Eyes with uveal melanoma have been shown to have multiple cytokines and chemokines in the aqueous humor. This study was conducted to determine whether a relation exists between the presence of these cytokines and the prognostic factors and survival of uveal melanoma.
Abstract
Purpose.
The presence of an inflammatory phenotype, characterized by an increased expression of HLA antigens and an immunologic infiltrate, carries a bad prognosis in uveal melanoma. This study was conducted to determine whether the aqueous humor (AqH) from eyes with uveal melanoma contains inflammatory cytokines and whether their presence is associated with inflammation.
Methods.
Immediately after enucleation, AqH was obtained from 37 eyes containing uveal melanoma. Samples were stored at −80°C until use. Fifteen different cytokines were measured with a multiplex bead array. Intratumoral macrophages were analyzed by immunohistochemistry and immunofluorescence staining. The presence of specific cytokines was compared with histopathologic, genetic, and clinical tumor characteristics, as well as patient survival.
Results.
Several cytokines showed significantly higher expression in the AqH of uveal melanoma–containing eyes than in the AqH of eyes undergoing cataract surgery. MCP-3 was associated with the presence of CD68+ macrophages. Correlations were found between some cytokine levels and a few known prognostic factors of uveal melanoma, but cytokine levels were not of predictive value for survival.
Conclusions.
Uveal melanoma–containing eyes often carry increased levels of inflammation-related cytokines in their AqH. However, the presence of most specific cytokines was not related to the presence of macrophages, clinical or histopathologic parameters, or prognosis.
Uveal melanoma is a malignancy in the eye that may give rise to metastases. Once metastases develop, survival is poor.1 Categories of patients can be identified that differ in prognosis. When enucleation is indicated for treatment, survival is worse than when local treatment can be applied.2 Many clinical and histopathologic parameters are known to be indicators of prognosis, including tumor size, the presence of epithelioid cells, loss of one copy of chromosome 3, and a specific mRNA footprint, as well as immunologic markers.3–5 The presence of an inflammatory infiltrate carries a poor prognosis and is a component of an inflammatory phenotype.6,7 Inflammatory infiltrates are characterized by an increased number of CD3+ and CD4+ lymphocytes as well as CD11b+ macrophages, and correspond with an increased expression of HLA class I and II expression on the tumor cells.8–11 We have described this combination as an inflammatory phenotype, which was associated with the presence of epithelioid cells and with monosomy of chromosome 3.7
Macrophages can be found in variable quantities in uveal melanoma. Mäkitie et al.12 classified them into three categories—low, medium, and high—and observed that a high density of tumor-associated macrophages (TAMs) correlates with decreased survival. The presence of macrophages was associated with hot spots of microvascular density.13 Vascular endothelial growth factor (VEGF) is often produced by macrophages, and it is possible that the production of VEGF by the infiltrating macrophages plays a role in the formation of blood vessels.14 It may be that VEGF also has a role in uveal melanoma. It has been shown to be present in tumors and also in the aqueous humor (AqH) and the vitreous of eyes with uveal melanoma.15,16 Missotten et al.16 showed that both the tumor and the surrounding retinal tissue are sources of VEGF.
Macrophages can be divided according to the so-called M1 and M2 paradigm, in which the M1-type macrophages appear to be more immunostimulatory, whereas the M2-type macrophages are involved in tissue repair, angiogenesis, and immunoregulation.17,18 These M2-type macrophages can be identified by their enhanced expression of the CD163 scavenger receptor.19 TAMs have been described to be mainly M2-type macrophages, which have a tumor-promoting role, owing especially to their proangiogenic and immunosuppressive capacities.17
Specific molecules (cytokines) are known to stimulate the influx of macrophages, and determining the presence of such cytokines in the eye may help in understanding why some tumors have many and others have few macrophages. Although fine-needle aspiration biopsies (FNABs) are currently used to discriminate between tumors with and without loss of one chromosome 3,20,21 hypothetically, studying a sample of AqH from a uveal melanoma-containing eye can provide a profile of cytokines that may also help to differentiate between “good” and “bad” tumors. Furthermore, if identified, such a set of known inflammatory cytokines could predict survival, since the inflammatory phenotype is associated with decreased survival. If AqH is a good method of predicting the prognosis of a certain tumor, treatment strategies could be customized for each patient.
We determined the presence of 15 cytokines related to inflammation in 37 eyes with uveal melanoma and attempted to find a correlation between the concentration of the individual cytokines and the standard prognostic parameters in uveal melanoma. As well, we compared the survival of patients with the presence of macrophage subtypes in 30 uveal melanoma samples.
Materials and Methods
Patient and Control Eyes
AqH samples were collected from 37 eyes that had been removed for uveal melanoma. Immediately after enucleation, approximately 150 μL of AqH was extracted with a 1-mL insulin syringe with a 23-gauge needle. The AqH was immediately stored in a freezer at −80°C. AqH samples from cataract patients were collected after the first paracentesis. These patients were included as control subjects, only when there was no history of ophthalmic disease or ongoing systemic disease that influences ocular status, such as diabetes mellitus or hypertension. In July 2009, patient data and survival were updated from the patients' charts and from the database of the Integral Cancer Center West, which records national death data. Death events were also obtained from the Central Bureau of Statistics of the Netherlands. The research protocol adhered to the tenets of the Declaration of Helsinki and was approved by the local Medical Ethics Committee.
