PKCα is a well known marker of retinal bipolar cells. PKCα-knockout mice show that this enzyme plays a specific role in bipolar cell response.
Abstract
Purpose.
Protein kinase (PKC)-α is abundant in retinal bipolar cells. This study was performed to explore its role in visual processing.
Methods.
PKCα-knockout (Prkca−/−) mice and control animals were examined by using electroretinography (ERG), light microscopy, and immunocytochemistry.
Results.
The Prkca−/− mice showed no signs of retinal degeneration up to 12 months of age, but ERG measurements indicated a decelerated increase in the ascending limb of the scotopic (rod-sensitive) b-wave as well as a delayed return to baseline. These results suggest that PKCα is an important modulator that affects bipolar cell signal transduction and termination. Confocal microscopy of retinal sections showed that PKCα co-localized with calbindin, which indicates a PKCα localization in close proximity to the horizontal cell terminals. In addition, the implicit time of the ERG c-wave originating from the retinal pigment epithelium (RPE) and the recovery of photoreceptors from bleaching conditions were substantially faster in the knockout mice than in the wild-type control animals.
Conclusions.
These results suggest that PKCα is a modulator of rod–bipolar cell function by accelerating glutamate-driven signal transduction and termination. This modulation is of importance in the switch between scotopic and photopic vision. Furthermore, PKCα seems to play a role in RPE function.
The visual process starts with the absorption of light by rods and cones in the retina. The second-order retinal neurons of both types of photoreceptors are the bipolar cells and horizontal cells. Rods are directly connected to ON-bipolar cells, which depolarize after the hyperpolarization of the rod photoreceptors in response to light. Cones transmit their signals to depolarizing ON- and hyperpolarizing OFF-bipolar cells. Rod bipolar cells receive input from rod photoreceptors, as light-dependent stimulation of rods reduces their release of the synaptic transmitter glutamate.1 This deactivates an ON-bipolar cell–specific metabotropic glutamate receptor, mGluR6, on the postsynaptic membrane, leading to depolarization of the bipolar cell2–5 and to an excitation of AII amacrine cells.6 The depolarization in light results from opening of cation channels by an unknown mechanism. The nature of the cation channel has long been elusive, but recent evidence suggests that a TRP-like channel, TRPM1, mediates synaptic function in ON-bipolar cells.7,8 In the dark, mGluR6 activates GαO proteins with high affinity.9–11 Thus, the activation of cation channels results from a reduction of levels of the active form of the GαO subunit, most likely the GαO-1 splice variant. However, the target protein of GαO-1 has not been identified, and the pathway leading to closure of cation channels is unknown.12
In the retina, PKC isoforms are found in bipolar,13 ganglion,14,15 amacrine,16 and pigment epithelial17 cells. PKCα is mainly expressed by rod bipolar cells and, to a lesser extent, by amacrine cells and cones.16,18–22 The role of PKCα, a Ser/Thr-specific protein kinase, in bipolar cell function is not understood. Its activation requires an increase in intracellular free Ca2+ and binding to diacylglycerol (DAG), which leads to a transfer of PKCα to the cell membrane. Immunohistochemical staining of rod bipolar cells has shown that PKCα is not only localized in the cell body but also in the dendrites and the synaptic terminals.18,23,24 Activation of PKCα by different phorbolesters potentiates the pool of releasable neurotransmitter vesicles at the synaptic site of the cell by regulation of the actin cytoskeleton.24–29 At the dendritic site, PKCα activation is known to reduce GABA-A–induced currents and to increase the run-down of GABA-A–induced currents.30–32 PKCα may provide a link between the stimulation of the mGluR6 receptor and the modulation of GABA-A receptors at the dendritic site of the bipolar cell.33 To establish the light-dependent physiological role of PKCα in bipolar cells, we investigated the consequence of PKCα deletion in knockout mice by means of immunocytochemistry and by ERG measurements.
