Abstract
Aim: TNF (tumor necrosis factor)-related apoptosis-inducing ligand (TRAIL), is a selective killer of tumor cells, although its potential is limited by the development of resistance. In this article, we investigated whether the polyherbal preparation Zyflamend® can sensitize tumor cells to TRAIL. Results: We found that Zyflamend potentiated TRAIL-induced apoptosis in human cancer cells. Zyflamend manifested its effects through several mechanisms. First, it down-regulated the expression of cell survival proteins known to be linked to resistance to TRAIL. Second, Zyflamend up-regulated the expression of pro-apoptotic protein, Bax. Third, Zyflamend up-regulated the expression of death receptors (DRs) for TRAIL. Up-regulation of DRs was critical as gene-silencing of these receptors significantly reduced the effect of Zyflamend on TRAIL-induced apoptosis. The up-regulation of DRs was dependent on CCAAT/enhancer-binding protein-homologous protein (CHOP), as Zyflamend induced CHOP, its gene-silencing abolished the induction of receptors, and mutation of the CHOP binding site on DR5 promoter abolished Zyflamend-mediated DR5 transactivation. Zyflamend mediated its effects through reactive oxygen species (ROS), as ROS quenching reduced its effect. Further, Zyflamend induced DR5 and CHOP and down-regulated the expression of cell survival proteins in nude mice bearing human pancreatic cancer cells. Innovation: Zyflamend can sensitize tumor cells to TRAIL through modulation of multiple cell signaling mechanisms that are linked to ROS. Conclusion: Zyflamend potentiates TRAIL-induced apoptosis through the ROS-CHOP-mediated up-regulation of DRs, increase in pro-apoptotic protein and down-regulation of cell survival proteins. Antioxid. Redox Signal. 16, 413–427.
Introduction
TNF (tumor necrosis factor)-related apoptosis-inducing ligand (TRAIL) was first identified in 1995 (47) and reported to selectively induce apoptosis in tumor cells—but not in most normal cells—through its action with two distinct death receptors (DRs), DR4 and DR5. Owing to this selectivity, this cytokine is a candidate for clinical investigation in cancer therapy. Although TRAIL signals via DR4 or DR5, most studies suggest that DR5 is the primary receptor leading to apoptosis (16). One difference between DR4 and DR5 is the capacity of p53 to induce DR5 transcription widely in response to deoxyribonucleic acid (DNA) damage in vitro and in vivo (48). In addition, wild-type TRAIL has a higher affinity for DR5 than for DR4 (45). Furthermore, a recent study has demonstrated that induction of DRs is cell type specific; so far, surface DR5 expression has been broadly found on TRAIL-sensitive tumor cell lines by flow cytometry and in primary tumors by immunohistochemistry. In contrast, DR4 has been found on the surface of selected tumor cell lines. For example, primary cells with chronic lymphocytic leukemia and mantle cell lymphoma almost exclusively express DR4 (28).
These receptors interact with the Fas-associated death domain (FADD), which leads to sequential activation of initiator caspase-8 and caspase-3. Although TRAIL can induce apoptosis in most cancer cells, the emergence of resistance to TRAIL is a major problem that limits its utility as a therapeutic agent. TRAIL resistance appears to be mediated through multiple mechanisms. One potential mechanism involves dysregulation of DR4 and DR5 (22). A second mechanism involves defects in effector caspases, such as caspase-3. A third mechanism of TRAIL resistance involves changes in proteins that affect caspase activation, including either inactivation of pro-apoptotic molecules (Bax, Bak, Bad, Bim, or Bid) or the overexpression of death inhibitors (cellular FADD-like interleukin-1β converting enzyme [FLICE] inhibitory protein [c-FLIP], Bcl-2, Bcl-xL, or inhibitor of apoptosis [IAP]). Whereas Bcl-2 and Bcl-xL bind to Bax and Bak and inhibit cytochrome c release by pore-forming proteins (Bid, Bik), IAPs directly bind and inhibit caspases-3, -7, and -9. Two different forms of the proteins c-FLIPL and c-FLIPS are known to prevent caspase-8 activation.
Innovation.
Although TNF (tumor necrosis factor)-related apoptosis-inducing ligand (TRAIL) is known to selectively kill cancer cells, the development of resistance to TRAIL is one of the major hurdles for its use as a cancer therapy. Thus agents that are safe, affordable, and easily available and can overcome the TRAIL resistance are urgently needed. In this study, for the first time, we demonstrate that Zyflamend®, a polyherbal preparation can sensitize tumor cells to TRAIL-induced apoptosis. Furthermore, the mechanism by which this polyherbal preparation sensitizes tumor cells to TRAIL was delineated. We found that Zyflamend mediated its effect through several mechanisms. First, it down-regulated the expression of cell survival proteins linked to resistance. Second, Zyflamend up-regulated the expression of pro-apoptotic protein. Third, Zyflamend induced the expression of death receptor (DR)-5. We found that up-regulation of the receptors was mediated through the expression of CCAAT/enhancer-binding protein-homologous protein (CHOP) and through generation of reactive oxygen species (ROS). The sensitization of cancer cells to TRAIL-induced apoptosis was abolished by ROS quenchers. Overall, our observations indicate that Zyflamend sensitizes cancer cells to TRAIL through the ROS-CHOP-DR5 mechanism. This study provides the evidence that this polyherbal preparation can be used for sensitization of cancer cells to TRAIL.
Natural products have been used as therapeutics for centuries and are thus envisioned as safe (31). As much as 70% of all drugs approved for cancer treatment between 1981 and 2002 were either natural products or based on natural products (31). Among agents with such potential, Zyflamend is a polyherbal formulation composed of 10 standardized herbal extracts (baikal skullcap, barberry, chinese goldthread, ginger, green tea, holy basil, hu zhang, oregano, rosemary, and turmeric) (38) (Table 1). Previously we reported that Zyflamend could inhibit TNF-induced nuclear factor-kappaB (NF-κB) activation in myelogenous leukemia cells (38). Because Zyflamend contains constituents that can suppress tumor cell proliferation, invasion, angiogenesis, and metastasis (3, 7, 10, 13, 19, 20, 29, 41, 44), we investigated whether Zyflamend could potentiate TRAIL-induced apoptosis in cancer cells. To test this possibility, we investigated the effect of Zyflamend on TRAIL-induced apoptosis and the mechanism by which Zyflamend mediates its effects. Our results demonstrate that Zyflamend potentiated the apoptosis induced by TRAIL by up-regulating the expression of DRs via reactive oxygen species (ROS)-dependent up-regulation of the cysteine-cysteine-alanine-alanine-threonine (CCAAT)/enhancer-binding protein-homologous protein (CHOP) pathway and by down-regulating anti-apoptotic proteins.
Table 1.
The Composition of Zyflamend
| Plant | Botanical name | Amt/serving* | Amount† | Active component |
|---|---|---|---|---|
| Baikal skullcap (root) | Scutellaria baicalensis | 10 mg | 1.19 μg | baicalein, baicalin, wogonin |
| Barberry (root) | Berberis vulgare | 20 mg | 2.38 μg | Berberine |
| Chinese goldthread (root) | Coptis Chinensis | 20 mg | 2.38 μg | Berberine |
| Ginger (rhizome) | Zingiber officinalis | 50 mg | 5.96 μg | gingerol, shogaol, paradol |
| Green tea (leaf ) | Camellia sinensis | 50 mg | 5.96 μg | epicatechin gallate, epigallocatechin,‡ epigallocatechin gallate‡ |
| Holy basil (leaf ) | Ocimum sanctum | 50 mg | 5.96 μg | eugenol, methylchavikol, rosmarinic acid, ursolic acid‡ |
| Hu zhang (root and rhizome) | Polygonum cuspidatum | 40 mg | 4.77 μg | resveratrol‡ |
| Oregano (leaf ) | Origanum vulgare | 20 mg | 2.38 μg | ursolic acid,‡ carvacrol, linalool, rosmarinic acid, thymol |
| Rosemary (leaf ) | Rosmarinus officinalis | 75 mg | 8.94 μg | borneol, camphor, cineol, rosmarinic acid |
| Turmeric (rhizome) | Curcuma longa | 55 mg | 6.56 μg | curcumin‡ |
One serving consists of 30 drops; †Amount present in 100 μg/mL Zyflamend; ‡Amount of active component out of total corresponding amount from column 4 (EGCG, 1,800 ng; catechin, 1,788–2,384 ng; ursolic acid, 2.682 ng; resveratrol, 28.62 ng; curcumin, 328–433 ng).