Pathologic Analysis
Histologic sections were prepared from tissues fixed in 4% neutral-buffered formalin for 48 hours and embedded in paraffin. Hematoxylin and eosin–stained 4-μm sections were reviewed by an ocular pathologist for confirmation of diagnosis, intraocular localization, cell type, largest basal diameter, prominence, and scleral invasion. Data regarding a group of 50 patients have been published elsewhere.7 From 37 of these cases, AqH was available for testing.
Cytokine Measurement in AqH
In AqH samples of 50 μL, we determined the cytokines IL-6, IL-10, bFGF, GM-CSF, IP-10, MCP-1, MIP-1α, RANTES, VEGF, MCP-3, MIF, TNF-β, TRAIL, ICAM-1, and VCAM-1 by array analysis (Bio-Plex Human Cytokine multiplex panel; Bio-Rad Laboratories, Veenendaal, The Netherlands), according to the manufacturer's instructions. Samples were analyzed with an array reader and software (Bio-Plex; Bio-Rad).
Histologic Analysis of Uveal Melanoma
Immunohistochemical (IHC) staining with mAb CD68 was performed by the alkaline phosphatase–monoclonal anti-alkaline phosphatase (APAAP) method.7,22 For immunofluorescence (IF) double staining, slides were incubated overnight at room temperature. Mouse anti-human CD68 mAb (1:50, clone 514H12; ab49777; Abcam, Cambridge, UK) was used to stain macrophages, and mouse anti-human CD163 mAb (1:100, clone 10D6; NCL-CD16; Novocastra, Newcastle-upon-Tyne, UK) was used to stain M2-type macrophages. IHC sections were counterstained with Mayer's hematoxylin (Klinipath, Duiven, The Netherlands) and embedded in Kaiser's glycerin.
As a negative control, the primary antibody was replaced by phosphate-buffered saline and bovine serum albumin 1%.
Immunofluorescence Staining Analysis
Images of the slides were obtained by confocal laser scanning microscope (model LSM510; Carl Zeiss Meditec, Jena, Germany) in a multitrack setting, in which the slide was scanned multiple times with a fixed pair of laser filters. Alexa-488 (staining CD68) was excited at 488 nm and detected with a 505- to 530-nm bandpass filter. Alexa-546 (staining CD163) was excited at 543 nm and detected with a 560- to 615-nm bandpass filter, yielding a two-color signal: green (CD68) and red (CD163). All images were 512 × 512 pixels; eight-bit depth; 368.5 × 368.5-μm stack size; and 0.72 × 0.72-μm scale. A 25×/0.80 objective (PH2 Plan-NEOFluar; Imm Korr; Carl Zeiss Meditec) was used. Images obtained were viewed and saved as merged images and as a set of two separate panels, in LSM files.
Assessment of Staining
Scoring of the slides stained with IHC and IF was performed by an investigator blinded with respect to clinical outcome. The procedure for the IHC evaluation is described elsewhere.7,12 For IF, the 10 most representative high-power scans (magnification, 250×) per slide were manually selected, to assess repeatability and validity. Each scan represented one square optical field (OF; area, 0137 mm2). Images of the sections were exported as JPG files with an image-converting software program (LSM Data Server, ver. 3.2.0.70; Carl Zeiss Meditec).
For digital analysis of the sections, we used an in-house software image-analysis program (Stacks; Department of Molecular Cell Biology, Leiden University Medical Center, Leiden, The Netherlands). The images with the green and red signals of CD68 and CD163, respectively, were first thresholded and in the resulting binary images, the presence of the subtype of macrophages was calculated by the number of pixels expressing green, red, or both colors.
Chromosome Analysis
Chromosome analysis was performed by karyotyping as well as by interphase FISH on nuclei isolated from 50-μm paraffin-embedded tissue sections, as described elsewhere.23
Statistics
A Student's t-test was performed to compare the two groups for differences in expression of cytokine levels. Linear regression was applied to observe which factors exactly determined differences in cytokine levels between the uveal melanoma and cataract patients. ANOVA trend testing was performed for comparing the mean expression among more ordinal groups. Spearman's rank correlation coefficient was used to analyze correlations between two numerical variables. Cox regression was performed to observe whether cytokine levels were associated with decreased survival. Differences at P < 0.05 were considered to be statistically significant.
Results
Cytokine Levels in AqH
We determined whether eyes with uveal melanoma contained more or other inflammatory cytokines in their AqH than did the normal eyes. We selected 15 cytokines and chemokines that could be related to the presence of inflammation and macrophage infiltration.
AqH was obtained from 37 eyes with uveal melanoma and 37 with cataract; the mean age of the control patients was 68.7 ± 12.4 years (±SEM) and that of the uveal melanoma patients was 58.5 ± 14.9 years (P = 0.002). The distribution of female and male patients was not significantly different (P = 0.16). In the control group, 40.5% were men, whereas 59.5% were women; in the patient group, the respective percentages were 56.8% and 43.2%. Mean follow-up time of the patients who underwent enucleation was 4.8 ± 2.3 years. Of the 37 patients, 45.9% were alive at the end of follow-up. Metastasis and subsequent death occurred in 40.5% (Table 1).
Table 1.