Methods
Mice
PKC Mutant Mice.
The knockout mice investigated in this study were originally generated to clarify the role of diacylglycerol-sensitive PKC isoforms such as PKCα in insulin-dependent glucose transport.34 Knockout mice were genotyped by Southern blot analysis and PCR, as described elsewhere.34
Experimental Mice.
Heterozygous Prkca−/+ chimeric germ line–transmitting mice, crossed into wild-type 129/SV mice, and male littermate offspring, homozygous for either knockout (−/−) or full retention (+/+) of the PKCα gene, were selected for experimental use. Note that the reporter gene construct and embryonic stem (ES) cells used for targeting were derived from the 129/SV mice; thus, crossing chimeras into the 129/SV mice yielded F1 heterozygous offspring on a pure 129/SV background. Consequently, the wild-type Prkca+/+ and the Prkca−/− mice were genetically similar, except for the targeted gene.
The ERG measurements were subsequently performed in two groups of animals. For the basic ERG, 11 knockout mice were compared to 10 control animals. Some additional electroretinographic examinations were not performed in all animals. The respective number of animals investigated is indicated in the description of each subexperiment. All animals were between 6 and 11 weeks old at the time of examination. Some control and knockout animals were recorded at the ages of 4 and 6 months, to prove that the changes found were persistent, which was the case. The mice were held in an animal laboratory in a 12-hour-dark/12-hour-light cycle. All experiments were approved by the local animal use committee and were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the Policies on the Use of Animals and Humans in Neuroscience Research.
Electroretinography
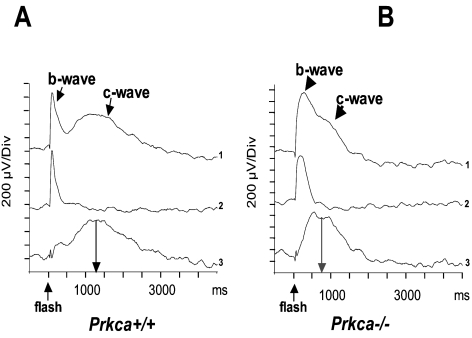
The methodology used to record the ERG is described elsewhere.35 The mice were kept in darkness for at least 2 hours before examination. The pupils were dilated by tropicamide 0.5% and atropine 1%. Xylazine 15 mg/kg body weight and ketamine 100 mg/kg were injected subcutaneously for anesthesia. A monopolar contact lens electrode served as the recording electrode. Silver-needle electrodes, fixed subcutaneously, served as the reference and ground. While the ERG was recorded, the mouse was placed in a commercially available Ganzfeld bowl (Toennies Multiliner Vision, Höchberg, Germany). The signal was amplified by 10,000 with a band-pass filter, in the range of 1 to 300 Hz. A high-pass of 1 Hz is the standard used in our laboratory, as the frequency content of the ERG-b-wave is normally more than 10 Hz. With the high-pass filter set to lower levels, the b-wave changes observed in the Prkca−/− mice were even more pronounced (see Fig. 4, c-wave recording, high-pass filter of 0.08 Hz).
Figure 4.
ERG c-wave recordings in Prkca+/+ and Prkca−/− mice. (A, B) Trace 1 is the original c-wave recording with a long-duration stimulus (200 ms). Trace 2, a short-duration stimulus (1 ms) evokes only a b-wave, but no c-wave. Subtracting traces 2 from 1 results in an isolated c-wave. The implicit time in knockout mice was significantly shorter than that in the control mice, whereas the amplitude did not show a significant difference.
Oscillatory potentials (OPs) were obtained by band-pass filtering from 100 to 300 Hz. In the dark-adapted state, a flash series consisting of eight steps starting at −4.0 log cds · m−2 and reached 0.48 log cds · m−2. The first two responses were averaged three times (flash interval: 2 seconds), responses three and four were averaged twice, and the final four responses were not averaged. Subsequently, the OPs were recorded (0.4 log cds · m−2, two responses, flash interval 15 seconds, rejection of the first response).