Results
The primary objective of this study was to determine whether Zyflamend, a polyherbal preparation that is consumed by thousands of people in the United States and considered safe (6) can potentiate TRAIL-induced apoptosis in cancer cells. If so, we sought to determine the mechanism by which Zyflamend modulates this effect. We used Zyflamend, as it contains multiple phytochemicals and nutraceuticals linked to cancer prevention and chemosensitization. The different components present in Zyflamend have been found safe (2, 4, 8, 14, 34). For most studies, we used human colorectal adenocarcinoma (HCT)-116 cells because the mechanism of TRAIL-induced apoptosis in this cell line has been extensively studied. We also used other cell lines to examine the specificity of Zyflamend effect.
Zyflamend potentiates TRAIL-induced apoptosis
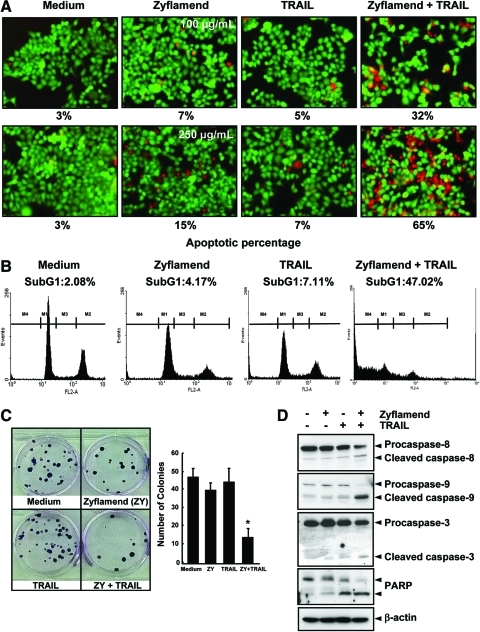
We used various assay systems to examine the effect of Zyflamend on apoptosis induced by TRAIL. First, we used the live/dead assay to determine whether Zyflamend can potentiate TRAIL-induced apoptosis in HCT-116 colon cancer cells. Results indicated that treatment with Zyflamend alone for up to 24 h did not induce any significant cell death. While Zyflamend and TRAIL alone induced cell death in the range of 7–15%, combined treatment of 2 increased cell death to 32% and 65% at 100 μg/mL and 250 μg/mL Zyflamend, respectively (Fig. 1A).
FIG. 1.
Zyflamend enhances TRAIL-induced HCT-116 cell death. (A) Cells were treated with 100 μg/mL (upper panel) or 250 μg/mL (lower panel) Zyflamend for 12 h, washed with PBS to remove Zyflamend and were then treated with 25 ng/mL TRAIL for 24 h. Cell death was determined by the live/dead assay. (B) Cells were treated with 100 μg/mL Zyflamend, 25 ng/mL TRAIL either alone or in combination for 24 h. Cells were then stained with propidium iodide, and the sub-G1 fraction was analyzed using FACS. (C) Cells were treated with 100 μg/mL Zyflamend for 12 h, washed with PBS to remove Zyflamend, and then treated with TRAIL (25 ng/mL) for 24 h. The cells were then reseeded in six-well plates and allowed to form colonies for 7 days, after which they were stained with crystal violet, as described in Materials and Methods. Each bar represents a mean number of colonies±SD of three experiments. (D) Cells were pre-treated with 100 μg/mL Zyflamend for 12 h and then washed with PBS to remove Zyflamend. Cells were then treated with TRAIL for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting using antibodies against caspase-3, caspase-8, caspase-9, and PARP. β-Actin was used as an internal control to verify equal protein loading. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Second, we measured apoptosis (at the sub-G1 stage) using the propidium iodide staining method by fluorescence-activated cell sorting (FACS). We found that Zyflamend alone and TRAIL alone induced 4.17% and 7.11% apoptosis, respectively, but the combination of the two enhanced the apoptosis to 47.02% (Fig. 1B).
Third, we determined whether Zyflamend could enhance the effect of TRAIL in a long-term colony-formation assay. We found that Zyflamend alone or TRAIL alone was minimally effective in inhibiting colony formation of HCT-116 cells, but the combination treatment significantly suppressed the colony-forming ability of these tumor cells (Fig. 1C).
Activation of caspases is another hallmark of apoptosis induced by most agents. Therefore, we investigated the effect of Zyflamend, TRAIL, and the combination on the activation of caspase-8, caspase-9, caspase-3, and poly (ADP-ribose) polymerase (PARP) cleavage (Fig. 1D). We found that although Zyflamend alone and TRAIL alone had little effect on caspase activation and PARP cleavage, the two together were highly effective.
Overall, these results indicate that Zyflamend can enhance TRAIL-induced apoptosis. Next, we investigated in detail how Zyflamend enhances TRAIL-induced apoptosis.
Zyflamend down-regulates the expression of cell survival proteins
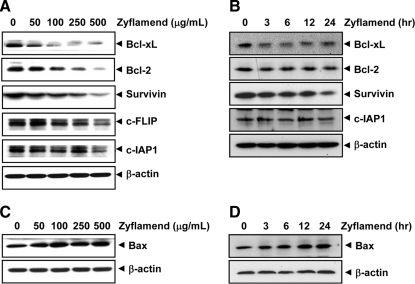
We examined whether Zyflamend had any effect on the expression of anti-apoptotic proteins. Zyflamend significantly down-regulated Bcl-xL, Bcl-2, and survivin and also slightly decreased c-FLIP and cellular inhibitor of apoptosis proteins (c-IAPs) in a concentration-dependent manner (Fig. 2A). Zyflamend also reduced the expression of these proteins in a time-dependent manner (Fig. 2B).
FIG. 2.
Zyflamend modulates anti-apoptotic and pro-apoptotic protein expression. HCT-116 cells were treated with the indicated concentrations of Zyflamend for 24 h (A, C) or with 250 μg/mL Zyflamend for the indicated times (B, D). Whole-cell extracts were prepared and analyzed by Western blotting using the relevant antibodies. β-Actin was used as an internal control to verify equal protein loading.
Zyflamend up-regulates the expression of pro-apoptotic protein
Whether Zyflamend could affect the expression of pro-apoptotic proteins was examined. Zyflamend enhanced the expression of pro-apoptotic Bax in a concentration- (Fig. 2C) and time-dependent manner (Fig. 2D).
Zyflamend up-regulates the death receptor
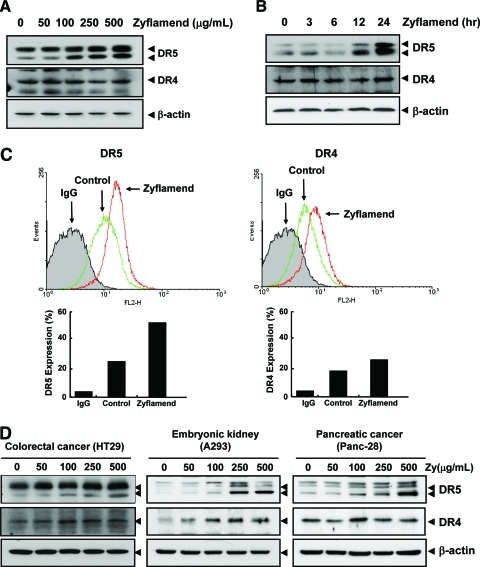
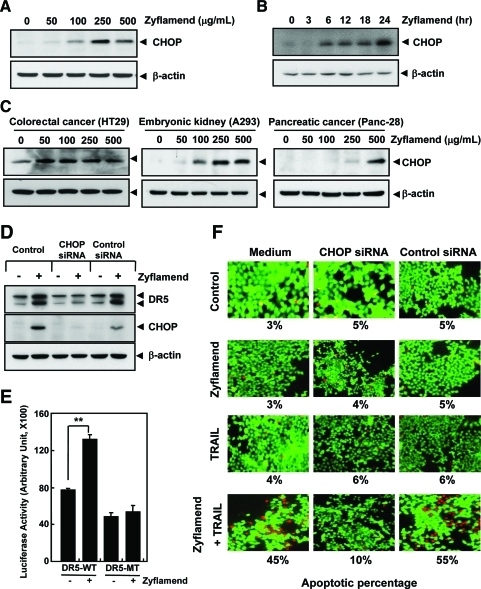
Another possible mechanism by which Zyflamend could affect TRAIL-induced apoptosis is through the modulation of DRs. To investigate this possibility, we examined the effect of Zyflamend on the expression of DR4 and DR5 in HCT-116 cells. HCT-116 cells were treated with various concentrations of Zyflamend for 24 h. Zyflamend induced DR5 in HCT-116 cells in a concentration- (Fig. 3A) and time-dependent manner (Fig. 3B). Zyflamend, however, failed to induce DR4 expression.