Patient and Tumor Characteristics
| Age, y (mean ± SD) | 58.5 ± 14.9 |
| Sex, n (%) | |
| Male | 21 (56.8) |
| Female | 16 (43.2) |
| Eye, n (%) | |
| Right | 21 (56.8) |
| Left | 16 (43.2) |
| Ciliary body involvement, n (%) | |
| Not present | 21 (56.8) |
| Present | 16 (43.2) |
| Largest basal diameter, mm (mean ± SD) | 13.4 ± 3.0 |
| Prominence, mm (mean ± SD) | 7.1 ± 2.7 |
| Tumor size according pTNM (7th edition), n (%) | |
| T1 | 4 (11.1) |
| T2 | 11 (30.6) |
| T3 | 20 (55.6) |
| T4 | 1 (2.8) |
| Cell type, n (%) | |
| Spindle | 9 (24.3) |
| Mixed + Epithelioid | 28 (75.7) |
| Macrophage Density (IHC), n (%) | |
| Low | 12 (32.4) |
| Medium | 15 (40.5) |
| High | 10 (27.0) |
| Microvascular density, n (%) | |
| First quartile | 10 (27.0) |
| Second quartile | 9 (24.3) |
| Third quartile | 7 (18.9) |
| Fourth quartile | 11 (29.7) |
| Chromosome 3 status, n (%) | |
| Disomy | 13 (35.1) |
| Monosomy | 24 (64.9) |
| HC10, % of tumor cells (mean ± SD) | 44.2 ± 32.8 |
| HCA2, % of tumor cells (mean ± SD) | 46.5 ± 31.7 |
| HLA-DR, % of tumor cells (mean ± SD) | 20.3 ± 21.3 |
| Follow-up, y (mean ± SD) | 4.8 ± 2.3 |
| Metastasis, n (%) | |
| Not present | 22 (59.5) |
| Present | 15 (40.5) |
| Survival status, n (%) | |
| Alive | 17 (45.9) |
| Death due to metastasis | 15 (40.5) |
| Death due to other causes | 5 (13.5) |
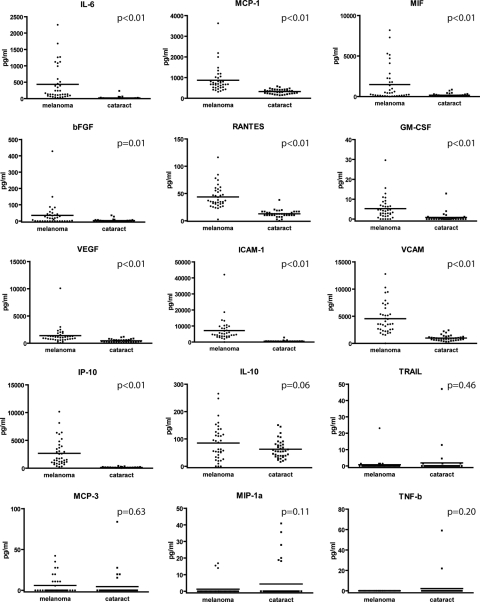
In comparison to eyes with cataract, eyes with uveal melanoma showed increased levels of almost all cytokines tested: IL-6 (P < 0.01), MCP-1 (P < 0.01), MIF (P < 0.01), bFGF (P = 0.01), RANTES (P < 0.01), GM-CSF (P < 0.01), VEGF (P < 0.01), ICAM-1 (P < 0.01), VCAM-1 (P < 0.01), and IP-10 (P < 0.01). The levels of TRAIL (P = 0.46), MCP-3 (P = 0.63), and MIP-1α (P = 0.11) were not significantly decreased in eyes with uveal melanoma compared with the levels of those cytokines in the control eyes (Fig. 1). TNF-β was expressed in only two samples from control patients.
Figure 1.
Expression of 15 different cytokines in AqH of eyes with uveal melanoma and control eyes with cataract. For most cytokines, the concentration of the cytokine was significantly higher in eyes with uveal melanoma. Horizontal line: the mean in each group.
Since there was a significant difference in age between the patients with uveal melanoma and the control group, we applied linear regression to study whether uveal melanoma or the difference in age between both groups contributed to the observed significant differences in expression of cytokine levels. After analysis, the presence of a tumor in the eye, not age, was found to determine the higher level of expression of cytokines (data not shown).
Mutual Correlation of Cytokines
Some cytokines correlated with each other—that is, patients who had a high level of a certain cytokine in their AqH often also had high levels of other cytokines and vice versa. GM-CSF, RANTES, VCAM-1, VEGF, and IL-6 constituted one group with correlations, as did MCP-1, MIP-1α, and MCP-3. This result indicates that, in uveal melanoma, an inflammatory state can exist with a coexpression of a cluster of cytokines (Supplementary Table S1, http://www.iovs.org/cgi/content/full/51/11/5445/DC1).
Comparison of Cytokine Concentrations with Clinical and Histopathologic Characteristics
We hypothesized that eyes with uveal melanoma showing the so-called inflammatory phenotype7 would have higher levels of inflammatory cytokines in the AqH than uveal-melanoma–containing eyes without this phenotype. Since we had investigated this set of uveal melanomas in a prior study,7 we knew that these tumors had different characteristics based on known prognostic factors. Therefore, we compared cytokine levels with a series of markers that either determined the inflammatory phenotype, such as the presence of macrophages and HLA expression (HCA2, HC10, and HLA-DR), or variables that specifically determined prognosis, such as the presence of ciliary body ingrowth, microvascular density, tumor size according to the pTNM classification (AJCC, 7th edition),24 epithelioid cells, and monosomy of chromosome 3.
