Using the adenosine A2A receptor (A2AR) KO model, the authors demonstrated that genetic inactivation of A2AR attenuates the development of oxygen-induced retinopathy. The finding also suggests the possibility that A2AR inactivation may be a therapeutic strategy for ROP by selectively targeting pathologic angiogenesis without affecting normal vasculogenesis in the retina.
Abstract
Purpose.
The adenosine A2A receptor (A2AR) modulates normal vascularization and pathologic angiogenesis in many tissues and may contribute to the pathogenesis of retinopathy of prematurity (ROP) characterized by abnormal retinal vascularization in surviving premature infants. Here, the authors studied the effects of the genetic inactivation of A2AR on normal retinal vascularization and the development of pathologic angiogenesis in oxygen-induced retinopathy (OIR), an animal model of ROP.
Methods.
After exposure to 75% oxygen for 5 days (postnatal day [P] 7–P12) and subsequently to room air for the next 9 days (P13–P21), we evaluated retinal vascular morphology by ADPase staining in retinal whole mounts, retinal neovascularization response by histochemistry in serial retinal sections, and retinal VEGF gene expression by real-time PCR analysis in A2AR knockout (KO) mice and their wild-type (WT) littermates.
Results.
At P17, A2AR KO mice displayed attenuated OIR compared with WT littermates, as evidenced by reduced vaso-obliteration and areas of nonperfusion in the center of the retina, reduced pathologic angiogenesis as evident by decreased non-ganglion cells and neovascular nuclei, and inhibited hypoxia-induced retinal VEGF gene expression. Notably, the attenuation of pathologic angiogenesis by A2AR inactivation was selective for OIR because it did not affect normal retinal vascularization during postnatal development.
Conclusions.
These findings provide the first evidence that A2AR is critical for the development of OIR and suggest a novel therapeutic approach of A2AR inactivation for ROP by selectively targeting pathologic but not developmental angiogenesis in the retina.
Retinopathy of prematurity (ROP) is a disease of surviving premature infants that disrupts normal processes of retinal vascularization.1 With advances in neonatology and increased survival of extremely premature infants, ROP has become a major cause of blindness in children of many parts of the world.1,2 ROP is caused at least in part by oxygen-induced damage to the developing retinal vasculature, resulting in characteristic pathologic changes including vaso-obliteration and proliferation of abnormal fibrovascular tissue at the border of the vascularized and nonvascularized retina.1 Current ROP therapies are limited to laser surgery and cryosurgery, which ablate the avascular retina anterior to the fibrovascular ridge to prevent the blindness caused by ROP.3 However, the largest multicenter clinical trial, the CRYO-ROP Study, involving 9751 infants has shown that even with cryoablation, almost half the treated eyes had a visual acuity of 6/60 or worse at the 10-year follow-up.3 Thus, there is a critical need to develop more effective and preferably noninvasive prophylactic and therapeutic strategies for ROP.
Normal retinal vascular development starts with the de novo formation of blood vessels from endothelial precursor cells (vasculogenesis). This is followed by the development of new blood vessels by budding from existing blood vessels (angiogenesis). A key event in the pathogenesis of ROP is oxygen damage to the developing retinal vasculature. ROP occurs in two distinct phases. First, the developing retina is exposed to a relatively hyperoxic oxygen environment that reduces hypoxia-driven stimuli to retinal blood vessel development (such as vascular endothelium growth factor [VEGF]).4,5 Consequently, the development of retinal vascularization is delayed, resulting in vaso-obliteration. Second, as avascular retina becomes critically hypoxic, increased VEGF production leads to pathologic angiogenesis1,6 and ultimately to traction retinal detachment and blindness. Oxygen-induced retinopathy (OIR) is an animal model of ROP that captures some characteristic pathophysiological features of ROP, including vaso-obliteration, abnormal vasoproliferation, and pathologic angiogenesis.1,4,5
Extracellular adenosine acts through multiple G-protein–coupled receptors (A1, A2A, A2B, and A3)7 to exert important control on blood vessel growth in various tissues, including retina, under normal and pathologic conditions.8,9 In the developing retina, immunoreactivity for adenosine and the A2A receptor (A2AR) are detected on endothelial cell precursors, angioblasts, and endothelial cells in formed blood vessels in the retina of neonatal animals.6 In a neonatal canine model of OIR, the extracellular adenosine level is markedly increased in hypoxic retinal tissues supporting the possible involvement of A2AR in retinal vasoproliferation in OIR.6,10 Based on these findings, it has been proposed that A2AR activity in the retina contributes to the modulation of normal retinal vascularization and to pathologic angiogenesis.6 This angiogenic role of A2AR is also consistent with increasing numbers of studies demonstrating that A2AR activation increases angiogenesis in various cell types and tissues, including liver,11 kidney,12 skin,13 and retina.14 This angiogenic effect of A2AR activation is likely mediated by increased expression of VEGF, a key regulator of tissue angiogenesis.10,15
However, the direct evidence for A2AR in the pathogenesis of OIR is lacking, and the effects of A2AR inactivation on normal and pathologic retinal vascular development are not clear. In fact, pharmacologic studies have suggested that the adenosine-mediated effect on retinal angiogenesis may be mediated through the A2B receptor (A2BR) rather than A2AR. In cultured human retinal endothelial cells, pharmacologic profiles are consistent with A2BR-mediated (but not with A1R- or A2AR-mediated) effects on growth factor expression and cell proliferation.15,16 In the mouse model of OIR, Mino et al.17 demonstrated that the A2BR antagonists inhibited OIR-associated neovascularization in vivo, whereas neither the A2AR antagonist ZM241385 nor the A1R antagonist CPDPX had any effect on neovascularization. These results obtained with the A2AR and A2BR agonists and antagonists are, however, intrinsically limited in their specificity. The exact role of the adenosine receptor subtypes, especially A2AR, in normal retinal vascular development and the pathogenesis of OIR remain to be determined.