FIG. 3.
Zyflamend induces expression of death receptors. HCT-116 cells were treated with the indicated concentrations of Zyflamend for 24 h (A) or with 250 μg/mL Zyflamend for the indicated times (B). Whole-cell extracts were prepared and analyzed for DR5 and DR4 by Western blotting. β-Actin was used as an internal control to verify equal protein loading. (C) HCT-116 cells were treated with 100 μg/mL Zyflamend for 24 h and then harvested for analysis of cell surface expression of DR5 and DR4 by FACS. (D) Zyflamend up-regulated DR5 in various types of cancer cells. Cells were treated with the indicated concentrations of Zyflamend for 24 h, and whole-cell extracts were prepared and analyzed by Western blotting using antibodies against DR5 and DR4. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. (Zy, Zyflamend) (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
We also analyzed the cell surface expression of DRs in cells treated with Zyflamend. Results showed that Zyflamend significantly increased the cell surface expression of DR5 but failed to have much effect on DR4 (Fig. 3C).
We investigated whether induction of DRs by Zyflamend is cell type specific. Colon cancer cells (HT29), pancreatic cancer cells (Panc-28), and embryonic kidney carcinoma cells (A293) were exposed to Zyflamend and then examined for DR5 and DR4 protein expression. Zyflamend induced DR5 but was unable to induce DR4 (Fig. 3D) in all three cell lines, clearly demonstrating that induction of DRs by Zyflamend is not cell type specific.
Induction of death receptors by Zyflamend is needed for TRAIL-induced apoptosis
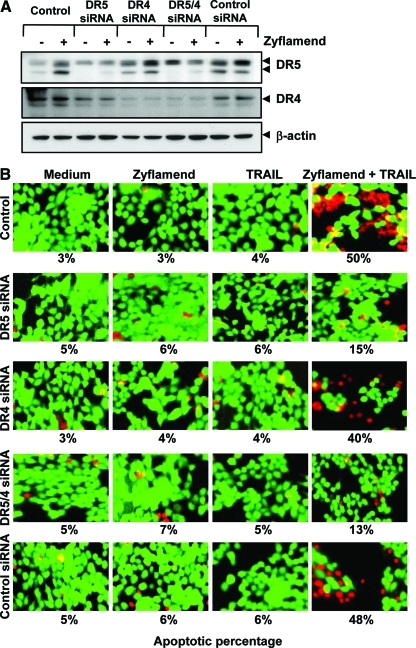
To determine the role of DR5 and DR4 in TRAIL-induced apoptosis, we used small interfering ribonucleic acids (siRNAs) specific to DR5 and DR4 to down-regulate the expression of these receptors. Transfection of cells with DR5 siRNA but not with the control siRNA reduced Zyflamend-induced DR5 expression (Fig. 4A).
FIG. 4.
Knockdown of death receptors abrogates Zyflamend's ability to enhance TRAIL-induced apoptosis. (A) HCT-116 cells were transfected with DR5 siRNA, DR4 siRNA, alone or in combination, and control siRNA. After 48 h, cells were treated with 250 μg/mL Zyflamend for 24 h, and whole-cell extracts were analyzed by Western blotting using DR5 and DR4 antibodies. (B) After 48 h of transfection with siRNA, cells were pre-treated with 100 μg/mL Zyflamend for 12 h and then incubated with 25 ng/mL TRAIL for 24 h. Cell death was determined by the live/dead assay. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
We next used the live/dead assay to examine whether the suppression of DR5 or DR4 by siRNA could abrogate the sensitizing effects of Zyflamend on TRAIL-induced apoptosis. We found that the effect of Zyflamend on TRAIL-induced apoptosis was remarkably abolished in cells transfected with DR5 siRNA but not in cells transfected with DR4 siRNA or control siRNA (Fig. 4B). These results suggest that DR5 plays a major role in TRAIL-induced apoptosis by Zyflamend.
Zyflamend-induced up-regulation of TRAIL receptors is mediated through activation of CCAAT/enhancer-binding protein-homologous protein
How Zyflamend induces DRs was investigated. It has been shown that the induction of DRs by certain agents is mediated through activation of CCAAT/enhancer-binding protein-homologous protein (CHOP) (40). We therefore investigated whether Zyflamend could induce the expression of CHOP. Cells were treated with the different concentrations of Zyflamend for 24 h and then examined for relative CHOP expression. We found that Zyflamend increased the expression of CHOP in a concentration- (Fig. 5A) and time-dependent (Fig. 5B) manner. Zyflamend also induced CHOP in pancreatic, kidney, and colorectal cancer cells (Fig. 5C).
FIG. 5.
Up-regulation of death receptors by Zyflamend is CHOP dependent. (A, B) HCT-116 cells were treated with the indicated concentrations of Zyflamend for 24 h (A) or with 250 μg/mL Zyflamend for the indicated times (B). Whole-cell extracts were prepared and analyzed by Western blotting using the relevant antibodies. β-Actin was used as an internal control to verify equal protein loading. (C) Zyflamend up-regulated CHOP in various types of cancer cells. Cells were treated with the indicated concentrations of Zyflamend for 24 h, and whole-cell extracts were prepared and analyzed by Western blotting using antibodies against CHOP. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. (D) Silencing of CHOP reversed the effect of Zyflamend on DR5 expression. Cells were transfected with either CHOP siRNA or control siRNA. After 48 h, cells were treated with 250 μg/mL Zyflamend for 24 h, and whole-cell extracts were subjected to Western blotting for CHOP and DR5. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. (E) Mutation of CHOP binding site on DR5 promoter abolishes Zyflamend-induced DR5 transactivation. A293 cells were transfected with DR5/-552 luciferase plasmid containing wild type or mutated CHOP binding site. After 24 h, cells were treated with Zyflamend for 24 h and luciferase activity was determined as described in materials and methods. Each bar represents means of luciferase activity±SD of three independent experiments (F) Gene silencing of CHOP abolishes the effect of Zyflamend on TRAIL-induced apoptosis in colon cancer cells. HCT-116 cells were transfected with CHOP siRNA for 48 h. Cells were then treated with 100 μg/mL Zyflamend for 12 h, washed with PBS, and then incubated with 25 ng/mL TRAIL for 24 h. Cell death was determined by the live/dead assay. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Mutation of CHOP binding site on DR5 promoter abolishes Zyflamend-induced DR5 transactivation
We examined whether the CHOP expression is essential for DR5 induction by Zyflamend. We transfected HCT-116 cells with CHOP siRNA and then treated the cells with Zyflamend. Results indicated that transfection of HCT-116 cells with CHOP siRNA completely suppressed Zyflamend-induced DR5 expression (Fig. 5D).
To confirm that the CHOP is required for DR5 induction by Zyflamend, we performed a mutational experiment using DR5 promoter plasmids that contained a wild-type or mutated CHOP binding site. A293 cells were transfected with wild-type or mutant plasmids before treatment with Zyflamend. Zyflamend was found to induce luciferase activity in cells transfected with wild-type plasmids. However, luciferase activity was significantly regressed in the cells transfected with mutant plasmids (Fig. 5E).
Induction of CHOP is required for potentiation of TRAIL-induced apoptosis by Zyflamend
We next examined whether expression of CHOP is needed for potentiation of TRAIL-induced apoptosis by Zyflamend. Results indicated that CHOP siRNA markedly reduced the effect of Zyflamend on TRAIL-induced apoptosis (from 45% to 10%) in HCT-116 cells, whereas treatment with control siRNA had no effect (Fig. 5F). These results suggest that potentiation of TRAIL-induced apoptosis by Zyflamend requires the expression of CHOP.