Macrophages in Uveal Melanoma.
The presence of macrophages was determined with standard CD68 IHC staining on paraffin-embedded sections in all 37 patients: 32% of the tumors had a low amount of macrophages according to the classification of Mäkitie et al.,12 41% had a medium amount, and 27% had a high amount (Table 1). From 30 of the 37 patients, sufficient AqH was available to perform IF on the markers CD68 and CD163 to determine the number of M2 macrophages, which express both antigens. IF was performed by analyzing the sections with image-calculation software, after visualization with confocal microscopy. The results on CD68+ cells obtained by binary analysis with the image-calculation software of the IF staining were in accordance with the CD68 IHC staining (ANOVA test, P < 0.01).
The density of CD163+ M2 macrophages was associated with the quantity of CD68+ macrophages, as determined by IHC staining (ANOVA test, P < 0.01) and also correlated significantly with CD68+ macrophage staining with IF (Spearman's ρ = 0.91, P < 0.01).
Cytokine levels were compared with the densities of CD68+, CD163+, and CD68+/CD163+ macrophages. A correlation was observed between CD68+ density and the level of the cytokine MCP-3 (P = 0.03 and P = 0.02, respectively; Table 2, Fig. 2). None of the other cytokines or chemokines showed a correlation with the intratumoral density of M2-type macrophages.
Table 2.
Correlation between Cytokines and CD68+ Macrophages or CD68+ CD163+ M2-type Macrophages
| Cytokines |
Test Applied | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | IL-10 | bFGF | GM-CSF | IP-10 | MCP-1 | MIP-1α | RANTES | VEGF | MCP-3 | MIF | TRAIL | ICAM-1 | VCAM-1 | ||
| CD68+ macrophages with IHC | 0.33 | 0.73 | 0.60 | 0.22 | 0.32 | 0.23 | 0.41 | 0.06 | 0.25 | 0.03 | 0.54 | 0.11 | 0.41 | 0.61 | ANOVA |
| CD68+ macrophages with IF | 0.80 | 0.09 | 0.99 | 0.08 | 0.40 | 0.68 | 0.26 | 0.41 | 0.14 | 0.02 | 0.97 | 0.42 | 0.08 | 0.33 | Spearman's correlation |
| CD68+ CD163+ M2 type macrophages IF | 0.77 | 0.13 | 0.67 | 0.13 | 0.22 | 0.79 | 0.68 | 0.48 | 0.14 | 0.07 | 0.73 | 0.62 | 0.18 | 0.61 | Spearman's correlation |
Correlations between cytokines and CD68+ macrophages were determined using IHC in 37 cases and IF staining in 30 cases. Macrophages determined by IHC were categorized as low, medium, and high concentrations, whereas with IF, the macrophages were scored as the area (pixels) that stained positive. P-values shown in bold are significant.
Figure 2.
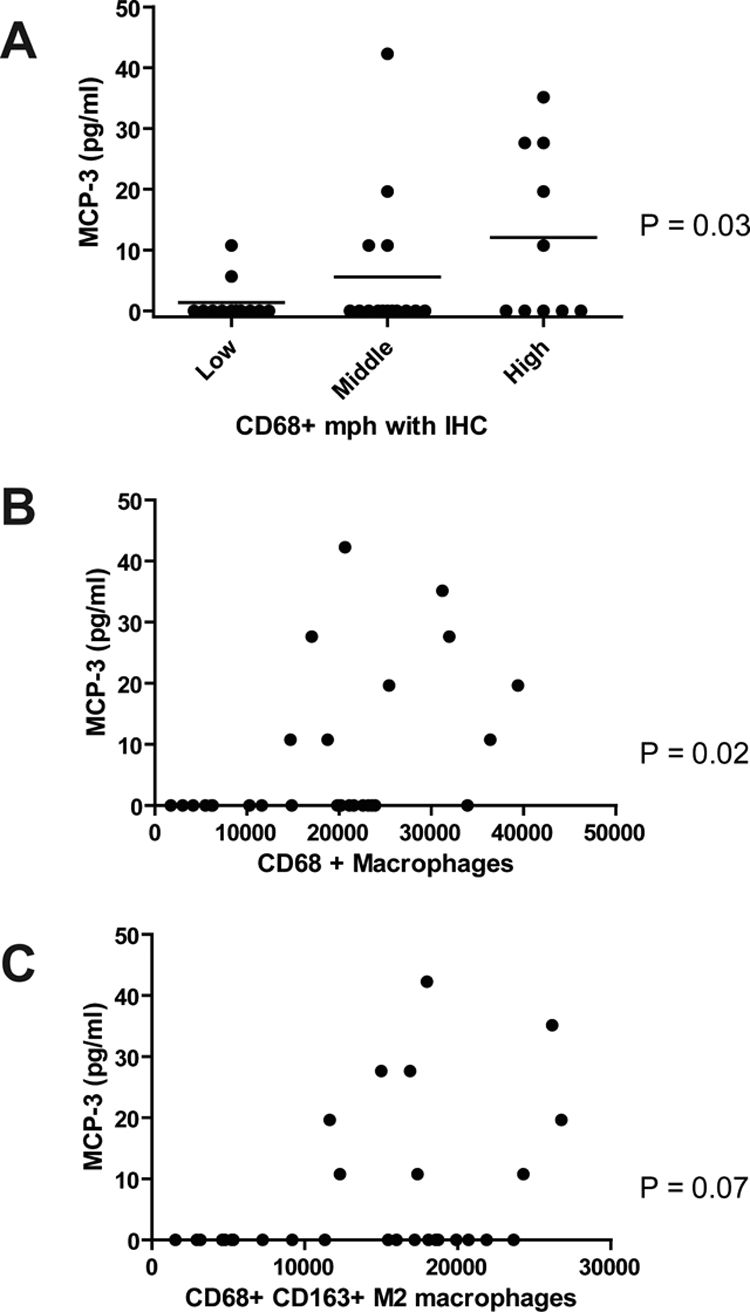
Expression of the cytokine MCP-3 versus the three groups of CD68 IHC (A, horizontal line represents the mean) and correlation with CD68 IF (B) and CD68 and CD163 IF (C) staining. Significant associations or correlations were observed between both types of CD68 staining and MCP-3.