The development of A2AR knockout (KO) mice in our laboratory, particularly A2AR KO mice in the congenic C57BL/6 background to avoid potential confounding effect of the genetic background,18–20 provides a unique opportunity to investigate the role of A2AR in normal retinal vascular development and in the pathogenesis of OIR. In this study, we examined the effect of genetic inactivation of A2AR on retinal vascular development and on pathologic angiogenesis in OIR using A2AR KO mice. By comparing retinal vascular development and pathologic angiogenesis in homozygous A2AR KO mice with their wild-type (WT) littermates, we provide the first direct evidence that the genetic inactivation of A2AR selectively attenuates OIR-associated pathologic angiogenesis and proliferative retinopathy without affecting normal vascularization in the retina.
Methods
Generation and Genotyping of A2AR KO Mice
A2AR KO mice were developed in our laboratory as described previously.18–20 Congenic A2AR KO mice in a C57BL/6 background were generated by backcrossing A2AR KO in mixed 129-Steel × C57BL/6 background to C57BL/6 mice for more than 10 generations. A2AR KO mice were bred at the Laboratory Animal Center at Wenzhou Medical College and used for this study. Heterozygous crossbreeding was used to generate homozygous A2AR KO (A2AR−/−), heterozygote A2AR KO (A2AR±), and wild-type (A2AR+/+) mice, all from the same breeding pairs. The A2AR−/−, A2AR±, and A2AR+/+ mice with matched sexes at age 12 days were used for this study. The genotypes of the mice were determined by PCR analysis of genomic DNA isolated from mouse tails, using the three primer sets targeted to the Neo-cassettes and the adjacent A2AR genes as described previously.18–20
Mouse Model of Oxygen-Induced Retinopathy
All experimental procedures were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Ethics Committee for Animal Use in Research and Education at Wenzhou Medical College. The mouse model of OIR was established according to the method described previously with the modification that the oxygen concentration was 75% rather than 95%.21 Briefly, WT and A2AR KO pups (OIR-WT and OIR-A2AR KO) at postnatal day (P) 7 were raised in a 75% ± 2% oxygen (hyperoxia) chamber together with their mothers for 5 days (P7–P12). Thereafter, the pups were returned to room air (normoxia) for 2, 5, or 9 days (P12–P14, P12–P17, or P12–P21, respectively). The animals were killed and the eye tissue samples were obtained at P14, P17, or P21, respectively. Meanwhile, separate sets of WT and A2AR KO mouse pups were housed in room air from P7 to P21 without exposure to hyperoxia and served as the control groups (room air, WT; room air, A2AR KO). All animals were killed by lethal injection of ketamine and xylazine. Both eyes of each were enucleated: the right eye was processed for retinal mounts with ADPase staining, and the left eye was processed for immunohistochemistry in retinal paraffin sections.
Retinal Vascular Morphology Using ADPase Staining
The right eyes were placed in the 10% neutral buffered formalin for 60 minutes. The cornea, lens, and vitreous were surgically removed from each eye, and each retina was dissected. Retinal tissues were then placed in the neutral buffered formalin overnight and processed for magnesium-activated ADPase staining as described by Lutty and McLeod.22 ADPase-stained retinas were serial flat-mounted on microscope slides in phosphate-buffered saline with a coverslip and photographed.
Assessment of Retinal Neovascularization Response
Quantification of neovascularization was performed according to the procedure described previously by Smith et al.21 The extent of neovascularization was evaluated by counting the number of neovascular nuclei, vessel lumens, and non-ganglion cells, respectively, and expressed as mean ± SD for six retinal cross-sections. Neovascular nuclei were defined as the nuclei of cells that extended beyond the inner limiting membrane of the retina into the vitreous. Vessel lumens were located in the inner plexiform layer, ganglion cell layer, nerve fiber layer, and vitreous. Non-ganglion cells were characterized by non-round nuclei, such as spindle and rhombus nuclei, between the internal plexiform layer and the inner limiting membrane. In this study, eyes of 12 mice from each group were examined and analyzed. For each eye, 20 retinal sections (excluding the optic nerve) were evaluated. All non-ganglion cells and neovascular nuclei were counted under 400× magnification with hematoxylin and eosin–stained retinal sections by an observer who was masked to the specific group assignment.
Immunohistochemistry in Serial Retinal Sections
To determine whether non-ganglion cells and neovascular cells were proliferative, we determined immunoreactivity for proliferating cell nuclear antigen (PCNA) in the retinal sections. Furthermore, to identify the nature of proliferating (PCNA+) cells, we made serial sections at 5-μm intervals. For two adjacent sections, the second section was flipped over before mounting, such that the second section was the mirror image of the first section. Then paired sections were respectively immunolabeled for PCNA and CD31, an endothelial marker, or PCNA and glial fibrillary acidic protein (GFAP), an astrocyte marker.