Induction of ROS generation by Zyflamend
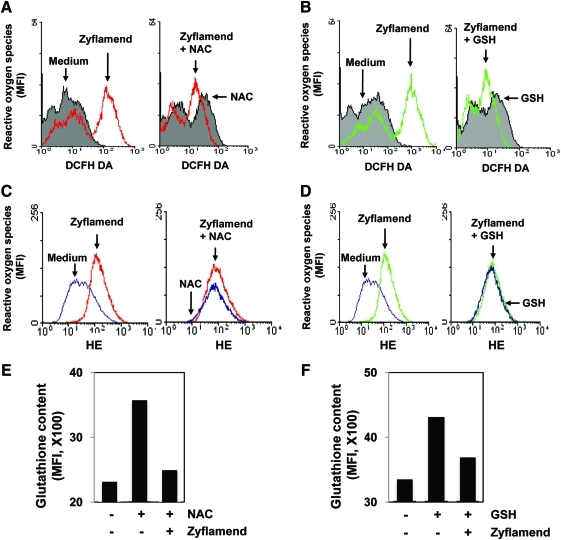
The nutraceuticals present in Zyflamend such as baicalein (46), baicalin (27), berberine (26), curcumin (15), epigallocatechin gallate (EGCG) (25), gingerol (32), resveratrol (29), rosmarinic acid (30), and ursolic acid (35), have been shown to possess pro-oxidant property. Because DR5 induction by certain agents requires ROS (15, 19), we sought to determine whether Zyflamend-induced DR5 induction occurs through a similar mechanism. We treated HCT-116 cells with Zyflamend and then measured changes in ROS levels inside the cells using dichlorodihydrofluorescein diacetate (DCF-DA) as a probe. Results indicated that Zyflamend significantly induced ROS generation in HCT-116 cells while pre-treatment of cells with N-acetyl-L-cysteine (NAC) (Fig. 6A) or glutathione (GSH) (Fig. 6B) significantly reduced ROS generation.
FIG. 6.
Zyflamend induces ROS generation. HCT-116 cells were pre-exposed to NAC (A and C) or GSH (B and D) for 1 h, washed off and then exposed to 250 μg/mL Zyflamend for 1 h. Cells were labeled with DCF-DA (A-B) or HE (C-D) and examined for ROS generation by FACS. (E-F) HCT-116 cells were pre-exposed to NAC (E) or GSH (F) for 1 h, washed off and then exposed to Zyflamend for 1 h, stained with monobromobimane and then examined for intracellular glutathione content by flow cytometer. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
To further confirm the ROS generating potential of Zyflamend, we used hydroethidine (HE) that basically measures superoxide radicals (9). Results indicated that Zyflamend significantly induced ROS generation in HCT-116 cells while pre-treatment of cells with NAC or GSH significantly reduced ROS generation (Figs. 6C, 6D).
Whether extracellular administration of NAC and GSH increases intracellular glutathione content was determined using monobromobimane (mBBr) by flow cytometry (9). Results indicated a significant increase in intracellular glutathione content by GSH and NAC treatment that was abrogated by Zyflamend treatment (Figs. 6E, 6F).
Induction of DR5 by Zyflamend is through generation of ROS
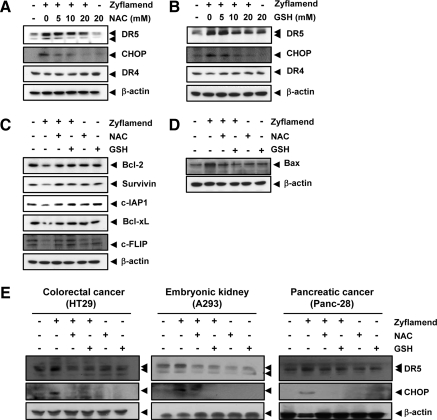
We next sought to determine whether the induction of DR5 by Zyflamend is regulated by ROS. We found that pre-treatment of HCT-116 cells with NAC (Fig. 7A) or GSH (Fig. 7B) reduced the Zyflamend-induced up-regulation of DR5 expression in a concentration-dependent manner. The expression level of DR did not change when cells were treated with the antioxidant NAC or GSH alone. These results suggest that ROS play an important role in the induction of DRs by Zyflamend. Moreover, the expression of CHOP was also abolished when cells were treated with the ROS scavengers. These results suggest that induction of CHOP by Zyflamend also requires ROS production.
FIG. 7.
Zyflamend induced up-regulation of DR5 and CHOP is mediated through generation of ROS. (A-B) HCT-116 cells were treated with indicated concentrations of NAC (A) or GSH (B) for 1 h, and then treated with 250 μg/mL Zyflamend for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting using indicated antibodies. β-Actin was used as a loading control. (C-D) Zyflamend-modulated anti-apoptotic and pro-apoptotic proteins expression is dependent on ROS. HCT-116 cells were pre-treated with NAC or GSH for 1 h and then treated with Zyflamend (250 μg/mL) for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting using the relevant antibodies. β-Actin was used as an internal control to verify equal protein loading. (E) Zyflamend-induced DR5 and CHOP expression in pancreatic cancer cells and human embryonic kidney cells is mediated through ROS. Cells were first treated with NAC or GSH for 1 h and then with 250 μg/mL Zyflamend for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting using indicated antibodies. β-Actin was used as a loading control.
Because ROS have been shown to control the expression of Bcl-2 family proteins (5, 24), we sought to determine whether ROS play a role in the modulation of Bcl-2 family proteins by Zyflamend. Results indicated that the down-regulation in the expression of Bcl-2, survivin, c-IAP-1, Bcl-xL and c-FLIP by Zyflamend was abolished when cells were pre-treated with NAC or GSH (Fig. 7C). We also found that Zyflamend failed to induce Bax expression when cells were pre-treated with ROS scavengers (Fig. 7D).
Whether Zyflamend-induced DR5 and CHOP in pancreatic cancer cells and embryonic kidney cells are mediated through ROS was investigated. A moderate increase in DR5 and CHOP expression was observed by Zyflamend that was reduced by NAC and GSH. NAC and GSH, alone were unable to induce DR5 and CHOP under similar conditions (Fig. 7E). These results suggest that Zyflamend might induce DR5 and CHOP in pancreatic cancer cells and embryonic kidney cells through ROS generation.
Potentiation of TRAIL-induced apoptosis by Zyflamend is ROS dependent
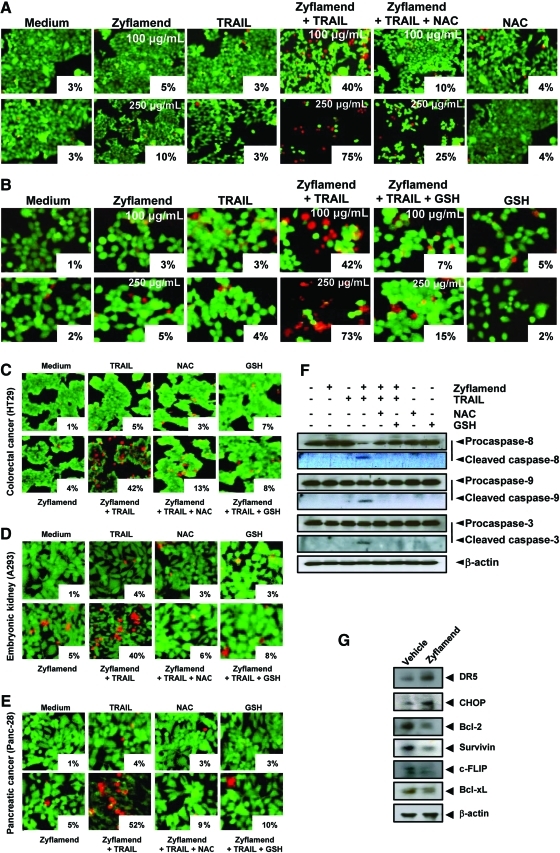
Finally, we sought to determine whether potentiation of TRAIL-induced apoptosis by Zyflamend is mediated through ROS. Results indicated that pre-treatment of HCT-116 cells with NAC or GSH significantly reduced the effect of Zyflamend on TRAIL-induced apoptosis (Fig. 8A-B). Similar effects were observed in pancreatic cancer cells and embryonic kidney cells (Fig. 8C-E). Concomitant with these observations, we found that pre-treatment with ROS scavengers significantly reduced caspase activation induced by Zyflamend and TRAIL in HCT-116 cells (Fig. 8F). Taken together, these results indicate that potentiation in TRAIL-induced apoptosis by Zyflamend requires ROS and is not cell type specific.
FIG. 8.