Other Prognostic Parameters in Uveal Melanoma.
We wondered whether there was a correlation between the presence of any of the cytokines and specific prognostic parameters (Table 3). Since TNF-β was expressed in only two patients, it was not included in the analysis. Correlations were observed between some individual cytokines and a few prognostic factors: increased IL-6 and MIF expression was found to correlate with ciliary body involvement (both P = 0.04) and the presence of the epithelioid cell type (P = 0.03). The presence of TRAIL and GM-CSF was associated with a higher HLA class II expression (HLA-DR staining), and, with regard to tumor size, a higher RANTES expression correlated with a larger basal diameter (P = 0.03) and the presence of measurable VEGF with a higher prominence (P = 0.02).
Table 3.
Correlations and Associations between Cytokines and Clinical and Histopathologic Parameters and between Cytokines and Survival
| Cytokines |
Test Applied | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | IL-10 | bFGF | GM-CSF | IP-10 | MCP-1 | MIP-1α | RANTES | VEGF | MCP-3 | MIF | TRAIL | ICAM-1 | VCAM-1 | ||
| Ciliary body ingrowth | 0.04 | 0.60 | 0.13 | 0.05 | 0.81 | 0.26 | 0.08 | 0.05 | 0.21 | 0.30 | 0.04 | 0.22 | 0.16 | 0.11 | t-Test |
| Largest basal diameter | 0.13 | 0.39 | 0.31 | 0.06 | 0.44 | 0.43 | 0.32 | 0.03 | 0.91 | 0.91 | 0.40 | 0.84 | 0.78 | 0.86 | Spearman's correlation |
| Prominence | 0.67 | 0.63 | 0.38 | 0.55 | 0.94 | 0.43 | 0.74 | 0.81 | 0.02 | 0.58 | 0.26 | 0.73 | 0.74 | 0.83 | Spearman's correlation |
| Tumor size pTNM (7th edition) | 0.71 | 0.40 | 0.10 | 0.98 | 0.48 | 0.54 | 0.88 | 0.50 | 0.77 | 0.99 | 0.31 | 0.92 | 0.99 | 0.51 | ANOVA |
| Presence of epithelioid cell type | 0.77 | 0.47 | 0.89 | 0.28 | 0.47 | 0.91 | 0.69 | 0.96 | 0.80 | 0.02 | 0.86 | 0.22 | 0.36 | 0.96 | t-Test |
| Microvascular density | 0.21 | 0.69 | 0.42 | 0.82 | 0.42 | 0.33 | 0.15 | 0.08 | 0.81 | 0.08 | 0.79 | 0.39 | 0.33 | 0.70 | ANOVA |
| Presence of monosomy 3 | 0.48 | 0.40 | 0.63 | 0.52 | 0.46 | 0.50 | 0.08 | 0.31 | 0.77 | 0.13 | 0.94 | 0.22 | 0.11 | 0.30 | t-Test |
| HLA class I with HC10 staining | 0.72 | 0.40 | 0.13 | 0.97 | 0.08 | 0.93 | 0.19 | 0.64 | 0.56 | 0.59 | 0.20 | 0.18 | 0.15 | 0.59 | Spearman's correlation |
| HLA class I with HCA2 staining | 0.91 | 0.39 | 0.74 | 0.78 | 0.56 | 0.39 | 0.85 | 0.60 | 0.15 | 1.00 | 0.49 | 0.13 | 0.12 | 0.08 | Spearman's correlation |
| HLA Class II with HLA-DR staining | 0.26 | 0.97 | 0.23 | 0.03 | 0.80 | 0.55 | 0.46 | 0.11 | 0.75 | 0.03 | 0.95 | 0.01 | 0.27 | 0.78 | Spearman's correlation |
| Survival analysis | 0.21 | 0.92 | 0.47 | 0.99 | 0.16 | 0.30 | 0.40 | 0.06 | 0.40 | 0.71 | 0.31 | 0.65 | 0.39 | 0.94 | Cox regression |
P-values shown in bold indicate a significant correlation.