After the c-wave (see below) was recorded, the background light (1.5 log cds · m−2) was turned on, and the photopic ERG was recorded (1.2 and 1.6 log cds · m−2, average of 10 recordings at 1.5 Hz). Subsequently, the animals were further light adapted for 10 minutes and the photopic ERG was recorded again.
The b-wave amplitude was determined from a-wave trough to b-wave peak, behind the last prominent OP. As the cone ERG is dominated by distinct OPs, a postrecording smoothing procedure was applied to assess amplitudes and implicit times.
ERG c-Wave
c-Wave recordings were taken with mice in the dark-adapted state, immediately after the scotopic ERG. A green light-emitting diode (LED) mounted in a Kooijman electrode (Roland Consult, Brandenburg, Germany) and located directly in front of the recording electrode served as the stimulus. The stimulus duration was 200 ms, and the stimulus energy was 1.8 log cds · m−2. As DC recording produced too many artifacts, a band-pass filter between 0.08 and 20 Hz was chosen. Beside this long-duration stimulus a brief LED stimulus (1 ms) was also delivered. This kind of stimulus evokes only a b-wave, not a c-wave. By subtraction of both responses the c-wave could be better identified and analyzed.
ERG a-Wave Analysis
The high-intensity stimuli suitable for the analysis of the dark adapted a-wave were generated in the Ganzfeld globe, which was equipped with a photoflash for this purpose (Ganzfeld; Toennies Multiliner Vision). This equipment provides flashes ranging in strength from 0.4 to 2.7 log cds · m−2. Five knockout and four control animals were dark adapted for at least 2 hours. Six flash energies were applied ranging from 1.5 to 2.7 log cds · m−2. At higher energies, the scotopic a-wave amplitude saturated. To prevent rod adaptation, no averaging was performed. The interstimulus interval was at least 2 minutes in duration. The analysis of the a-wave data is related to the Lamb-Pugh model of phototransduction,36–40 rendering Rmax as the saturated amplitude and S as the amplification constant of the rod photoreceptor.
Dark Adaptation
The ability to adapt the visual system to low light levels (dark adaptation) is mainly based on retinal processing.41 Adaptation from a rod-desensitizing background light can therefore be estimated by looking at the recovery of ERG responses. Since adaptation is due to receptoral and postreceptoral processes, b- and a-wave recovery was investigated. After a dark adaptation of 2 hours, an ERG response at –2.0 log cds · m−2 was recorded. Subsequently, the mice were light adapted for 5 minutes at 1.85 log cds · m−2. To investigate the b-wave recovery, we delivered the −2.0 log cds · m−2 stimulus 1 minute after turning off the light and every 2 minutes afterward. As soon as a b-wave could be identified, the amplitude was plotted up to 19 minutes after the background light was turned off. The identification of the first detectable b-wave was performed by two independent investigators. Twenty minutes after the light exposure, the scotopic mixed rod–cone response (stimulus 0.48 log cds · m−2) was recorded and compared between knockout (nine animals) and control mice (nine animals).
The investigation of the a-wave recovery as a parameter of photoreceptor adaptation was accomplished in an independent step. After dark adaptation, a 1.95 log cds · m−2 response was recorded. The bleaching was the same as with the b-wave (1.85 log cds · m−2 for 5 minutes). Subsequently, the 1.95 log cds · m−2 stimulus was delivered immediately after the light was turned off and every 3 minutes thereafter up to 21 minutes. This longer interstimulus interval was chosen first to minimize rebleaching and second because the potential was clearly visible at the first measurement. For a- and b-wave recordings, statistical analysis was performed with repeated-measures ANOVA. In the case of the b-wave, the analysis started with the first detectable b-wave. The mixed cone–rod response recorded after 20 minutes was tested by the two-tailed t-test.