Potentiation of TRAIL-induced apoptosis by Zyflamend is ROS dependent. (A-E) NAC and GSH reversed cell death induced by the combination of Zyflamend and TRAIL in cancer cells. Cells were pre-treated with NAC or GSH for 1 h before treatment with Zyflamend. After 12 h, cells were washed with PBS and then treated with TRAIL for 24 h. Cell death was determined by the live/dead assay. (F) Whole-cell extracts prepared from treated cells were analyzed by Western blotting using antibodies against caspase-8, caspase-9, and caspase-3. β-Actin was used as an internal control to verify equal protein loading. (G) Zyflamend induces expression of DR5, CHOP and down-regulates anti-apoptotic proteins in vivo. The protein extract obtained from the tumor tissues of vehicle treated and Zyflamend treated nude mice were analyzed by Western blot analysis using indicated antibodies. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Zyflamend up-regulates DR5 and suppresses anti-apoptotic proteins expression in pancreatic tumors in vivo
Whether Zyflamend modulates DR5 and CHOP in vivo was investigated. The Western blot analysis showed that tissues from vehicle-treated animals expressed little DR5 and CHOP, whereas those from Zyflamend-treated animals exhibited a significant increase in the protein expression (Fig. 8G). We further investigated whether Zyflamend has the potential to modulate cell survival proteins in vivo. We found that cell survival protein, such as Bcl-2, survivin, c-FLIP, and Bcl-xL, were constitutively expressed in tumor tissues, whereas Zyflamend treatment significantly reduced the expression of cell survival proteins (Fig. 8G).
Discussion
In the current study, we found that the polyherbal formulation Zyflamend potentiated TRAIL-induced apoptosis, as indicated by intracellular esterase activity, DNA strand breaks, and activation of caspases-3, -8, and -9. It is well documented that the DR-mediated apoptotic signaling pathway requires recruitment of FADD and caspase-8, which results in caspase-8 activation and subsequent activation of its downstream caspase cascades and apoptosis (17). Our results showed that neither TRAIL alone nor Zyflamend alone induced apoptosis in HCT-116 cells. However, the combination of Zyflamend with TRAIL induced activation and cleavage of caspase-8, caspase-9 and caspase-3, which are critical for DR-mediated apoptosis. Activation of caspase cascade and cleavage of PARP is the hallmark of apoptosis, which in turn causes DNA fragmentation and cell death. The cell-intrinsic pathway involves mitochondrial disruption by pro-apoptotic Bcl-2 family members and consequent release of factors such as cytochrome c that promote activation of caspase-9 (18). Our study showed that Zyflamend induces up-regulation of Bax and down-regulation of survivin, Bcl-2, and Bcl-xL, and activation of caspase-9. Therefore, Zyflamend activated both the major extrinsic and intrinsic apoptosis signaling pathways—leading to enhancement of TRAIL-induced apoptosis.
We investigated in detail how Zyflamend could enhance the sensitivity of colon cancer cells to TRAIL-induced apoptosis. We found that Zyflamend significantly up-regulated the expression of the TRAIL receptor DR5 but did not have much effect on DR4. TRAIL-induced apoptosis appears to require expression of one or both of its death domain-containing receptors, DR4 or DR5 (12) and is mediated via a FADD-dependent pathway (39).
We found that silencing of DR4 had only a minimal effect on TRAIL-induced apoptosis. This result indicates that enhancement of apoptosis by Zyflamend is mediated through the induction of DR5 over that of DR4. Furthermore, because wild-type TRAIL has a higher affinity for DR5 than for DR4 (45), it seems logical to observe a major role of DR5 in the enhancement of TRAIL-induced apoptosis by Zyflamend. CHOP, known as growth arrest- and DNA damage-inducible gene 153, is a transcription factor that is up-regulated following multiple stimuli. It has been shown, for example, that the induction of DRs by certain agents (i.e., tunicamycin, silibinin) is mediated through the activation of CHOP (40, 43). Of note, our results showed that Zyflamend induced the expression of CHOP. The role of CHOP in inducing DR5 by Zyflamend was confirmed by gene silencing and DR5 promoter analyses. The suppression of CHOP by siRNA abrogated the effects of Zyflamend on TRAIL-induced apoptosis. These results indicate that CHOP-dependent DR5 up-regulation contributes to the sensitizing effect of Zyflamend on TRAIL-induced apoptosis. It has not been reported whether CHOP induces DR5 transcription widely in response to damage, but it has been reported that one difference between DR4 and DR5 is the capacity of p53 to induce DR5 transcription widely in response to DNA damage in vitro and in vivo (48). The effect of CHOP in preferring DR5 to DR4 in HCT-116 cells remains to be elucidated.
We found that perhaps the most important upstream signal linked to the modulation of TRAIL signaling is ROS. Zyflamend was found to induce ROS, and scavenging of ROS abrogated the effect of Zyflamend on the induction of DRs and CHOP and also abolished the potentiation of TRAIL-induced apoptosis by Zyflamend. These results are similar to that reported with curcumin for induction of DR5 (15). Our findings also corroborate with previous findings on quercetin (19) and zerumbone (50). The major components of Zyflamend that have the potential to act as pro-oxidants are baicalein (46), baicalin (27), berberine (26), curcumin (15), epigallocatechin gallate (EGCG) (25), gingerol (32), resveratrol (29), rosmarinic acid (30), and ursolic acid (35). Although difficult to establish accurately, it is likely that the pro-oxidant property of Zyflamend is due to all these components.
Besides induction of DRs, our results also established that Zyflamend induces the up-regulation of Bax and down-regulation of survivin, Bcl-2, and Bcl-xL protein, which might have led to the promotion of TRAIL-induced apoptosis. Down-regulation of c-FLIP by Zyflamend may also lead to the enhancement of TRAIL-induced apoptosis. Recently it has been shown that withaferin A also enhances TRAIL-induced apoptosis through down-regulation of c-FLIP (23). How Zyflamend mediates the down-regulation of anti-apoptotic proteins and up-regulation of pro-apoptotic protein is not clear. However, previous reports have suggested that the expression of Bcl-2 (24), Bcl-xL (24), and Bax (5) are controlled by ROS. Concurring with these observations, we also found that down-regulation in the expression of Bcl-2, Bcl-xL, and up-regulation in Bax is controlled by ROS. In addition, results also indicated that the modulation of survivin and c-FLIP expression by Zyflamend is controlled by ROS. The anti-apoptotic proteins (Bcl-2, Bcl-xL, and survivin) are known to be regulated by pro-inflammatory transcription factor NF-κB. Because Zyflamend has been shown to suppress NF-κB activation (38), it is likely that this polyherbal preparation is inhibiting anti-apoptotic proteins though suppression of NF-κB activation.
Zyflamend is a polyherbal preparation that is being consumed by thousands of people in America as an effective anti-inflammatory agent. There are 10 different plant extracts present in Zyflamend (Table 1), the main plants being turmeric, rosemary, hu zhang, and green tea. The main nutraceuticals present in Zyflamend are baicalein, berberine, curcumin, EGCG, eugenol, gingerol, resveratrol, ursolic acid, and wogonin (Table 1). Most of these agents have been reported to possess anti-inflammatory and anti-cancer properties (3, 7, 10, 13, 20, 29, 41, 44, ). For instance, 100 μg/mL of Zyflamend (the concentration employed in the present study) contains 6.56 μg of turmeric, which has 5–6.6 % of curcumin (328–433 ng/ml) (36). In a previous study, 30 μM (11.16 μg/ml) of curcumin was found to enhance TRAIL-induced apoptosis (15). Thus in our studies curcumin at 25x–34x lower concentration in Zyflamend was effective in potentiating the effect of TRAIL. Similarly, we found that EGCG (42), ursolic acid (1, 35) and resveratrol (11, 37) present in Zyflamend were at concentrations 10 to 4,000 times lower than their reported effective concentrations. These observations suggest that most of these nutraceuticals mediate their effects in a synergistic manner.
There are a limited number of studies about the safety of Zyflamend in animals and humans. Zyflamend components, such as turmeric (14), rosemary (2), green tea (8), ginger (4), and baikal skullcap-wogonin (34), have been shown to be safe in animals and humans. None of the reports indicated any adverse effects of the other five components. One study examined the effect of Zyflamend in men with prostatic intraepithelial neoplasia (PIN) (6). Zyflamend, when administered orally for 18 months to men with high-grade PIN, had no serious adverse effects. Based on these published reports, we speculate that Zyflamend can be used safely in cancer patients who develop resistance to TRAIL.