Predictive Value of Cytokines for Survival
We also studied whether cytokine levels were predictive of survival. With Cox regression, we determined the hazard ratio for a decreased survival in the presence of increased expression of any of the cytokines. None of the cytokines was predictive of survival (Table 3).
Discussion
Other studies have indicated alterations in the blood–aqueous barrier in eyes containing uveal melanoma. Küchle et al.,25 using a laser flare cell meter, showed that eyes containing uveal melanoma had an increased aqueous flare compared with eyes containing a benign nevus and with normal eyes. An increased flare correlated with higher tumor height, serous retinal detachment, tumor necrosis, and lymphocytic tumor infiltration. Proteomic analysis of AqH showed a difference in protein constitution between AqH from melanoma-containing eyes and eyes undergoing surgery for cataract,26 but the identity of the precise proteins is as yet unknown. Therefore, changes in the AqH take place when a uveal melanoma develops in the eye. We demonstrated in the current study that, in uveal melanoma-containing eyes, several chemotactic cytokines are highly expressed in the AqH, when compared with their expression in control eyes.
Many studies have elaborated on the expression of cytokines in the AqH of eyes with uveitis, which primarily is an inflammatory disease. In idiopathic acute anterior uveitis, AqH shows increased levels of various proinflammatory cytokines such as IL-6, MCP-1 (CCL2), and IFN-γ.27 Other cytokines, such as TGFβ2 and CXCL12, have been found to be decreased in cases of severe inflammation.28 In a study using a multiplex immunoassay, van Kooij et al.29 not only observed increased levels of IL-6 and -8 in the AqH of uveitis patients, but also of soluble vascular cell adhesion molecule (sVCAM), IP-10, and RANTES. Several leukocyte-attracting chemokines have been found specifically in acute inflammation: IL-8 (CXCL8, a recruiter of neutrophils), MCP-1 (CCL2), and MIP-1β (CCL4), both of which are known to attract monocytes.30 In the present study many of these uveitis-related cytokines were also increased in the AqH of uveal melanoma eyes.
The presence of intraocular lymphoma is associated with specific cytokine levels that differ in concentration from those in uveitis. In lymphoma, the ratio of IL-6 to IL-10 is lower than 1.0, whereas in uveitis it is vice versa. In our cases of uveal melanoma, on average, the concentration of IL-6 was higher than that of IL-10, indicating that the ratio observed with lymphoma is not the general rule in malignancies. The cytokine pattern in uveal melanoma is an indicator of inflammation, but it is not specific.
Although, in general, the AqH of uveal melanoma–containing eyes showed a pattern corresponding to inflammation, quite a lot of variation was observed in the cytokine levels. When correlating individual cytokine levels with clinical parameters and prognostic parameters, including those that are part of the inflammatory phenotype, we noticed that almost no cytokine was associated with any of those. The occurrence of some sporadic associations may have been the result of our setting P = 0.05 for significant associations. Since we tested 15 cytokines in our set, it is likely that a significant association would have been found, without a biologically relevant explanation. Similar to Missotten et al.,16 we found a correlation of the cytokine VEGF with tumor prominence. We recently showed that in uveal melanoma, VEGF is upregulated by ischemia.31 It may be that thicker tumors have less oxygenation, leading to an increased VEGF production. One could doubt whether the levels of cytokines in the AqH are a good representation of the presence of cytokines in the posterior eye. However, Boyd et al.15 showed good correlation between VEGF levels in AqH and vitreous.
Nevertheless, some of the strong associations found in our cytokine set have also been described for other cell types. We found that increased levels of GM-CSF and TRAIL correlated with HLA-DR expression. GM-CSF is one of the stimulating cytokines for myeloid cells, such as macrophages, causing them to express MHC class II (HLA-DR) at a high level.32 This effect helps to stimulate antigen presentation to CD4+ T-helper cells and TRAIL secretion, killing hostile cells by apoptosis.33
Furthermore, we found that the presence of the proinflammatory cytokine IL-6 correlated with GM-CSF. Recently, an article has been published on GM-CSF production by activated T cells, leading to recruitment of macrophages producing IL-6.34 This proinflammatory cytokine attracts more inflammatory cells, leading to a self-perpetuating macrophage–T-cell loop in inflammatory environments. We know from a previous study in our laboratory that macrophage infiltration, HLA expression, and CD3+ T cell lymphocytes are associated with decreased survival.11 We found that the cytokines are not associated with HLA expression and macrophage infiltration. It is known that T lymphocytes are capable of producing cytokines35,36; therefore, the levels of cytokines could be representative of T-lymphocyte infiltration.
As chemotactic attraction of immune cells into the eye is mediated by cytokines, we expected that the level of infiltrating macrophages and the specific tumor-promoting subtype of M2 macrophages would be associated with a higher expression of the chemotactic cytokines in the AqH. We found no correlation between the amount of cytokines expressed in the AqH and the number and subtype of macrophages. Apparently, the inflammatory cytokines found in the AqH do not determine the macrophage infiltration in a tumor or vice versa.