Preparation of Tissue and Immunocytochemistry
Adult wild-type (C57BL/6) and Prkca−/− mice (129/SV) were deeply anesthetized by intraperitoneal injection of ketamine and xylazine. The procedures were approved by the local animal care committees and are in accordance with the German law for animal experiments (Tierschutzgesetz) and the ARVO Statement. The eyes were enucleated and transferred into a Petri dish containing carboxygenated Ames medium (Sigma, Taufkirchen, Germany). After removal of the cornea and lens, the posterior eyecups were immersion fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4) for 15 minutes. After fixation, the retinas were cryoprotected in 30% sucrose, embedded in gelatin, and sectioned vertically (15 μm) with a cryostat. After several washes in PB, vertical sections were incubated in a solution containing 10% normal goat serum (NGS), 1% bovine serum albumin (BSA), and 0.5% Triton X-100 in PB for 1 hour, and they were subsequently transferred for an overnight incubation to a solution containing primary rabbit anti-calbindin D28K (Swant, Bellinzona, Switzerland), rabbit anti-mGluR6 (Neuromics, Edina, MN), rabbit anti-Pcp242 (Bradley M. Denker, Harvard Medical School, Boston, MA), mouse anti-kinesin II (clone K2.4; Covance Research Products Inc., Berkeley, CA), and mouse anti-PKCα (MC5 clone; Biodesign International, Saco, Maine), diluted 1:500 and 1:100, respectively, in 3% NGS, 1% BSA, and 0.5% Triton X-100 in PB. Finally, a mixture of secondary antibodies, dissolved in 1% NGS, 1% BSA, and 0.5% Triton X-100 in PB, was applied for 2 hours at room temperature. Secondary antibodies were conjugated to AlexaFluor 488 or AlexaFluor 568 (Molecular Probes, Eugene, OR). Micrographs of fluorescent specimens were taken with a confocal microscope (TCS SL; Leica, Wetzlar, Germany) equipped with an argon (Ar) and a helium-neon (HeNe) laser. Scanning was performed with 40×/1.25 and 63×/1.32 objectives (Plan-Apochromat; Nikon Instruments, Inc., Melville, NY) at a resolution of 1024 × 1024 pixels. Different wavelength scans were performed sequentially to rule out cross talk between the green and red channels.
Results
PKCα and Survival of Retinal Neurons
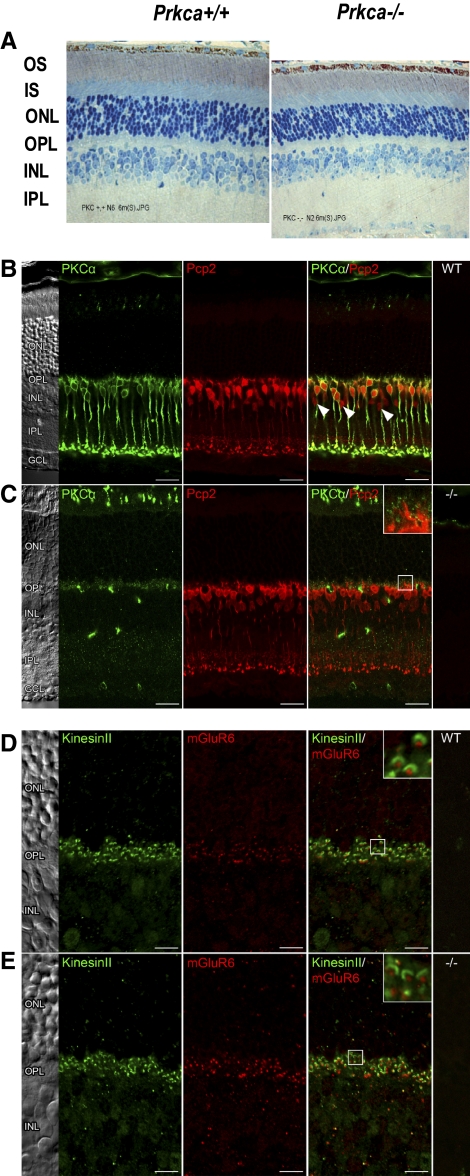
To generate a Prkca−/− mouse, we replaced exon 2 of the PKCα gene with a neomycin cassette by using targeted deletion.34 The Prkca−/− mouse, originally generated to investigate the role of DAG/Ca2+-sensitive PKCα in insulin-stimulated glucose transport in adipocytes and skeletal muscle, is viable and propagates normally, and its life expectancy does not appear to be restricted. As expected, immunocytochemical staining of Prkca−/− retinal sections with anti-PKCα antibody were negative (Fig. 1C), whereas wild-type retina showed prominent