For immunohistochemistry, retinal paraffin sections were deparaffinized with xylene, followed by rehydration in a series of washes with reducing ethanol concentrations, and finally with dH2O. Subsequently, sections were boiled in a microwave oven to allow recovery of antigen. The sections were then treated with 0.3% H2O2 for 30 minutes and blocked in 3% goat serum. These sections were then incubated overnight at 4°C with one of the following primary antibodies: rat anti-mouse PCNA (1:200; Signet Laboratory, Dedham, MA), rat anti-mouse CD31 (1:50 dilution, clone 6.64; ImClone Systems, New York, NY), and rat anti-mouse GFAP (1:100; Chemicon, Temecula, CA). After washing in PBS, the slides were incubated with a biotinylated horse peroxidase-conjugated secondary antibody and visualized with diaminobenzidine (Sigma, St. Louis, MO). All slides were counterstained with hematoxylin.
Quantification of VEGF Gene Expression in Retina
Total RNA was extracted from the retinas using reagent (Trizol; Invitrogen, Carlsbad, CA). Residual genomic DNA was removed by incubating the RNA with DNase (Promega, Madison, WI). Reverse transcription–polymerase chain reaction (RT-PCR) was carried out in a thermal cycler (PCR System PIC-200; Bio-Rad, Hercules, CA). For each assay, 1 μg mRNA was reverse-transcribed using 200 U reverse transcriptase (Promega) in a final volume of 20 μL. The reaction mixtures were incubated at 25°C for 10 minutes and at 42°C for 1 hour and terminated by heating at 70°C for 5 minutes.
For real-time PCR, 50 ng cDNA was used for both VEGF and a control gene (β-actin) RT-PCR amplification. Forward and reverse primers for VEGF and β-actin were as follows: 5′-GAAAGGGTCAAAAACGAAAGC-3′, 5′-CGCTCTGAACAAGG CTCAC A-3′, 5′-CTACAATGAGCTGCGTGTGG-3′, and 5′-ACCAGAGGCATACAGGG ACA-3′, respectively (Invitrogen). The PCR reaction was carried out using a PCR kit (Applied Biosystems, Foster City, CA), and 10 μmol/μL concentration of each upstream and downstream primer per reaction. The PCR protocol included 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles at 95°C for 15 seconds, 60°C for 1 minute. All experimental results were normalized to the internal control β-actin. The relative abundance of the VEGF mRNA was obtained by ΔΔ-CT method.
Statistical Analysis
Data are expressed as mean ± SD. Comparison among the groups was analyzed by ANOVA, followed by post hoc comparison with the least significant difference test (equal variances assumed) or Dunnett's test (equal variances not assumed). P < 0.05 was considered statistically significant.
Results
Genetic Inactivation of A2AR Did Not Affect Normal Development of Vascularization in Retina
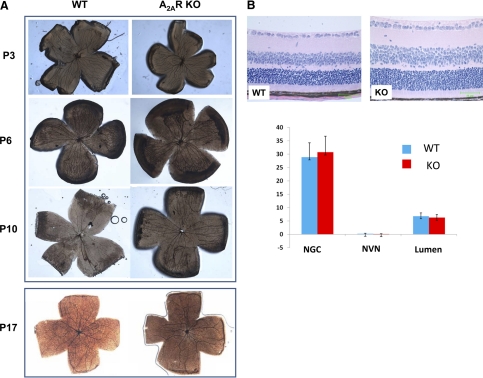
Given that the postnatal development of retinal vessels undergoes several distinct development changes in the mouse, we examined the effect of A2AR KO on normal vascularization in the retina in room air at four developmental stages—P3, P6, P10, and P17—using the flat-mounted whole retina stained with ADPase. Consistent with previous reports on the normal development of retinal blood vessels of C57BL/6 mice during early development,23 we observed similar distinct postnatal developmental stages in WT mice. At P3, large primitive vessels developed radially from the optic disc to the ora serrata and formed an initial vascular network in the superficial retinal layer. At P6, secondary deeper vascular networks were formed within deeper retinal layers that became thinner and straighter gradually with the regression of the superficial large vessels from the optic disc. At P10, a capillary network was developed in the intermediate layer of the retina as further branches of the secondary vascular network, with the number of superficial large vessels decreasing. At P17, the superficial vessels disappeared, and an arboreous pattern of arteries formed within the retina. Importantly, the early developing processes (P3, P6, and P10) of the retinal vessels were indistinguishable between A2AR KO mice and WT mice (Fig. 1A). There was little difference in the rate of the deeper vascular growth between A2AR KO mice and WT mice (Fig. 1A). For example, in WT mouse retina, the deeper vessels spread approximately 50% by P6 and approximately reached the ora serrata by P10, and arboreous patterns of arteries formed within the retinas by P17 in both WT mice and A2AR KO mice (Fig. 1A). At the cellular level, the retinas of both WT and A2A KO mice (Fig. 1B) showed normal vessel growth and retinal cellular organization, and no cells extended from the inner limiting membrane into the vitreous (i.e., no neovascular nuclei) by hematoxylin and eosin staining at P17 (Fig. 1B). Quantitative analysis showed that the number of neovascular nuclei, non-ganglion cells, and new vessel lumens were indistinguishable between WT and A2AR KO mice under room air conditions (Fig. 1B). Thus, the genetic inactivation of A2AR did not affect normal vascularization of the retina during postnatal development.
Figure 1.