An explanation of our finding that cytokines are not representative of the presence of (M2-type) macrophages and the inflammatory phenotype of the tumor can be that not the tumor but surrounding tissue secretes the cytokines in the AqH (e.g., the retinal pigment cells or leukocytes),37,38 due to the inflammatory process. We have observed that both tumor cells and the surrounding tissue can produce VEGF, leading to high amounts in the eye.16 Apparently, the presence of melanoma leads to the production of an abundance of proinflammatory cytokines that promote the tumor's survival by creating a new microenvironment that clearly differs between a diseased eye and a cataractous eye. This expression would be a point of interest from a therapeutic viewpoint, since targeting the production of cytokines could then become a treatment for the tumor in the eye.
Another explanation of the absence of correlations between cytokines and leukocytes could be that macrophages and other immune cells do not require cytokines to be recruited into the eye. Macrophages and other cells of the innate system may also respond to ischemic or necrotic tumor cells, leading to infiltration macrophages, for example, by HIF-1α triggering.39
In our study of whether a higher expression of cytokines is associated with a better or worse survival, we observed no prognostic value of individual cytokines in this dataset. We were not able to identify a specific set of cytokines that would determine the bad or good prognosis of a tumor. We will therefore continue to rely on obtaining tumor tissue to determine important prognostic factors such as monosomy of chromosome 3 or mRNA gene expression analysis.4,40
Supplementary Material
Footnotes
Supported by Netherlands Organization for Scientific Research (NWO) Mozaiek Grant 017.003.059, Stichting Blindenhulp, and Stichtung Nederlands Oogheelkundig Onderzoek.
Disclosure: L.V. Ly, None; I.H.G. Bronkhorst, None; E. van Beelen, None; J. Vrolijk, None; A.W. Taylor, None; M. Versluis, None; G.P.M. Luyten, None; M.J. Jager, None
References
- 1. Gragoudas ES, Egan KM, Seddon JM, et al. Survival of patients with metastases from uveal melanoma. Ophthalmology. 1991;98:383–389 [DOI] [PubMed] [Google Scholar]
- 2. Virgili G, Gatta G, Ciccolallo L, et al. Survival in patients with uveal melanoma in Europe. Arch Ophthalmol. 2008;126:1413–1418 [DOI] [PubMed] [Google Scholar]
- 3. Mooy CM, De Jong PT. Prognostic parameters in uveal melanoma: a review. Surv Ophthalmol. 1996;41:215–228 [DOI] [PubMed] [Google Scholar]
- 4. Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004;64:7205–7209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prescher G, Bornfeld N, Hirche H, et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347:1222–1225 [DOI] [PubMed] [Google Scholar]
- 6. de la Cruz PO, Jr., Specht CS, McLean IW. Lymphocytic infiltration in uveal malignant melanoma. Cancer. 1990;65:112–115 [DOI] [PubMed] [Google Scholar]
- 7. Maat W, Ly LV, Jordanova ES, et al. Monosomy of chromosome 3 and an inflammatory phenotype occur together in uveal melanoma. Invest Ophthalmol Vis Sci. 2008;49:505–510 [DOI] [PubMed] [Google Scholar]
- 8. Blom DJ, Luyten GP, Mooy C, et al. Human leukocyte antigen class I expression: marker of poor prognosis in uveal melanoma. Invest Ophthalmol Vis Sci. 1997;38:1865–1872 [PubMed] [Google Scholar]
- 9. Dithmar S, Crowder J, Jager MJ, Vigniswaran N, Grossniklaus HE. HLA class I antigen expression correlates with histological cell type in uveal melanoma (in German). Ophthalmologe. 2002;99:625–628 [DOI] [PubMed] [Google Scholar]
- 10. Ericsson C, Seregard S, Bartolazzi A, et al. Association of HLA class I and class II antigen expression and mortality in uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42:2153–2156 [PubMed] [Google Scholar]
- 11. Waard-Siebinga I, Hilders CG, Hansen BE, van Delft JL, Jager MJ. HLA expression and tumor-infiltrating immune cells in uveal melanoma. Graefes Arch Clin Exp Ophthalmol. 1996;234:34–42 [DOI] [PubMed] [Google Scholar]
- 12. Mäkitie T, Summanen P, Tarkkanen A, Kivela T. Tumor-infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42:1414–1421 [PubMed] [Google Scholar]
- 13. Toivonen P, Makitie T, Kujala E, Kivela T. Microcirculation and tumor-infiltrating macrophages in choroidal and ciliary body melanoma and corresponding metastases. Invest Ophthalmol Vis Sci. 2004;45:1–6 [DOI] [PubMed] [Google Scholar]
- 14. Campochiaro PA. Ocular versus extraocular neovascularization: mirror images or vague resemblances. Invest Ophthalmol Vis Sci. 2006;47:462–474 [DOI] [PubMed] [Google Scholar]
- 15. Boyd SR, Tan D, Bunce C, et al. Vascular endothelial growth factor is elevated in ocular fluids of eyes harbouring uveal melanoma: identification of a potential therapeutic window. Br J Ophthalmol. 2002;86:448–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Missotten GS, Notting IC, Schlingemann RO, et al. Vascular endothelial growth factor a in eyes with uveal melanoma. Arch Ophthalmol. 2006;124:1428–1434 [DOI] [PubMed] [Google Scholar]