staining in bipolar cells and a subset of amacrine cells (Fig. 1B). Regarding the retinal architecture, the thickness of Prkca−/− outer (ONL) and inner (INL) nuclear layers appeared normal (Fig. 1A). However, in some of the examined Prkca−/− animals, mild atrophy was observed in the INL, but staining of the Prkca−/− retinas for Pcp2 (Purkinje cell protein-2) showed no changes in the number of rod ON-bipolar cells in 9- to 12-month-old animals (Fig. 1C). In addition, staining of the Prkca−/− retinas for mGluR6 and kinesin revealed no changes in the synaptic connections between the rods and bipolar cells (Figs. 1D, 1E). The Prkca−/− photoreceptor function was not impeded (a-wave, described later), and the pupillary light reflex of knockout and control mice in the dark, determined by infrared pupillography (AmTech, Dossenheim, Germany), was indistinguishable (data not shown).
Figure 1.
Localization of PKCα in the retina. (A) Light microscopy of 6-month-old retina sections. In some of the 6/6 examined animals, there was some minor atrophy of the IPL. OS, photoreceptor outer segments; IS, inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer. (B) Wild-type retinal sections, and (C) Prkca−/− retinal sections stained with anti-PKCα antibody and with anti-Pcp antibody. Rod bipolar cells in the control mouse (B) stained intensely for PKCα; no labeling was found in the knockout retina (C). However, anti-Pcp2 staining revealed a normal number of rod ON-bipolar cells in the Prkca−/− retinas. (D) Wild-type retinal sections and (E) Prkca−/− retinal sections stained with anti-mGluR6 antibody and with anti-kinesin antibody, to show the synaptic connection between rod and bipolar cells. No differences between the control mouse (D) and the knockout retina (E) indicate no changes in the synaptic connections. Sections (A–D) were from 1- to 9-month-old retinas.
Localization of PKCα
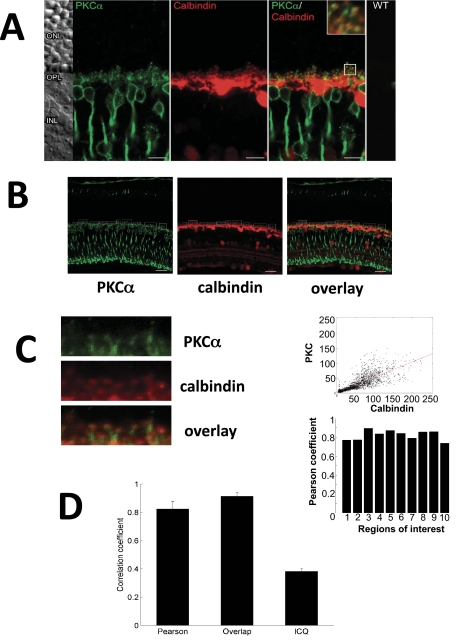
Tissues from PKCα-deficient mice offer the possibility of more detailed analysis of PKCα localization, because they can be used as a negative control that prevents positive staining due to nonspecific binding by antibodies. Vertical sections of retinas from wild-type mice were stained against PKCα (Fig. 2A) and double-labeled with antibodies against calbindin, which is a marker of horizontal cells (Fig. 2B). Analysis of the merged image revealed yellow dots, which indicate the localization of PKCα in horizontal cell synaptic endings. Quantification by pixel analysis (Fig. 2C) showed a significant localization of PKCα close to horizontal cell terminals. In addition, the high degree of localization of PKCα in dendrites with close contact to horizontal cells was quantified by Pearson's correlation coefficient of the merged image analysis (Fig. 2D).
