Abstract
Background
Medicinal plants in Uganda and other developing countries have been scientifically demonstrated to have medicinal benefits but few or none have been translated to products for clinical use. Most herbal products developed by local herbalists and sold to the public are not standardized and lack efficacy and safety data to support use.
Objective
To formulate from two Ugandan medicinal plants a herbal product for wound management and test its preclinical safety and efficacy using rat models.
Methods
Thirty (30) Wistar albino rats were randomly divided into three groups and wounds were surgically created on the mid-dorsal region. The wounds were treated topically with distilled water (group I), Jena® (group II)and Neomycin sulfate cream (group III). The effects of the treatments on rate of wound closure, epithelialisation time and histological organization of tissue were assessed.
Results
The herbal formulation (Jena) had a significantly higher rate of wound closure than neomycin (p<0.05) which itself was better than distilled water. Epithelialisation time was also significantly shorter for the herbal product (p<0.01). Histological picture revealed more collagen fibers, less inflammation and better tissue remodeling for rats treated with herbal product.
Conclusion
The herbal formulation Jena® systematically designed and formulated based on two Ugandan medicinal plants is according to this study better than neomycin and probably other imported products for wound management in Uganda. We recommend its trial in a clinical setting as an alternative in wound management.
Keywords: Ugandan, Herbal formulation, wound, management
Introduction
A wound is defined as a break in the epithelial integrity of skin or tissue which may be caused by physical, chemical or microbial agents1. Wound healing is a complex but dynamic process of restoring damaged tissue that involves several interrelated events2. Faster healing of wounds is paramount because the skin is the organ through which the body interacts with the environment3. Many plants have been found to have wound healing activities owing to the vast array of medicinal compounds synthesized by these medicinal plants4,5,6. Some plant extracts have been formulated for clinical use in wound management and have proved safe and efficacious2,7. However, in Uganda and in other developing countries, little has been done to develop and prove efficacy of herbal products yet the demand and consumption of herbal products is on the rise worldwide. Indigenous people in Uganda prefer herbal formulations for treatment of various clinical ailments including wounds. This trend has particularly been fueled by the high cost of conventional treatments and the general belief that herbal products are free of side of effects. In this study two indigenous plants Zanthoxylum chalybeum family Rutacea and Warbugia ugandensis family Canellaceae that have previously been shown to have invitro antioxidants and antimicrobial activities respectively8,9 were used in the formulation of herbal product named Jena®. Antimicrobial property helps to keep the wound area sterile while antioxidants enhance tissue regeneration at the wound site5. Z. chalybeum and W. ugandensis are plants used widely in African traditional medicine for management of various illness. An ethnobotanical survey done among the Masai community it Kenya reports the use of Z.chalybeum and W.Ugandensis for management of various diseases including internal wounds and skin diseases10. Z.chalybeum extract systemic safety has also been previously demonstrated11 but there is no safety data following topical application. The herbal product in this study was experimentally developed at the Natural Chemotherapeutics Research Laboratory to contain 20% extract of W.ugandensis and 80% extract of Z.chalybeum. The study was conducted to evaluate the wound healing activity of the herbal formulation in comparison to neomycin sulfate cream an antibacterial formulation commonly prescribed in Uganda for management of wounds, cuts and burns.
Methods
Study materials
Neomycin sulfate cream was purchased from a registered pharmacy in Kampala, Uganda. Z.chalybeum roots and leaves were obtained from Eastern Uganda, identified by botanist and deposited at the Natural Chemotherapeutics Research Laboratory (NCRL) herbarium under specimen number NCJ 256 while W.ugandensis leaves were obtained from the NCRL medicinal garden. The dry plant materials were powdered and extracted using ethanol by macerating for 48 h. Ethanol was then removed using rotary evaporator at low temperature (55°C) and the semi solid concentrate used in the formulation of the test product.
