. doi: 10.1186/1471-2482-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lessin MS, Chan M, Catallozzi M, et al. Selective use of ultrasonography for acute appendicitis in children. Am J Surg. 1999;177:193–196. doi: 10.1016/s0002-9610(99)00002-1. [DOI] [PubMed] [Google Scholar]
- 5.Andersson M, Andersson RE. The appendicitis inflammatory response score: a tool for the diagnosis of acute appendicitis that outperforms the Alvarado score. World J Surg. 2008;32:1843–1849. doi: 10.1007/s00268-008-9649-y. [DOI] [PubMed] [Google Scholar]
- 6.Martin JF, Trowbridge EA, Salmon G, Plumb J. The biological significance of platelet volume: its relationship to bleeding time, platelet thromboxane B2 production and megakaryocyte nuclear DNA concentration. Thromb Res. 1983;32:443–460. doi: 10.1016/0049-3848(83)90255-4. [DOI] [PubMed] [Google Scholar]
- 7.Bath PM, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis. 1996;7:157–161. [PubMed] [Google Scholar]
- 8.Bath P, Algert C, Chapman N, Neal B PROGRESS Collaborative Group, author. Association of mean platelet volume with risk of stroke among 3134 individuals with history of cerebrovascular disease. Stroke. 2004;35:622–626. doi: 10.1161/01.STR.0000116105.26237.EC. [DOI] [PubMed] [Google Scholar]
- 9.Endler G, Klimesch A, Sunder-Plassmann H, et al. Mean platelet volume is an independent risk factor for myocardial infarction but not for coronary artery disease. Br J Haematol. 2002;117:399–404. doi: 10.1046/j.1365-2141.2002.03441.x. [DOI] [PubMed] [Google Scholar]
- 10.Kapsoritakis AN, Koukourakis MI, Sfiridaki A, et al. Mean platelet volume: a useful marker of inflammatory bowel disease activity. Am J Gastroenterol. 2001;96:776–781. doi: 10.1111/j.1572-0241.2001.03621.x. [DOI] [PubMed] [Google Scholar]
- 11.Albayrak Y, Albayrak A, Albayrak F, et al. Mean Platelet Volume: A New Predictor in Confirming Acute Appendicitis Diagnosis. Clin Appl Thromb Hemost. doi: 10.1177/1076029610364520. [DOI] [PubMed] [Google Scholar]
- 2.Lanzkowsky P. Manual of Pediatric Hematology and Oncology. 4th ed. California, Elsevier: 2005. [Google Scholar]
- 13.Thompson CB, Eaton KA, Princiotta SM, Rushin CA, Valeri CR. Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br J Haematol. 1982;50:509–519. doi: 10.1111/j.1365-2141.1982.tb01947.x. [DOI] [PubMed] [Google Scholar]
- 14.Martin J. The relationship between megakaryocyte ploidy and platelet volume. Blood Cells. 1989;15:108–121. [PubMed] [Google Scholar]
- 15.Cole JL, Marzec UM, Gunthel CJ, et al. Ineffective platelet production in thrombocytopenic human immunodeficiency virus-infected patients. Blood. 1998;9:3239–3246. [PubMed] [Google Scholar]
- 16.Chatterji AK, Lynch EC, Garg SK, Amorosi EL, Karpatkin S. Circulating large platelets. N Engl J Med. 1971;284:1440–1441. doi: 10.1056/NEJM197106242842517. [DOI] [PubMed] [Google Scholar]
- 17.Van der Loo B, Martin JF. Megakaryocytes and platelets in vascular disease. Baillieres Clin Haematol. 1997;10:109–123. doi: 10.1016/s0950-3536(97)80053-4. [DOI] [PubMed] [Google Scholar]
- 18.Canpolat FE, Yurdakök M, Armangil D, Yiðit S. Mean platelet volume in neonatal respiratory distress syndrome. Pediatr Int. 2009;51:314–316. doi: 10.1111/j.1442-200X.2009.02820.x. [DOI] [PubMed] [Google Scholar]
- 19.Mercan R, Demir C, Dilek I, Asker M, Atmaca M. Mean platelet volume in acute coronary syndrome. Van Tip Dergisi. 2010;17(3):89–95. [Google Scholar]
- 20.Kisacik B, Tufan A, Kalyoncu U, et al. Mean platelet volume (MPV) as an inflammatory marker in ankylosing spondylitis and rheumatoid arthritis. Joint Bone Spine. 2008;75(3):291–294. doi: 10.1016/j.jbspin.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Yüksel O, Helvaci K, Ba°ar O, et al. An overlooked indicator of disease activity in ulcerative colitis: mean platelet volume. Platelets. 2009;20:277–281. doi: 10.1080/09537100902856781. [DOI] [PubMed] [Google Scholar]
- 22.Shah A, Morgan G, Rose JD, Fifield R, Rhodes J. Platelet number and size in relation to serum orosomucoid concentration in Crohn's disease. Med Lab Sci. 1989;46:79–80. [PubMed] [Google Scholar]
- 23.Järemo P, Sandberg-Gertzen H. Platelet density and size in inflammatory bowel disease. Thromb Haemost. 1996;75:560–561. [PubMed] [Google Scholar]
- 24.Kayahan H, Akarsu M, Ozcan MA, et al. Reticulated platelet levels in patients with ulcerative colitis. Int J Colorectal Dis. 2007;22:1429–1435. doi: 10.1007/s00384-007-0330-y. [DOI] [PubMed] [Google Scholar]
- 25.Makay B, Türkyilmaz Z, Unsal E. Mean platelet volume in children with familial Mediterranean fever. Clin Rheumatol. 2009;28:975–978. doi: 10.1007/s10067-009-1148-5. [DOI] [PubMed] [Google Scholar]
- 26.Danese S, Motte Cd Cde L, Fiocchi C. Platelets in inflammatory bowel disease: clinical, pathogenic, and therapeutic implications. Am J Gastroenterol. 2004;99:938–945. doi: 10.1111/j.1572-0241.2004.04129.x. [DOI] [PubMed] [Google Scholar]
- 27.Shafi SM, Afsheen M, Reshi FA. Total leucocyte count, C-reactive protein and neutrophil count: diagnostic aid in acute appendicitis. Saudi J Gastroenterol. 2009;15:117–120. doi: 10.4103/1319-3767.48969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang LT, Prentiss KA, Simon JZ, Doody DP, Ryan DP. The use of white blood cell count and left shift in the diagnosis of appendicitis in children. Pediatr Emerg Care. 2007;23:69–76. doi: 10.1097/PEC.0b013e31802d1716. [DOI] [PubMed] [Google Scholar]