- 17. Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–727 [DOI] [PubMed] [Google Scholar]
- 18. Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686 [DOI] [PubMed] [Google Scholar]
- 19. Shabo I, Stal O, Olsson H, Dore S, Svanvik J. Breast cancer expression of CD163, a macrophage scavenger receptor, is related to early distant recurrence and reduced patient survival. Int J Cancer. 2008;123:780–786 [DOI] [PubMed] [Google Scholar]
- 20. Midena E, Bonaldi L, Parrozzani R, et al. In vivo detection of monosomy 3 in eyes with medium-sized uveal melanoma using transscleral fine needle aspiration biopsy. Eur J Ophthalmol. 2006;16:422–425 [DOI] [PubMed] [Google Scholar]
- 21. Shields CL, Materin MA, Teixeira L, et al. Small choroidal melanoma with chromosome 3 monosomy on fine-needle aspiration biopsy. Ophthalmology. 2007;114:1919–1924 [DOI] [PubMed] [Google Scholar]
- 22. Cordell JL, Falini B, Erber WN, et al. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984;32:219–229 [DOI] [PubMed] [Google Scholar]
- 23. Maat W, Jordanova ES, Zelderen-Bhola SL, et al. The heterogeneous distribution of monosomy 3 in uveal melanomas: implications for prognostication based on fine-needle aspiration biopsies. Arch Pathol Lab Med. 2007;131:91–96 [DOI] [PubMed] [Google Scholar]
- 24. Edge SB, Byrd DR, Compton CC, Fritz AG. AJCC Cancer Staging Manual. 7th ed New York: Springer; 2009;547–560 [Google Scholar]
- 25. Kuchle M, Nguyen NX, Naumann GO. Quantitative assessment of the blood-aqueous barrier in human eyes with malignant or benign uveal tumors. Am J Ophthalmol. 1994;117:521–528 [DOI] [PubMed] [Google Scholar]
- 26. Missotten GS, Beijnen JH, Keunen JE, Bonfrer JM. Proteomics in uveal melanoma. Melanoma Res. 2003;13:627–629 [DOI] [PubMed] [Google Scholar]
- 27. Chang JH, McCluskey PJ, Wakefield D. Acute anterior uveitis and HLA-B27. Surv Ophthalmol. 2005;50:364–388 [DOI] [PubMed] [Google Scholar]
- 28. Curnow SJ, Falciani F, Durrani OM, et al. Multiplex bead immunoassay analysis of aqueous humor reveals distinct cytokine profiles in uveitis. Invest Ophthalmol Vis Sci. 2005;46:4251–4259 [DOI] [PubMed] [Google Scholar]
- 29. van Kooij B, Rothova A, Rijkers GT, Groot-Mijnes JD. Distinct cytokine and chemokine profiles in the aqueous of patients with uveitis and cystoid macular edema. Am J Ophthalmol. 2006;142:192–194 [DOI] [PubMed] [Google Scholar]
- 30. Curnow SJ, Murray PI. Inflammatory mediators of uveitis: cytokines and chemokines. Curr Opin Ophthalmol. 2006;17:532–537 [DOI] [PubMed] [Google Scholar]
- 31. El Filali M, Missotten GS, Maat W, et al. Regulation of VEGF-A in uveal melanoma. Invest Ophthalmol Vis Sci. 2010;51:2329–2337 [DOI] [PubMed] [Google Scholar]
- 32. Fleetwood AJ, Dinh H, Cook AD, Hertzog PJ, Hamilton JA. GM-CSF- and M-CSF-dependent macrophage phenotypes display differential dependence on type I interferon signaling. J Leukoc Biol. 2009;86:411–421 [DOI] [PubMed] [Google Scholar]
- 33. Herbeuval JP, Lambert C, Sabido O, et al. Macrophages from cancer patients: analysis of TRAIL, TRAIL receptors, and colon tumor cell apoptosis. J Natl Cancer Inst. 2003;95:611–621 [DOI] [PubMed] [Google Scholar]
- 34. Hashimoto M, Hirota K, Yoshitomi H, et al. Complement drives Th17 cell differentiation and triggers autoimmune arthritis. J Exp Med. 2010;207:1135–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dearman RJ, Moussavi A, Kemeny DM, Kimber I. Contribution of CD4+ and CD8+ T lymphocyte subsets to the cytokine secretion patterns induced in mice during sensitization to contact and respiratory chemical allergens. Immunology. 1996;89:502–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilczynski JR, Tchorzewski H, Glowacka E, et al. Cytokine secretion by decidual lymphocytes in transient hypertension of pregnancy and pre-eclampsia. Mediators Inflamm. 2002;11:105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Elner VM, Burnstine MA, Strieter RM, Kunkel SL, Elner SG. Cell-associated human retinal pigment epithelium interleukin-8 and monocyte chemotactic protein-1: immunochemical and in-situ hybridization analyses. Exp Eye Res. 1997;65:781–789 [DOI] [PubMed] [Google Scholar]
- 38. Kramer M, Monselise Y, Bahar I, et al. Serum cytokine levels in active uveitis and remission. Curr Eye Res. 2007;32:669–675 [DOI] [PubMed] [Google Scholar]
- 39. Lewis CE, De Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res. 2007;67:8429–8432 [DOI] [PubMed] [Google Scholar]
- 40. Tschentscher F, Husing J, Holter T, et al. Tumor classification based on gene expression profiling shows that uveal melanomas with and without monosomy 3 represent two distinct entities. Cancer Res. 2003;63:2578–2584 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.