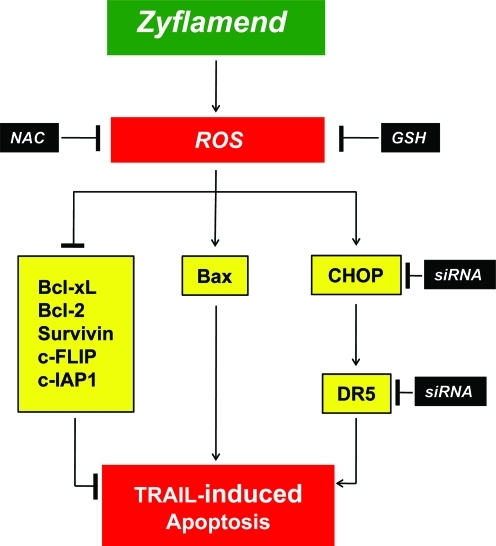
Overall, our results indicate that Zyflamend can enhance TRAIL-induced apoptosis in human cancer cells through (i) the up-regulation of DR5 in an ROS-CHOP-dependent manner, (ii) down-regulation of anti-apoptotic proteins and up-regulation of pro-apoptotic protein in an ROS-dependent manner (Fig. 9). Our results also indicate the efficacy of Zyflamend in nude mice implanted with pancreatic cancer cells. However, further in vivo studies using combinations of Zyflamend and TRAIL preceding human clinical trials are required to fully realize the potential of this polyherbal preparation.
FIG. 9.
A schematic diagram showing the mechanisms by which Zyflamend sensitizes cancer cells to TRAIL-induced apoptosis. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Materials and Methods
Materials
The Zyflamend® used in our studies was a standardized preparation supplied by NewChapter Inc. Zyflamend was dissolved in dimethyl sulfoxide (DMSO) as a 100 mg/ml stock solution, stored at −20°C, and then diluted further in cell culture medium as needed. Soluble recombinant human TRAIL was purchased from PeproTech. Penicillin, streptomycin, RPMI 1640, Dulbecco's modified Eagle medium (DMEM), and fetal bovine serum (FBS) were purchased from Invitrogen. Tris, glycine, NaCl, sodium dodecyl sulfate (SDS), bovine serum albumin (BSA), N-acetyl-L-cysteine (NAC), glutathione (GSH), and β-actin antibody were obtained from Sigma-Aldrich. Antibodies against DR4, poly (ADP-ribose) polymerase (PARP), Bcl-2, Bcl-xL, Bax, caspase-3, caspase-8, cIAP-1, c-FLIP, CHOP, and caspase-9 were obtained from Santa Cruz Biotechnology. Anti-DR5 was purchased from ProSci, Inc. Antibodies against survivin and phycoerythrin (PE)-conjugated DR5 and DR4 were obtained from R & D Systems. Anti-c-FLIP was purchased from Imgenex. Dichlorodihydrofluorescein diacetate (DCF-DA), hydroethidine (HE), and monobromobimane (mBBr) were purchased from Molecular Probes.
Cell lines
HCT-116, HT29 (human colon adenocarcinoma), A293 (human embryonic kidney carcinoma), and Panc-28 (pancreatic cancer) cell lines were obtained from American Type Culture Collection. HT29 cells were cultured in RPMI 1640 medium, HCT-116, Panc-28, and A293 cells were cultured in DMEM. All media were supplemented with 10% FBS and penicillin/streptomycin.
Live/dead assay
To measure apoptosis, we used the Live/Dead® assay kit (Invitrogen), which determines intracellular esterase activity and plasma membrane integrity. In brief, treated or untreated cells were stained with the live/dead reagent (5 μM ethidium homodimer and 5 μM calcein-AM) and incubated at 37°C for 30 min. Cells were then analyzed under a fluorescence microscope (Labophot-2; Nikon).
Clonogenic assay
HCT-116 cells were treated with Zyflamend (100 μg/mL) for 12 h. The medium was changed, and cells were treated with TRAIL for 24 h and allowed to form colonies. After 7 days, colonies were stained with 0.3% crystal violet, and the number of colonies was counted.
Western blot analysis
Proteins were resolved by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to nitrocellulose membranes, blotted with the relevant antibody, and detected by enhanced chemiluminescence reagent (GE Healthcare) (50).
Analysis of cell surface expression of DR4 and DR5
Cells were treated with Zyflamend for 24 h and washed with phosphate buffered saline (PBS) containing 0.5% BSA. Cells were then stained with phycoerythrin (PE)-conjugated mouse monoclonal anti-human DR5 or DR4 for 45 min at 4°C according to the manufacturer's instructions. Cells were then washed and resuspended in 0.5% BSA-supplemented PBS for analysis by FACS (FACSCalibur, BD Biosciences) using an excitation wavelength of 488 nm (50).
Transfection with siRNA
HCT-116 cells were plated in each well of six-well plates for Western blot or in a four-well chamber slide for the live/dead assay and allowed to adhere for 24 h. On the day of transfection, 12 μL of Hiperfect transfection reagent (Qiagen) was added to 50 nM siRNA or scRNA in a final volume of 100 μL of culture medium. After 48 h of transfection, cells were treated with Zyflamend for 12 h, washed, and then exposed to TRAIL for 24 h.
Assay for luciferase activity
The DR5 promoter DNA constructs used in this study have been described before (33, 49). Cell transfection with the pGL3 basic luciferase reporter plasmids was conducted using calcium phosphate transfection kit (Invitrogen) essentially following the manufacturer's instructions. In brief, A293 cells were seeded and co-transfected with 0.05 μg/μL of the β-galactosidase as a normalization control and 3 μg of firefly luciferase constructs containing the wild-type or mutant DR5 promoter region. After 12 h, media was changed and cells were left for another 12 h. Cells were then treated with 250 μg/mL Zyflamend for 24 h. To measure luciferase activity, 50 μg of whole cell protein was mixed with 200 μl of substrate (1 mM luciferin salt, 3 mM adenosine triphosphate (ATP) and 15 mM MgSO4 in 30 mM HEPES buffer, pH 7.8) in each well of 96 well plates. We used Victor 3 microplate reader (Perkin Elmer Life and Analytical Sciences) to measure luciferase activity.
Propidium iodide staining for DNA fragmentation
Cells were pre-treated with Zyflamend for 12 h and then exposed to TRAIL for 24 h. Propidium iodide staining was then performed to analyze DNA content (50).
Measurement of reactive oxygen species
Cells were pre-incubated with 20 μM dichlorodihydrofluorescein diacetate (DCF-DA) for 30 min at 37°C before they were pre-treated with NAC or GSH for 1 h, washed off, and then were treated with Zyflamend. After 1 h, the increase in fluorescence resulting from oxidation of DCF-DA to DCF was measured by FACS with an excitation wavelength of 488 nm, emission was collected with a 530/30 nm bandpass filter. The mean fluorescence intensity (MFI) of DCF among different samples was compared. To measure the superoxide production, cells were pre-incubated with NAC or GSH for 1 h, washed off, and treated with Zyflamend for 1 h. Cells were washed and loaded with 1 μM HE for 45 min at 37°C and the increase in fluorescence resulting from oxidation of HE to ethidium bromide was measured as MFI by FACS (FACSCalibur, BD Biosciences) at 575/26 nm.
Intracellular glutathione measurement
To measure the effect of NAC and GSH on intracellular glutathione content, HCT-116 cells were treated with NAC or GSH for 1 h before Zyflamend treatment. Cells were then washed and loaded with monobromobimane (9). Fluorescence of mBB-sulfhydryl adducts was measured by flow cytometry with an excitation wavelength of 405 nm, and emission was collected with a 440/40 nm bandpass filter. Data were analyzed from 10,000 cells at a flow rate of 250–300 cells/s.
Animal study
The pancreatic MIA PaCa-2 cells, stably transfected with luciferase were orthotopically implanted in athymic nu/nu mice as described before (21). One week after tumor implantation, mice were randomized into the following treatment groups (n=6/group) based on the bioluminescence measured with an in vivo imaging system (IVIS 200, Xenogen Corp.): (a) untreated control (olive oil, 100 μL by gavage, daily); (b) Zyflamend (1g/kg once daily orally). Therapy was continued for 4 weeks, and the animals were euthanized 1 week later. Primary tumors in the pancreas were excised, snap-frozen in liquid nitrogen, and stored at −80°C. The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee at MD Anderson Cancer Center.