Genetic inactivation of A2ARs does not affect normal vascularization in the retina during postnatal development. (A) Postnatal development of retina vascularization of WT mice (left) and A2AR KO mice (right) at P3, P6, P10, and p17 was determined by ADPase staining using flat-mounted whole retina. (B) Normal retinal cellular organization; no cells extended from the inner limiting membrane into the vitreous (i.e., no neovascular nuclei) by hematoxylin and eosin staining at P17. Bar graph shows the results of quantitative analysis on the numbers of neovascular nuclei (NVN), non-ganglion cells (NGCs), and new vessel lumens in WT and A2AR KO mice in room air conditions.
Oxygen Exposure Led to Vasculo-obliteration and Pathologic Angiogenesis in WT Mice
In the room air WT mice (P7–P21 exposure to room air), retinal vascularization was nearly completed by P12. Both the superficial and the deep vascular layers from the optic disc to the periphery were well developed at P12 to P17 (Fig. 2, left). No further changes were observed at P21. By contrast, for the OIR-WT group, hyperoxic exposure from P7 to P12 significantly hindered the growth of the retinal vasculature when examined at P12 (day 1 returning from 75% oxygen chamber to the room air). After 5 days of exposure to 75% oxygen, retinas from the OIR-WT group clearly showed oxygen-induced vaso-obliteration changes (large areas of the central retina showing nonperfusion). However, from P14 to P17 with continuous exposure to room air for 2 and 5 days, respectively, the oxygen-induced central avascular areas gradually decreased, and the radial large vessels appeared tortuous and dilated. It should be noted that compared with other OIR models that used 95% oxygen,24 the 75% oxygen-induced vaso-obliteration detected in the present study was comparatively mild. At P17, the neovascular tuft was observed. At P21, the OIR-related proliferative changes in retina were slightly reduced compared with the retina at P17. These results demonstrated that the nonperfusion in the retina induced by hyperoxia at P12 was gradually reversed after the return to room air and that OIR-related neovascularization progressed between P12 and P17 in the retina. Thereafter, these OIR changes in the retina regressed slightly but remained elevated at P21.
Figure 2.
Oxygen exposure leads to vaso-obliteration in retina of WT mice. WT mice were initially exposed to 75% oxygen in a hyperoxic chamber for 5 days (P7–P12) and subsequently to room air (21% oxygen) for the next 9 days (P13–P21) (OIR, right), or they were exposed to room air throughout P7 to P21 (room air, left), as described in Methods. Left: normal retinal vascularization during P12 to P21 in room air. Right: retinal vaso-obliteration after exposure to hyperoxia.
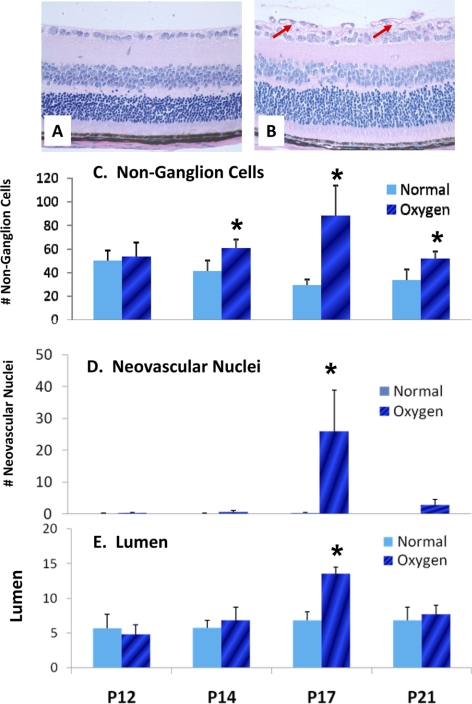
At the cellular level, in the retinas of the room air WT group, most retinal cells between the internal plexiform layer and the inner limiting membrane were ganglion cells with large, round nuclei, and a few were non-ganglion cells with small, non-round nuclei (Fig. 3A). Distinct cell types were largely organized and distributed in a single layer, and no nuclei were detected on the vitreal side of the inner limiting membrane. By contrast, the number of retinal cells of the OIR-WT group increased dramatically at P17, especially non-ganglion cells and neovascular nuclei, compared with those of the room air WT group (Fig. 3B). In the retinas of the OIR-WT group, various cell types showed disorganization with distribution in two or more layers both within and beyond the inner limiting membrane. At P12, the OIR-WT and the room air WT retinas had similar non-ganglion cells by quantitative cell counting. However, compared with the room air WT group, non-ganglion cells increased by 1.50-fold higher in the OIR-WT group at P14 (P < 0.05; n = 6), peaked by 3.14-fold higher at P17 (P < 0.001; n = 20), and regressed relatively but remained higher by 1.55-fold higher at P21 (P < 0.05; n = 6; Fig. 3C). Quantitative analysis also showed a marked increase in neovascular nuclei at P17 (P < 0.001; n = 20; Fig. 3D). Similarly, neovascular lumens were significantly increased in the OIR-WT group at P17 compared with those in the room air WT group (Fig. 3E). Consistent with the results from retinal-mounted slides (Fig. 2), these findings demonstrate that OIR-induced neovascularization peaked at P17. Of note, though OIR-associated proliferation of non-ganglion cells was detected as early as P14 and remained persistently enhanced until P21 with a peak at P17, increased proliferation of neovascular nuclei was detected transiently, albeit dramatically, only at P17.
Figure 3.