Figure 2.
Co-localization of PKCα and calbindin in the dendrites of ON-bipolar cells. (A) Double staining of a retinal section with antibodies against PKCα and rod antibodies against calbindin (horizontal cell marker). In the merged image, areas of co-localization can be identified (yellow pixels). (B) Selection of margins for pixel analysis. Left: staining against PKCα; middle: staining against calbindin; right: merged image. (C) Left: magnification of an area with merger of the images of anti-PKCα and anti-calbindin staining; top right: analysis of merged pixels on this area; bottom right: Pearson's correlation coefficient for the 10 selected margins. (D) Pearson's correlation coefficient of the merged image analysis. The coefficient of 82% ± 1.6% (overlap coefficient 0.90 ± 0.019; Li's intensity correlation coefficient of 0.38 ± 0.0067) indicates a high degree of localization of PKCα in dendrites with close contact to horizontal cells.
The Prkca−/− Rod Pathway Response
Scotopic ERG b-Wave.
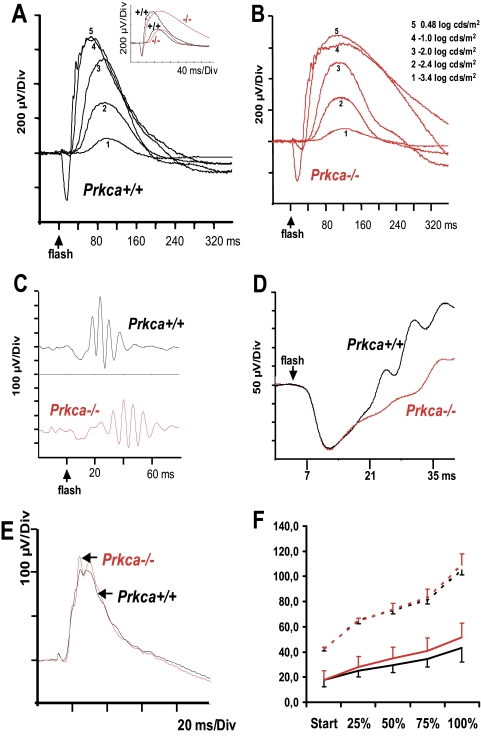
To correlate the loss of PKCα activity with functional defects in the bipolar cells, we first measured the scotopic (dark-adapted) ERG in normal and knockout mice. The scotopic ERG allows the analysis of the rod system where the leading edge of the a-wave arises from the light-induced hyperpolarization of rods, and the b-wave is primarily generated by the depolarization of rod bipolar cells. The scotopic ERGs of knockout and control mice show a typical a- and b-wave pattern (averaged data of 10 control and 11 Prkca−/− mice; Figs. 3A, 3B), with very similar amplitudes at low stimulus levels. However, the time to reach the peak of the b-wave (peak time)—in particular the decline of the b-wave to the baseline—was prolonged in the Prkca−/− retina. This effect was pronounced at high flash intensities (i.e., −1.0, 0, and 0.48 log cds · m−2), but a broadening of the b-wave was observed at all flash intensities.
Figure 3.