Statistical analysis
Experiments were repeated a minimum of 3 times with consistent results. Data presented are the mean±standard deviation (SD). Statistical analysis was carried out using a two-tailed unpaired Student's t-test. Values of p<0.05 and p<0.01 was considered statistically significant.
Abbreviations Used
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- BSA
bovine serum albumin
- c-FLIP
cellular FLICE inhibitory protein
- CHOP
CCAAT/enhancer-binding protein-homologous protein
- c-IAPs
cellular inhibitor of apoptosis proteins
- CCAAT
cysteine-cysteine-alanine-alanine-threonine
- DCF-DA
dichlorodihydrofluorescein diacetate
- DMEM
Dulbecco's minimum essential medium
- DMSO
dimethyl sulfoxide
- DNA
deoxyribonucleic acid
- DRs
death receptors
- EGCG
epigallocatechin gallate
- FACS
fluorescence-activated cell sorting
- FADD
Fas-associated death domain
- FBS
fetal bovine serum
- FLICE
FADD-like interleukin-1β converting enzyme
- GSH
glutathione
- HCT
human colorectal adenocarcinoma
- HE
hydroethidine
- IAP
inhibitor of apoptosis
- mBB
monobromobimane
- MFI
mean fluorescence intensity
- NAC
N-acetyl-L-cysteine
- NF-κB
nuclear factor-kappaB
- PAGE
polyacrylamide gel electrophoresis
- PARP
poly (ADP-ribose) polymerase
- PBS
phosphate buffered saline
- PE
phycoerythrin
- PIN
prostatic intraepithelial neoplasia
- ROS
reactive oxygen species
- SD
standard deviation
- SDS
sodium dodecylsulfate
- siRNA
small interfering ribonucleic acid
- TNF
tumor necrosis factor
- TRAIL
TNF-related apoptosis-inducing ligand
Acknowledgments
Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported in part by NewChapter Incorporated, a core grant from the National Institutes of Health (CA-16 672), a program project grant from National Institutes of Health (NIH CA-124787-01A2), a grant from the Center for Targeted Therapy at the M.D. Anderson Cancer Center. The authors thank Michael Worley and the Department of Scientific Publications for carefully editing the manuscript. They thank Drs. H.G. Wang at the Penn State College of Medicine Cancer Institute and S.Y. Sun at the Emory University School of Medicine for providing DR5 constructs.
Author Disclosure Statement
No competing financial interests exist among the authors.
References
- 1.Abe F. Yamauchi T. Nagao T. Kinjo J. Okabe H. Higo H. Akahane H. Ursolic acid as a trypanocidal constituent in rosemary. Biol Pharm Bull. 2002;11:1485–1487. doi: 10.1248/bpb.25.1485. [DOI] [PubMed] [Google Scholar]
- 2.Anadon A. Martinez-Larranaga MR. Martinez MA. Ares I. Garcia-Risco MR. Senorans FJ. Reglero G. Acute oral safety study of rosemary extracts in rats. J Food Prot. 2008;4:790–795. doi: 10.4315/0362-028x-71.4.790. [DOI] [PubMed] [Google Scholar]
- 3.Atsumi T. Murakami Y. Shibuya K. Tonosaki K. Fujisawa S. Induction of cytotoxicity and apoptosis and inhibition of cyclooxygenase-2 gene expression, by curcumin and its analog, alpha-diisoeugenol. Anticancer Res. 2005;6B:4029–4036. [PubMed] [Google Scholar]
- 4.Borrelli F. Capasso R. Aviello G. Pittler MH. Izzo AA. Effectiveness and safety of ginger in the treatment of pregnancy-induced nausea and vomiting. Obstet Gynecol. 2005;4:849–856. doi: 10.1097/01.AOG.0000154890.47642.23. [DOI] [PubMed] [Google Scholar]
- 5.Byun JY. Kim MJ. Eum DY. Yoon CH. Seo WD. Park KH. Hyun JW. Lee YS. Lee JS. Yoon MY, et al. Reactive oxygen species-dependent activation of Bax and poly(ADP-ribose) polymerase-1 is required for mitochondrial cell death induced by triterpenoid pristimerin in human cervical cancer cells. Mol Pharmacol. 2009;4:734–744. doi: 10.1124/mol.109.056259. [DOI] [PubMed] [Google Scholar]
- 6.Capodice JL. Gorroochurn P. Cammack AS. Eric G. McKiernan JM. Benson MC. Stone BA. Katz AE. Zyflamend in men with high-grade prostatic intraepithelial neoplasia: results of a phase I clinical trial. J Soc Integr Oncol. 2009;2:43–51. [PubMed] [Google Scholar]
- 7.Choi YH. Baek JH. Yoo MA. Chung HY. Kim ND. Kim KW. Induction of apoptosis by ursolic acid through activation of caspases and down-regulation of c-IAPs in human prostate epithelial cells. Int J Oncol. 2000;3:565–571. [PubMed] [Google Scholar]
- 8.Chow HH. Hakim IA. Pharmacokinetic and chemoprevention studies on tea in humans. Pharmacol Res. 2011;2:105–112. doi: 10.1016/j.phrs.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cossarizza A. Ferraresi R. Troiano L. Roat E. Gibellini L. Bertoncelli L. Nasi M. Pinti M. Simultaneous analysis of reactive oxygen species and reduced glutathione content in living cells by polychromatic flow cytometry. Nat Protoc. 2009;12:1790–1797. doi: 10.1038/nprot.2009.189. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda K. Hibiya Y. Mutoh M. Koshiji M. Akao S. Fujiwara H. Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J Ethnopharmacol. 1999;2:227–233. doi: 10.1016/s0378-8741(98)00162-7. [DOI] [PubMed] [Google Scholar]
- 11.Fulda S. Debatin KM. Resveratrol-mediated sensitisation to TRAIL-induced apoptosis depends on death receptor and mitochondrial signalling. Eur J Cancer. 2005;5:786–798. doi: 10.1016/j.ejca.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Griffith TS. Lynch DH. TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol. 1998;5:559–563. doi: 10.1016/s0952-7915(98)80224-0. [DOI] [PubMed] [Google Scholar]
- 13.Huang SS. Zheng RL. Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett. 2006;2:271–280. doi: 10.1016/j.canlet.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Joshi J. Ghaisas S. Vaidya A. Vaidya R. Kamat DV. Bhagwat AN. Bhide S. Early human safety study of turmeric oil (Curcuma longa oil) administered orally in healthy volunteers. J Assoc Physicians India. 2003;51:1055–1060. [PubMed] [Google Scholar]
- 15.Jung EM. Lim JH. Lee TJ. Park JW. Choi KS. Kwon TK. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5) Carcinogenesis. 2005;11:1905–1913. doi: 10.1093/carcin/bgi167. [DOI] [PubMed] [Google Scholar]
- 16.Kelley RF. Totpal K. Lindstrom SH. Mathieu M. Billeci K. Deforge L. Pai R. Hymowitz SG. Ashkenazi A. Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J Biol Chem. 2005;3:2205–2212. doi: 10.1074/jbc.M410660200. [DOI] [PubMed] [Google Scholar]
- 17.Kelley SK. Ashkenazi A. Targeting death receptors in cancer with Apo2L/TRAIL. Curr Opin Pharmacol. 2004;4:333–339. doi: 10.1016/j.coph.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Kerr JF. Wyllie AH. Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;4:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JY. Kim EH. Park SS. Lim JH. Kwon TK. Choi KS. Quercetin sensitizes human hepatoma cells to TRAIL-induced apoptosis via Sp1-mediated DR5 up-regulation and proteasome-mediated c-FLIPS down-regulation. J Cell Biochem. 2008;6:1386–1398. doi: 10.1002/jcb.21958. [DOI] [PubMed] [Google Scholar]
- 20.Kim SO. Kundu JK. Shin YK. Park JH. Cho MH. Kim TY. Surh YJ. [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-kappaB in phorbol ester-stimulated mouse skin. Oncogene. 2005;15:2558–2567. doi: 10.1038/sj.onc.1208446. [DOI] [PubMed] [Google Scholar]
- 21.Kunnumakkara AB. Sung B. Ravindran J. Diagaradjane P. Deorukhkar A. Dey S. Koca C. Tong Z. Gelovani JG. Guha S. Krishnan S. Aggarwal BB. Zyflamend suppresses growth and sensitizes human pancreatic tumors to gemcitabine in an orthotopic mouse model through modulation of multiple targets. Int J Cancer. 2011 doi: 10.1002/ijc.26442. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SH. Shin MS. Kim HS. Lee HK. Park WS. Kim SY. Lee JH. Han SY. Park JY. Oh RR, et al. Somatic mutations of TRAIL-receptor 1 and TRAIL-receptor 2 genes in non-Hodgkin's lymphoma. Oncogene. 2001;3:399–403. doi: 10.1038/sj.onc.1204103. [DOI] [PubMed] [Google Scholar]