Oxygen exposure leads to pathologic angiogenesis in the retinas of WT mice. After initial exposure to 75% oxygen for 5 days (P7–P12) and subsequent exposure to room air for the next 9 days (P13–P21), the OIR-WT mice developed proliferative retinopathy characterized by the disorganized proliferation of non-ganglion cells and neovascular nuclei in the retina. Histology of retinal cellular structure in room air WT (A) and OIR-WT (B) mice at P17. Quantitative analysis of non-ganglion cells (C), neovascular nuclei (D), and number of nuclei on the vitreal side of the inner limiting membrane (new vessel lumen; E) from P12 to P21. *P < 0.05; OIR-WT mice compared with room air WT mice (n = 8 per group) on the same postnatal day.
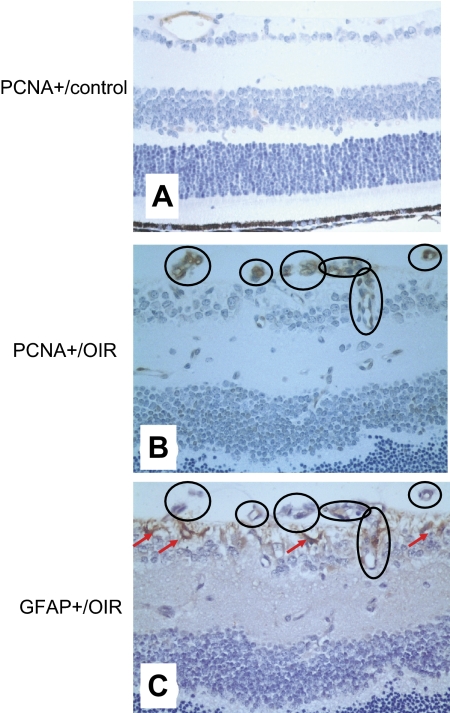
To confirm OIR-related cell proliferation, we performed immunohistochemistry of PCNA, a cell cycle marker for cell proliferation, in retinal sections. In the room air WT group, only scattered PCNA+ immunoreactivity was detected in normal vascular structures of the retina (Fig. 4A). However, in the OIR-WT group, PCNA+ cells were dramatically increased in most neovascular cells but not in non-ganglion cells of the retina (Fig. 4B). To further explore the nature of proliferative (PCNA+) cells, we examined the immunoreactivities for CD31 or GFAP in association with PCNA+ cells in mirror images of adjacent retinal sections. Interestingly, most PCNA+ cells do not express GFAP (indicated by the circles) in these mirror images. Instead, many PCNA+ cells appeared to be colocalized with proliferative endothelial cells, as demonstrated by their locations in the vitreal side of the inner limiting membrane (Fig. 4C). This indicates that most proliferative cells were of endothelial origin and that many non-ganglion cells were probably of astrocytic origin.
Figure 4.
PCNA+ cells are not colocalized with GFAP+ cells but appear to be associated with proliferative endothelial cells. PCNA+ cells (A, B) and GFAP+ cells (C) were determined in the retinas of the room air WT (A) and OIR-WT (B, C) by immunohistochemistry at P17 in two adjacent, mirror image sections. (The second section was flipped over before mounting so that it was the mirror image of the first section.) With these mirror image sections, we were able to merge these images of PCNA+ cells (B) and GFAP+ cells (C) in the retina to determine whether they were different cells or the same cells (circles). In the room air WT group, only scattered PCNA+ immunoreactivity was detected in the normal vascular structures of the retina (A). However, in the OIR-WT group, PCNA+ cells were dramatically increased, and most PCNA+ cells did not express GFAP (circles) in the mirror images. Instead, many PCNA+ cells appeared to be colocalized with proliferative endothelial cells, as indicated by their locations in the vitreal side of the inner limiting membrane (C, arrows).
Hypoxia-Induced Pathologic Angiogenesis Was Attenuated in A2AR KO Mice
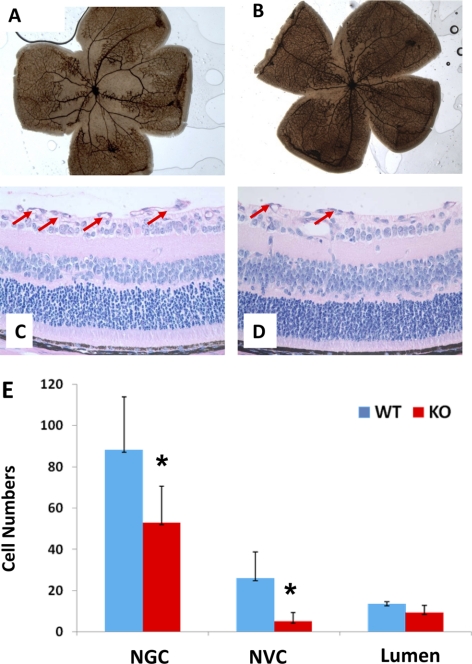
Next, we examined the effect of A2AR KO on oxygen-induced vaso-obliteration and abnormal angiogenesis by comparing the OIR-KO group with the OIR-WT group. After exposure to hyperoxia for 5 days, the retinas of the OIR-WT mice developed central vaso-obliteration and nonperfusion. The formation of peripheral neovascular tufts was detected in a large area of retina (Fig. 5A). By contrast, the retinas of the OIR-A2AR KO mice displayed much less nonperfusion area at the center and fewer peripheral tufts in the retinas at P17 (Fig. 5B).
Figure 5.