Identification and finger printing of active ingredients
Qualitative test was done on the on the formulation to identify, fingerprint and assess stability of the major classes of active ingredients in it. A method described in Practical Manual for Analysis of Vegetable Drugs was used with little modification12. A portion of the formulation was used directly in the detection of polyuronides, reducing compounds, saponins, tannins, alkaloids and steroid glycosides as detailed below;
Polyuronides
To a test tube containing (10ml) was added drops of water leading to formation of a thick precipitate. The precipitate obtained was placed on the filter paper and on staining with hematoxylin formed a blue precipitate for presence of polyuronides.
Reducing compounds
1ml of formulation was diluted with water (2ml) in test tube. Fehling's solutions I (1ml) and Fehling's solution II (1ml) were added and heated in water bath at 90°C forming a brick-red precipitate.
Saponins
A diluted solution of the formulation (2 ml) was placed in a test tube and shaken for 15 minutes. A soapy like column of about 2cm formed above liquid level.
Tannins
To the formulation (1ml) was added water (2ml) and a 3 drops of ferric chloride. A blackish blue color formed.
Alkaloid salts
The formulation (15ml) was evaporated to dryness in an oven at 55°C and residue dissolved in 10% v/v Hydrochloric acid (10 mL). 10 % v/v ammonia solution (10ml) was added to precipitate the alkaloids and then extracted with ether (15ml). The ether portion was evaporated to dryness and hydrochloric acid (1.5ml) added. To 0.5ml of the acidic solution was added 2–3 drops of Mayer's reagents forming opalescence precipitate.
To detect Steroid glycosides, Anthracenosides , coumarins and flavonosides, 25ml of the formulation was mixed in 10% v/v hydrochloric acid (15ml), refluxed for 30minutes, cooled and extracted with diethyl ether (36ml) in portions of 12ml each.
Steroid glycosides
To a residue obtained by evaporating to dryness ether extract (10 ml) was added acetic anhydride (0.50 ml) and chloroform (0.50 ml) and transferred into a dry tube. Conc. Sulphuric acid (2 ml) was added by means of a pipette at the bottom of the tube forming reddish-brown ring at the contact zone of the two layers.
Anthracenosides
The ether extract (4 mL) was added to conc. Sulphuric acid (2 mL) and shaken with 25% v/v ammonia solution (2ml) forming cherished-red solution on the top layer.
Coumarin derivatives
To a residue obtained by evaporating ether extract (5 mL) was added hot water (2ml) to dissolve. 10% v/v ammonium solution (0.5 ml) was then added forming a blue fluorescence solution under UV.
Flavonosides
The residue obtained by evaporating ether extract (5ml) was heated in 50% methanol (2 mL). Metallic magnesium (0.5g) and conc. Hydrochloric acid (5 drops) was added forming a red solution.
For stability of the product, the presence of the active ingredients in the formulation stored in an opaque container at room temperatures (25°C to 30C°) was tested again after 12 months using the same procedures.
Experimental animals
Male Wistar Albino rats weighing180–250g were provided from the same colony by Faculty of Veterinary Medicine Makerere University Kampala and housed individually under standard laboratory conditions (temperature 25±1°C, 12 hour light and 12 hour dark cycle, fed on standard pellet diet and Water).
Experiment design
Thirty (30) rats were randomly selected and grouped into three treatment groups (I, II and III) of ten animals per group. Circular excision wounds were created humanely in the mid-dorsal region of rats under Ketamine anaesthesia (50mg/kg) using the methods adopted from similar studies13. Treatment of the wounds on animals was started twenty hours after excision and the study products were applied topically twice a day. Distilled water and neomycine were used as negative (natural healing) and positive (aided) controls respectively. The wound contraction was determined every third day of treatment by subtracting the areas determined at day 3, 6 and 9 from the area at baseline13. Reduction in wound area was taken as the measure for wound contraction with wound area on day 1 as reference point. Epithelialization time was monitored by recording the number of days taken for the scar to fall off. On day 7, three animals per treatment group were sacrificed humanely under diethyl ether anaesthesia, their wound tissue excised and fixed in formalin (10%) for histological examination. Sections were stained with Trichome stain and examined under power X40 and X100 for collagen formation and inflammation. Comparison of tissue appearance was made between controls and experimental product.