- 23.Lee TJ. Um HJ. Min do S. Park JW. Choi KS. Kwon TK. Withaferin A sensitizes TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of death receptor 5 and down-regulation of c-FLIP. Free Radic Biol Med. 2009;12:1639–1649. doi: 10.1016/j.freeradbiomed.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Li D. Ueta E. Kimura T. Yamamoto T. Osaki T. Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. 2004;8:644–650. doi: 10.1111/j.1349-7006.2004.tb03323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li GX. Chen YK. Hou Z. Xiao H. Jin H. Lu G. Lee MJ. Liu B. Guan F. Yang Z, et al. Pro-oxidative activities and dose-response relationship of (-)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: a comparative study in vivo and in vitro. Carcinogenesis. 2010;5:902–910. doi: 10.1093/carcin/bgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CC. Yang JS. Chen JT. Fan S. Yu FS. Yang JL. Lu CC. Kao MC. Huang AC. Lu HF, et al. Berberine induces apoptosis in human HSC-3 oral cancer cells via simultaneous activation of the death receptor-mediated and mitochondrial pathway. Anticancer Res. 2007;5A:3371–3378. [PubMed] [Google Scholar]
- 27.Lu HF. Hsueh SC. Ho YT. Kao MC. Yang JS. Chiu TH. Huamg SY. Lin CC. Chung JG. ROS mediates baicalin-induced apoptosis in human promyelocytic leukemia HL-60 cells through the expression of the Gadd153 and mitochondrial-dependent pathway. Anticancer Res. 2007;1A:117–125. [PubMed] [Google Scholar]
- 28.MacFarlane M. Kohlhaas SL. Sutcliffe MJ. Dyer MJ. Cohen GM. TRAIL receptor-selective mutants signal to apoptosis via TRAIL-R1 in primary lymphoid malignancies. Cancer Res. 2005;24:11265–11270. doi: 10.1158/0008-5472.CAN-05-2801. [DOI] [PubMed] [Google Scholar]
- 29.Manna SK. Mukhopadhyay A. Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;12:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 30.Murakami K. Haneda M. Qiao S. Naruse M. Yoshino M. Prooxidant action of rosmarinic acid: transition metal-dependent generation of reactive oxygen species. Toxicol In Vitro. 2007;4:613–617. doi: 10.1016/j.tiv.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Newman DJ. Cragg GM. Snader KM. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003;7:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 32.Nigam N. Bhui K. Prasad S. George J. Shukla Y. [6]-Gingerol induces reactive oxygen species regulated mitochondrial cell death pathway in human epidermoid carcinoma A431 cells. Chem Biol Interact. 2009;1:77–84. doi: 10.1016/j.cbi.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Oh YT. Liu X. Yue P. Kang S. Chen J. Taunton J. Khuri FR. Sun SY. ERK/ribosomal S6 kinase (RSK) signaling positively regulates death receptor 5 expression through co-activation of CHOP and Elk1. J Biol Chem. 2010;53:41310–41319. doi: 10.1074/jbc.M110.153775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng J. Qi Q. You Q. Hu R. Liu W. Feng F. Wang G. Guo Q. Subchronic toxicity and plasma pharmacokinetic studies on wogonin, a natural flavonoid, in Beagle dogs. J Ethnopharmacol. 2009;2:257–262. doi: 10.1016/j.jep.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 35.Prasad S. Yadav VR. Kannappan R. Aggarwal BB. Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent up-regulation of death receptors: evidence for the role of reactive oxygen species and JNK. J Biol Chem. 2011;7:5546–5557. doi: 10.1074/jbc.M110.183699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Prasad S. Aggarwal BB. Turmeric, the golden spice: from traditional medicine to modern medicine. In: Benzie IFF, editor; Wachtel-Galor S., editor. Herbal Medicine: Biomolecular and Clinical Aspects. Boca Raton, FL: CRC Press; 2011. pp. 259–284. [PubMed] [Google Scholar]
- 37.Qian G. Leung SY. Lu G. Leung KS. Optimization and validation of a chromatographic method for the simultaneous quantification of six bioactive compounds in Rhizoma et Radix Polygoni Cuspidati. J Pharm Pharmacol. 2008;1:107–113. doi: 10.1211/jpp.60.1.0014. [DOI] [PubMed] [Google Scholar]
- 38.Sandur SK. Ahn KS. Ichikawa H. Sethi G. Shishodia S. Newman RA. Aggarwal BB. Zyflamend, a polyherbal preparation, inhibits invasion, suppresses osteoclastogenesis, and potentiates apoptosis through down-regulation of NF-kappa B activation and NF-kappa B-regulated gene products. Nutr Cancer. 2007;1:78–87. doi: 10.1080/01635580701268295. [DOI] [PubMed] [Google Scholar]
- 39.Schneider P. Thome M. Burns K. Bodmer JL. Hofmann K. Kataoka T. Holler N. Tschopp J. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity. 1997;6:831–836. doi: 10.1016/s1074-7613(00)80401-x. [DOI] [PubMed] [Google Scholar]
- 40.Shiraishi T. Yoshida T. Nakata S. Horinaka M. Wakada M. Mizutani Y. Miki T. Sakai T. Tunicamycin enhances tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human prostate cancer cells. Cancer Res. 2005;14:6364–6370. doi: 10.1158/0008-5472.CAN-05-0312. [DOI] [PubMed] [Google Scholar]
- 41.Shishodia S. Majumdar S. Banerjee S. Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003;15:4375–4383. [PubMed] [Google Scholar]
- 42.Siddiqui IA. Malik A. Adhami VM. Asim M. Hafeez BB. Sarfaraz S. Mukhtar H. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene. 2008;14:2055–2063. doi: 10.1038/sj.onc.1210840. [DOI] [PubMed] [Google Scholar]
- 43.Son YG. Kim EH. Kim JY. Kim SU. Kwon TK. Yoon AR. Yun CO. Choi KS. Silibinin sensitizes human glioma cells to TRAIL-mediated apoptosis via DR5 up-regulation and down-regulation of c-FLIP and survivin. Cancer Res. 2007;17:8274–8284. doi: 10.1158/0008-5472.CAN-07-0407. [DOI] [PubMed] [Google Scholar]
- 44.Tjendraputra E. Tran VH. Liu-Brennan D. Roufogalis BD. Duke CC. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg Chem. 2001;3:156–163. doi: 10.1006/bioo.2001.1208. [DOI] [PubMed] [Google Scholar]
- 45.Truneh A. Sharma S. Silverman C. Khandekar S. Reddy MP. Deen KC. McLaughlin MM. Srinivasula SM. Livi GP. Marshall LA, et al. Temperature-sensitive differential affinity of TRAIL for its receptors. DR5 is the highest affinity receptor. J Biol Chem. 2000;30:23319–23325. doi: 10.1074/jbc.M910438199. [DOI] [PubMed] [Google Scholar]
- 46.Wang J. Yu Y. Hashimoto F. Sakata Y. Fujii M. Hou DX. Baicalein induces apoptosis through ROS-mediated mitochondrial dysfunction pathway in HL-60 cells. Int J Mol Med. 2004;4:627–632. [PubMed] [Google Scholar]
- 47.Wiley SR. Schooley K. Smolak PJ. Din WS. Huang CP. Nicholl JK. Sutherland GR. Smith TD. Rauch C. Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;6:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 48.Wu GS. Burns TF. McDonald ER., III Jiang W. Meng R. Krantz ID. Kao G. Gan DD. Zhou JY. Muschel R, et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet. 1997;2:141–143. doi: 10.1038/ng1097-141. [DOI] [PubMed] [Google Scholar]
- 49.Yamaguchi H. Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;44:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 50.Yodkeeree S. Sung B. Limtrakul P. Aggarwal BB. Zerumbone enhances TRAIL-induced apoptosis through the induction of death receptors in human colon cancer cells: Evidence for an essential role of reactive oxygen species. Cancer Res. 2009;16:6581–6589. doi: 10.1158/0008-5472.CAN-09-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]