Hypoxia-induced pathologic angiogenesis in the retina is attenuated in A2AR KO mice. OIR-induced vaso-obliteration in the OIR-WT (A) and the OIR-KO (B) mice were assessed in the retina at P17 by ADPase staining, as described in Methods. Similarly, OIR-induced infiltration of non-ganglion cells and neovascular nuclei from inner limiting membrane into vitreous in the OIR-WT (C) and the OIR-KO (D) mice were assessed by histology, as described in Methods. (E) Quantitative analysis of the number of non-ganglion cells, neovascular nuclei, and new vessel lumens in the OIR-WT and the OIR-KO mice. *P < 0.001; OIR-WT mice compared with OIR-KO mice (n = 20 for each group).
At the cellular level, the retinas of the OIR-WT mice displayed dramatic neovascular reactions as the number of non-ganglion cells and neovascular nuclei increased significantly at P17 compared with that of the room air WT group (Fig. 3). Importantly, compared with the OIR-WT group (Fig. 5C), oxygen-induced infiltration of non-ganglion cells and neovascular nuclei from the inner limiting membrane into the vitreous were significantly reduced in A2AR KO mice (Fig. 5D). The numbers of non-ganglion cells, neovascular nuclei, and new vessel lumens in A2AR KO mice were decreased by 20%, 60%, and 69%, respectively, compared with those of WT littermates (Fig. 5E). Thus, oxygen-induced abnormal angiogenesis, particularly the infiltration of non-ganglion cells and neovascular nuclei from the inner limiting membrane into vitreous, was strikingly reduced in the OIR-A2AR KO group compared with the OIR-WT group at P17.
A2AR Inactivation Attenuated Hypoxia-Induced VEGF Gene Expression in the Retinas of OIR Mice
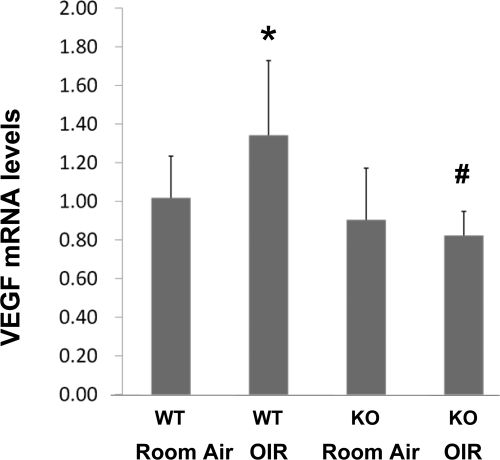
To explore the mechanism underlying the attenuated OIR in A2AR KO mice, we investigated whether A2AR affects OIR by modulating VEGF gene expression in the retina. We determined the levels of VEGF mRNA in the retinas of WT and KO mice at P17 in normal conditions (the room air WT and the room air-A2AR KO group) and after exposure to hyperoxia and consequential hypoxia (the OIR-WT and the OIR-A2AR KO group) by real-time PCR. After exposure to room air, the VEGF mRNA levels in retina were indistinguishable between those of WT and A2AR KO mice (P > 0.05, n = 8 in each group; Fig. 6). However, after exposure to hyperoxia followed by normoxia, the VEGF mRNA level at P17 was increased only in the OIR-WT group. Hypoxia-induced increase of retinal VEGF mRNA level was completely blocked in the OIR-A2AR KO group at P17 (P < 0.01, compared with the changes in WT groups, n = 8 in each group; Fig. 6). Of note, the VEGF mRNA in the OIR-A2AR KO group was slightly but not significantly decreased compared with that of the room air-A2AR KO group (Fig. 6). Thus, in parallel with the attenuated proliferative OIR histology in A2AR KO mice, hypoxia-induced expression of VEGF gene in retina was significantly attenuated in A2AR KO mice.
Figure 6.
A2AR inactivation attenuates hypoxia-induced VEGF gene expression in the retinas of OIR mice. VEGF mRNA levels in the retinas of room air-WT mice, room air-A2AR KO mice, OIR-WT mice, and OIR-A2AR KO mice were determined by real-time PCR. Data were presented as mean ± SD. *P < 0.05; OIR-WT mice compared with the room air WT group (n = 8 for each group). #P < 0.01; OIR-WT mice compared with OIR-KO mice (n = 8 for each group).
Discussion
Using the A2AR KO model, this study uncovers a critical role for A2AR in the development of pathogenic angiogenesis in a mouse model of OIR. To best demonstrate the protective effect of A2AR inactivation, we adapted a relatively mild hyperoxic model (75% rather than 90% oxygen) that produced characteristic OIR pathologic conditions, including initial development of vaso-obliteration and subsequent abnormal angiogenesis in the retinas of WT mice. Given that adenosine levels and expression of 5′ nucleotidase (an enzyme to convert adenosine monophosphate to adenosine) and A2AR expression are low at the inner retina during vaso-obliteration,6,14,25 it is unlikely that A2AR inactivation would have any major effect at this pathologic phase. As vaso-obliteration and avascular areas develop and hypoxia- and ischemia-driven proliferative pathologies become predominant, adenosine levels, expression of the 5′ nucleotidase, and A2AR are markedly increased in newly formed vasculatures, as demonstrated previously by immunohistochemistry in a dog model of OIR.6,14,25 At this phase, the numbers of neovascular nuclei increased by >20-fold from P12 to P17 in the OIR-WT group (Fig. 3 this study,21). This abnormal increase in neovascular nuclei is largely abolished in A2AR KO mice, indicating the requirement of A2AR activation for the development of abnormal angiogenesis during OIR. Although retinal neovascular nuclei represented the most increased cell population during OIR, non-ganglion cells seemed to represent an early indicator of proliferative responses during OIR because non-ganglion cell levels increased as early as P14 and remained elevated throughout the postnatal stages examined (P14, P17, and P19) in the OIR-WT group. Similarly, the increase in non-ganglion cells was largely abolished in A2AR KO mice. Interestingly, our analysis of immunoreactivity for three types of non-ganglion cells, CD31+ endothelial cells, GFAP+ astrocytes, and PCNA+/GFAP−/CD31− angioblasts, suggested that neovascular cells (PCNA+/GFAP−) originate not from astrocytes but from proliferative vascular endothelial cells, as indicated by their locations in the vitreal side of the inner limiting membrane. This indicates that A2AR activity modulates angiogenesis in the retina during OIR by affecting largely the proliferation of endothelial cells rather than glial cells.