Statistical analysis
The data for wound contraction rate and epithelisation time were analysed by student t-tests using Stata Version 10 Computer software. The statistical significance was set for p <0.05.
Ethical consideration
This study was cleared by the Natural Chemotherapeutics Research Laboratory (NCRL) Pharmacology Department Ethics Committee under study number NCRL/09-2. All the study animals were handled humanely as per the NCRL guidelines for studies involving laboratory animals.
Results
Major phytochemicals in the formulation
Eleven (11) classes of phytochemicals were identified in the herbal formulation (see table 1). These phytochemicals were found to be stable over 12 months at room temperatures ( 25°C to 30C°) indicating shelf life of more than 12 months.
Table 1.
Phytochemicals identified in the herbal formulation
| Phytochemical group |
Result | Phytochemical group |
Result |
| Tannins | (++) | Anthocyanin pigments |
(++) |
| Saponins | (++) | Steroids glycosides | (++) |
| Reducing compounds |
(++) | Alkaloids | (++) |
| Anthracenosides | (++) | Glucides | (++) |
| Coumarin | (++) | Flavonosides | (++) |
derivatives
Polyuronides (+)
Present in small quantities (+)
present in abundance (++)
Wound healing effects of the formulation
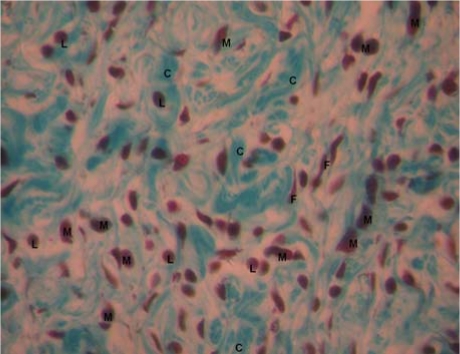
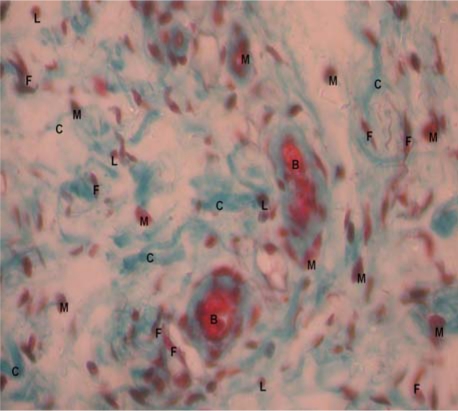
The wound contraction rates were in the order of Jena® herbal drops >>> neomycin > distilled water (See table 1 & 2). Jena® herbal drops had better wound contraction rate than neomycin by day 3 and 6, (49.7±4.7 vs 28.2±5.2), p=0.013 and (61.2±9.6 vs 44.4±11.4), p=0.021 respectively (see table 2). Epithelialisation time was significantly shorter for Jena herbal drops than for neomycin sulfate cream (6.5±0.8 vs9.5±1.1), p=0.0002. Histological examination of wound tissue of rats from the three treatment groups revealed that wounds treated with the herbal formulation had better remodeling, more collagen and less tissue infiltration with macrophages and lymphocytes (see figures 1,2, &3). No signs of any adverse drug reaction occurred in any experimental animal indicating safety of the products tested.
Table 2.
Wound contraction rates for Neomycin and distilled water
| Distilled Water | Neomycine | ||||
| Mean±SD | 95% CI | Mean±SD | 95% CI | P-value | |
| Day 3 | 17.8±8.1 | (10.3,25.3) | 28.2±5.2 | (15.5,40.9) | 0.10 |
| Day 6 | 40.9±5.7 | (35.6,46.2) | 44.4±11.4 | (33.9,54.9) | 0.24 |
| Day 9 | 63.1±5.6 | (57.3.1,69.0) | 68.7±15.4 | (59.7,77.7) | 0.08 |
Neomycin had better wound contraction rate than distilled but the difference was not statistically significant.