In further support of A2AR-modulation of OIR, we demonstrated that the expression of VEGF mRNA in the retina was markedly induced in the OIR-WT mice, but this effect was essentially abolished in the OIR-A2AR KO mice. This strongly suggested that activation of A2AR is critical for the induction of VEGF in OIR. Given that VEGF is considered as a major factor in triggering neovascularization in the hypoxic retina,5,10,15 our finding provides the molecular link for A2AR modulation of OIR. It is important to point out that A2AR activity can act synergistically with hypoxia inducible factor to control VEGF expression.26 In addition, VEGF may work in concert with other factors to promote angiogenesis because VEGF alone seems to be insufficient to induce retinal neovascularization.6 This distinct effect of the A2AR on VEGF is consistent with previous findings that A2AR activity can affect VEGF expression through a cAMP signaling pathway and CREB phosphorylation.10
Our finding of the critical role of A2AR in OIR is not only supported by a well-documented angiogenesis effect of A2AR activation in various tissues11–14 but is in agreement with an early report that immunoreactivities for both adenosine and A2AR were significantly increased at the edges of forming vasculature and in the intravitreal neovascular formations in a canine model of OIR.14 However, our finding is notably different from those of previous pharmacologic studies showing that the systemically administered A2BR antagonists enprofylline and IPDX reduced neovascularization in a mouse model of OIR whereas the A1R or A2AR antagonists CPX and ZM241385, respectively, did not.17 The discrepancy between that study and ours may be partially attributed to the different approaches of pharmacologic versus genetic inactivation to the A2AR. Pharmacologic studies of A2AR are limited intrinsically by their partial specificity and partial inhibition and by limited permeability of the retinal tissues. By contrast, A2AR KO mice inactivate A2AR with complete specificity and completion inactivation, but this genetic inactivation is also associated with potential developmental compensatory changes. It is possible that pathologic angiogenesis of OIR may involve both A2AR and A2BR; additional experiments with A2BR KO mice could clarify this issue.
Based on the finding that the intensity of A2AR immunoreactivity increased at the edge of forming vasculature during postnatal development, it was suggested that A2AR might play a modulating role in retinal vessel development.6,14 One of the critical concerns in developing an A2AR-based strategy for treating ROP is that A2AR activity may be necessary for the normal development of retinal vascularization, thus hampering the recovery of retinal vascularization. However, retinal vessel development is indistinguishable between A2AR KO mice and WT littermates in room air, indicating that A2AR activation is not required for normal development of retinal vessels in mice. It is possible that the increased expression of A2AR immunoreactivity at the edge of vessel development may be coincident with retinal vessel development or may be a response to local retinal vessel development. The differential effects of the A2AR on normal retinal vessel development and OIR-related vascularization indicate the distinct mechanisms underlying the control of retinal vessel development. Consistent with this notion, adenosine has been shown in dogs to stimulate endothelial cell migration and tube formation, two primary events for retinal vasculature development, but not the proliferation of retinal microvascular endothelial cells.27
The lack of effect of A2AR deletion on the normal development of retinal vessels provides a potential therapeutic use of A2AR antagonist-based strategy by targeting hypoxia-induced pathologic angiogenesis in OIR without affecting normal retinal vascularization. This feature also permits the local control of blood vessel growth by adenosine accumulating locally during hypoxia (rather than a general increase in vessel proliferation in the body). It would be interesting to investigate whether A2AR inactivation can exert prophylactic or therapeutic effects against OIR and perhaps ultimately ROP.
In summary, using the A2AR KO model, we demonstrated that genetic inactivation of A2AR attenuates the development of OIR pathology, as evidenced by reduced vaso-obliteration and nonperfusion areas in the center of retina, reduced pathologic angiogenesis, and inhibited hypoxia-induced VEGF gene expression. Notably, A2AR inactivation selectively attenuates OIR-related pathologic angiogenesis but does not affect normal retinal vascularization during postnatal development. These findings provide the first direct evidence that the A2AR is critical to the development of OIR and suggest the exciting possibility that A2AR inactivation may be a potential therapeutic strategy for ROP by selectively targeting pathologic, but not developmental, angiogenesis in the retina.
Acknowledgments
The authors thank Jiang-Hong An, Jun Gao, and Guo-Rong Chen for help with pathology examination, Ge Zheng for participation in the early development of the oxygen-induced retinopathy model, Robert Green (Mount Sinai School of Medicine, New York, NY) for critical review of the manuscript, and Yuan-Yuan Zhou (University of Nottingham, Ningbo, China) for help with manuscript preparation.