Figure 1.
Histological section of wounds treated with Jena herbal formulation at day 7 of treatment(X40)
M: macrophages; L: Lymphocytes: C: Collagen fibers: F: Fibroblasts.
Figure 2.
Histological section of wounds treated with Neomycin sulfate at day 7 (X40)
M: macrophages; L: Lymphocytes: C: Collagen fibers: F: Fibroblasts.
Figure 3.
Histological section of wounds treated with Distilled water at day 7 (X40)
M: macrophages; L: Lymphocytes: C: Collagen fibers: F: Fibroblasts; B: Blood vessel
Discussion
On the basis of the results obtained in this study, it is clear that some locally available medicinal plants can be formulated for clinical application and are potentially better than the conventional drugs in use. In this study Jena herbal formulation was superior to neomycin in both wound contraction rates and epithelialisation time. The observed faster wound healing activity of this herbal product may be attributed the phytomedicines previously reported in the medicinal plants used in its formulation. W. ugandensis water extract has been demonstrated to elicit powerful effects against both Escherischia coli and Staphylococcus aureus8, which are common bacterial infections in wounds that show resistance to most antibacterial agents in clinical use. Z. chalybuem another plant used in the formulation of the herbal product has also been demonstrated to have powerful antioxidant effects9, which have been shown to play a major role in wound healing14,15.
Although most medicinal plants have been shown to have antibacterial and antioxidants properties which are useful in promoting wound healing, few local plant extracts have been formulated into clinically useful products. Jena herbal formulation is therefore a clear demonstration that extracts of plants locally available have great potential and can be formulated for management of simple ailments such as wounds and cuts. The finding from this study are consistent with studies on polyherbal products reported in other countries in which polyherbal products have been shown to be of clinical benefit16. Considering that Uganda is very rich in biodiversity, herbal formulations such as Jena are not only of clinical benefit to Ugandans but also of potential economic benefit.
Conclusion
In this study, Jena herbal formulation significantly accelerated wound healing in normal Wistar albino rats. The herbal product indicated good safety profile in experimental animals and better efficacy than neomycin. We therefore recommend that the herbal formulation be tried in clinical setting.
Table 3.
Wound contraction rates for herbal formulation and neomycin
| Jena | Neomycin | ||||
| Mean±SD | 95% CI | Mean±SD | 95% CI | P -value | |
| Day 3 | 49.7±4.7 | (38.3,61.2) | 28.2±5.2 | (15.5,40.9) | 0.013 |
| Day 6 | 61.2±9.6 | (52.3,70.1) | 44.4±11.4 | (33.9,54.9) | 0.021 |
| Day 9 | 80.7±9.1 | (71.1,90.2) | 68.7±15.4 | (59.7,77.7) | 0.057 |
The herbal formulation had better wound contraction rates by day 3 and 6.
Table 4.
Epithelialisation times compared with distilled water
| EPT (Mean±SD) | 95%CI. | P-value | |
| Distilled Water (blank) | 11.7±2.3 | (9.3,14.0) | - |
| Neomycin (standard) | 9.5±1.1* | (8.4,10.6) | 0.017 |
| Jena herbal formulation (test) | 6.5±0.8** | (5.6,7.4) | 0.0006 |
p<0.0001,
p<0.01.
The herbal formulation reduced epithelisation time by 45% compared to control (distilled water) while neomycin caused 19% reduction.
Acknowledgement
Special thanks go to the Director and staff of Natural Chemotherapeutics Research Laboratory for the technical and material support provided that enabled conduction of this noble pioneering work of scientific herbal product development in Uganda. We also thank the Head and staff of the Department of Anatomy Faculty of Veterinary Medicine that helped in taking of microscopic photographs of the histology tissues.
References
- 1.Abdulla MA, Hassan D, Hapipa MA. Acceleration of wound healing potential of Lantana camara Leaf extract in experimental rats. Research J Med Sc. 2009;3(2):75–79. [Google Scholar]
- 2.Sachin J, Jain N, Tiwari A, Balekar N, Jain DK. Simple evaluation of wound healing activity of a Polyherbal formulation of roots of Ageratum conyzoides Linn. Asian J Research Chem. 2009;2(2):135–138. [Google Scholar]
- 3.Shikha S, Nidhi M. Evaluation of Polyherbal formulation for wound healing activity. Der Pharmacia Lettre. 2009;1(1):157–161. [Google Scholar]
- 4.Raina R, Prawez S, Verma PK, Pankaj NK. Medicinal Plants and their Role in Wound Healing. VetScan. 2008;3(1):1–7. [Google Scholar]
- 5.Shetty S, Udupa S, Udupa L. Evaluation of antioxidant and wound healing effects of alcoholic and aqueous extract of Ocimum sanctum linn. in rats. Evid Based Complement Altern Med. 2008;5:95–101. doi: 10.1093/ecam/nem004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suntar IP, Koca U, Esra KA, Yýlmazer D, Alper M. Assessment of Wound Healing Activity of the Aqueous Extracts of Colutea cilicica Boiss. & Bal. Fruits and Leaves. Evid Based Complement Alternat Med. 2009 doi: 10.1093/ecam/nep190. (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esimone CO, Ibezim EC, Chah KF. The wound healing effect of herbal ointments formulated with Napoleona imperialis. J pharm and allied sci. 2005;3(1):294–299. [Google Scholar]
- 8.Olila D, Olwa-Odyek, Opuda AJ. Antibacterial and antifungal activities of extracts of Zanthoxylum chalybeum and Warburgia ugandensis, Ugandan medicinal plants. Afr Health Sci. 2001;1(2):66–72. [PMC free article] [PubMed] [Google Scholar]
- 9.Torunn S, Wangensteen H, Katuura E, Lye KA, Berit SP. Antioxidant and anti-plasmodial activity of extracts from three Ugandan medicinal plants. J Med Plants Res. 2010;4(18):1916–1923. [Google Scholar]
- 10.Kiringe JW. A Survey of Ethnomedicinal Health Remedies used by the Maasai of Southern Kaijiado District, Kenya. Ethnobotany Res & Appl. 2006;4:061–073. [Google Scholar]
- 11.Ogwang PE, Tumusiime R, Agwaya M, Mugisha G, Nambatya GK, Galiwango B, Waako P. Repeat-dose effects of Zanthoxylum chalybeum root bark extract: A traditional medicinal plant used for various diseases in Uganda. African J Pharm and Pharmacol. 2008;2(6):101–105. [Google Scholar]
- 12.Culei I. Methodology for the analysis of vegetable drugs: Practical Manual on the industrial Utilisation of Medicinal and Aromatic Plants. 1st ed. Romania: Center Building; 1982. [Google Scholar]
- 13.Nayak SB, Sandiford S, Maxwell A. Evaluation of the wound-healing activity of ethanolic extract of Morinda citrifolia L. leaf. Evid Based Complement Alternat Med. 2007;7(4):1–6. doi: 10.1093/ecam/nem127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiemersma ET, Bronzwaer S, Outi L, Degener JE, Schrijnemakers P, Bruinsma N, et al. Methicillin-resistant Staphylococcus aureus in Europe,1999–2002. Emerging Infectious Diseases Report. 2004;10(9):1–4. doi: 10.3201/eid1009.040069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta A, Singh RL, Raghubir R. Antioxidant status during cutaneous wound healing in immunocompromised rats. Mol & Cellular Biochem. 2002;241(2):1–7. doi: 10.1023/a:1020804916733. [DOI] [PubMed] [Google Scholar]
- 16.Charde MS, Hemke AT, Fulzele SV, Satturwar PP, Kasture AV, Dorle AK. Investigation of the wound healing activity of Tilvadi ghrita: a herbal formulation. Indian J Trad Knowledge. 2002;3(3):247–252. [Google Scholar]