Footnotes
Supported by National Natural Science Foundation of China Grant 30470563; the 973 program 2006CB503900, China; and US Public Health Service Grant NS41083.
Disclosure: X.-L. Liu, None; R. Zhou, None; Q.-Q. Pan, None; X.-L. Jia, None; W.-N. Gao, None; J. Wu, None; J. Lin, None; J.-F. Chen, None
References
- 1. Fleck BW, McIntosh N. Pathogenesis of retinopathy of prematurity and possible preventive strategies. Early Hum Dev. 2008;84:83–88 [DOI] [PubMed] [Google Scholar]
- 2. Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82 [DOI] [PubMed] [Google Scholar]
- 3. Clark D, Mandal K. Treatment of retinopathy of prematurity. Early Hum Dev. 2008;84:95–99 [DOI] [PubMed] [Google Scholar]
- 4. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487 [DOI] [PubMed] [Google Scholar]
- 5. Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028 [DOI] [PubMed] [Google Scholar]
- 6. Lutty GA, McLeod DS. Retinal vascular development and oxygen-induced retinopathy: a role for adenosine. Prog Retin Eye Res. 2003;22:95–111 [DOI] [PubMed] [Google Scholar]
- 7. Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology, XXV: nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552 [PMC free article] [PubMed] [Google Scholar]
- 8. Adair TH, Cotten R, Gu JW, et al. Adenosine infusion increases plasma levels of VEGF in humans. BMC Physiol. 2005;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patz A. Studies on retinal neovascularization: Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1980;19:1133–1138 [PubMed] [Google Scholar]
- 10. Takagi H, King GL, Robinson GS, Ferrara N, Aiello LP. Adenosine mediates hypoxic induction of vascular endothelial growth factor in retinal pericytes and endothelial cells. Invest Ophthalmol Vis Sci. 1996;37:2165–2176 [PubMed] [Google Scholar]
- 11. Day YJ, Li Y, Rieger JM, Ramos SI, Okusa MD, Linden J. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia-reperfusion injury. J Immunol. 2005;174:5040–5046 [DOI] [PubMed] [Google Scholar]
- 12. Okusa MD. A(2A) adenosine receptor: a novel therapeutic target in renal disease. Am J Physiol Renal Physiol. 2002;282:F10–F18 [DOI] [PubMed] [Google Scholar]
- 13. Montesinos MC, Gadangi P, Longaker M, et al. Wound healing is accelerated by agonists of adenosine A2 (G alpha S-linked) receptors. J Exp Med. 1997;186:1615–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taomoto M, McLeod DS, Merges C, Lutty GA. Localization of adenosine A2A receptor in retinal development and oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2000;41:230–243 [PubMed] [Google Scholar]
- 15. Grant MB, Tarnuzzer RW, Caballero S, et al. Adenosine receptor activation induces vascular endothelial growth factor in human retinal endothelial cells. Circ Res. 1999;85:699–706 [DOI] [PubMed] [Google Scholar]
- 16. Grant MB, Davis MI, Caballero S, Feoktistov I, Biaggioni I, Belardinelli L. Proliferation, migration, and ERK activation in human retinal endothelial cells through A(2B) adenosine receptor stimulation. Invest Ophthalmol Vis Sci. 2001;42:2068–2073 [PubMed] [Google Scholar]
- 17. Mino RP, Spoerri PE, Caballero S, et al. Adenosine receptor antagonists and retinal neovascularization in vivo. Invest Ophthalmol Vis Sci. 2001;42:3320–3324 [PubMed] [Google Scholar]
- 18. Chen JF, Huang Z, Ma J, et al. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Day YJ, Huang L, McDuffie MJ, et al. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang QY, Wei C, Yu L, et al. Adenosine A2A receptors in bone marrow-derived cells but not in forebrain neurons are important contributors to 3-nitropropionic acid-induced striatal damage as revealed by cell-type-selective inactivation. J Neurosci. 2006;26:11371–11378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111 [PubMed] [Google Scholar]
- 22. Lutty GA, McLeod DS. A new technique for visualization of the human retinal vasculature. Arch Ophthalmol. 1992;110:267–276 [DOI] [PubMed] [Google Scholar]
- 23. Connolly SE, Hores TA, Smith LE, D'Amore PA. Characterization of vascular development in the mouse retina. Microvasc Res. 1988;36:275–290 [DOI] [PubMed] [Google Scholar]
- 24. Browning J, Wylie CK, Gole G. Quantification of oxygen-induced retinopathy in the mouse. Invest Opthalmol Vis Sci. 1997;38:1168–1174 [PubMed] [Google Scholar]
- 25. Lutty GA, Merges C, McLeod DS. 5′ Nucleotidase and adenosine during retinal vasculogenesis and oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2000;41:218–229 [PubMed] [Google Scholar]
- 26. Adair TH. Growth regulation of the vascular system: an emerging role for adenosine. Am J Physiol Regul Integr Comp Physiol. 2005;289:R283–R296 [DOI] [PubMed] [Google Scholar]
- 27. Lutty GA, Mathews MK, Merges C, McLeod DS. Adenosine stimulates canine retinal microvascular endothelial cell migration and tube formation. Curr Eye Res. 1998;17:594–607 [PubMed] [Google Scholar]