Scotopic (A–D) and photopic (E) ERG recordings of the control and Prkca−/− mice. (A) Superimposed control recordings (Prkca+/+), mean of 10, at 5 different flash intensities. (B) Superimposed recordings of Prkca−/− animals, mean of 11. At higher flash energies (traces 4 and 5), the prolongation of the b-wave was clearly visible. (A, inset) Direct comparison of Prkca+/+ (black) and Prkca−/− (red) at −2.4 and 0.48 log cds · m−2, respectively. (C) The OPs, the constitutive part of the ascending limb of the b-wave, were only slightly smaller in the knockout mice, but were considerably delayed. (D) The Prkca+/+ and Prkca−/− a-waves evoked by high flash energies were indistinguishable (flash energy, 2.1 log cds · m−2, mean of four control and five knockout mice). (E) Photopic ERG after 10 minutes of light adaptation (means of 10 Prkca+/+ and 11 Prkca−/−) (F) Course of the normalized latencies (i.e., the same starting point) at different positions of the ascending limb of the b-wave. Rod response (dashed lines): P = 0.71 (repeated-measures ANOVA); rod-cone response (solid line): P = 0.013 (repeated-measures ANOVA). y-axis: latencies in milliseconds.
Superimposing the averaged data recorded at a flash energy of −2.4 log cds · m−2 and 0.48 log cds · m−2 evoking a rod response and a combined rod–cone response, respectively, highlighted the difference in b-wave descent in the knockout and control animals, but also showed an altered ascending limb of the b-wave (Fig. 3A, Fig. 3F, insets) resulting in a different time to peak.
To quantify both, we analyzed the effects of the mainly rod-driven response at −2.4 log cds · m−2 and the combined rod–cone response (0.48 log cds · m−2). The differences between the peak times in the Prkca−/− and Prkca+/+ mice were 22.8 ms (rod response) and 44.2 ms (rod–cone response), respectively (Table 1). The prolonged peak time of the Prkca−/− rod response was mainly due to the later onset of the b-wave (42.4 ms, Prkca−/−, and 28.6 ms, Prkca+/+; Table 2). In contrast, in the combined rod–cone response, the shallower slope of the ascending limb of the b-wave prevailed (Fig. 3F). When the ascending limbs were divided into four proportions (25%, 50%, 75%, and 100%), the respective times to reach these points were significantly different between the Prkca−/− and Prkca+/+ mice, in the case of the 0.48 log cds · m−2 response, but not with the −2.4 log cds · m−2 response (Table 2, Fig. 3F). A comparison of the decline of the b-waves showed that the time to reach 50% of the maximum b-wave amplitude on the way to baseline differed between the Prkca−/− and Prkca+/+ mice by 18.3 ms (rod response) and 121 ms (rod-cone response; Table 1). This result shows that the prolongation of b-wave deactivation prevails at higher stimulus energies, where rods and cones contribute to the response.
Table 1.
Time Characteristics of the b-Wave Decline and b-Wave Peak Time of the Scotopic ERG
| Scotopic b-Wave Amplitude | Time at 50% Half Maximum Amplitude (ms ± SEM) | Peak Time (ms ± SEM) |
|---|---|---|
| Rod response Prkca−/−* | 203.1 ± 3 | 153.3 ± 4 |
| Rod response Prkca+/+* | 184.8 ± 5 | 130.5 ± 4 |
| P value, t-test | 0.0023 | 0.0004 |
| Rod-cone response, −/−† | 293.1 ± 8 | 140.9 ± 6 |
| Rod-cone response, +/+† | 172.1 ± 6 | 96.7 ± 6 |
| P value, t-test | 0.0001 | 0.0001 |
−2.4 log cds · m−2.
0.48 log cds · m−2.
Table 2.
Time Characteristics of the Ascending Limb of the Scotopic ERG b-Wave
| Proportion of Ascending Limb | Rod Response*Prkca−/− | Rod Response*Prkca+/+ | Rod-Cone Response†Prkca−/− | Rod-Cone Response†Prkca+/+ |
|---|---|---|---|---|
| Start | 42.4 ± 7.1 | 28.6 ± 5.3 | 17.7 ± 1.4 | 16.3 ± 1.6 |
| 25% | 64.4 ± 8.2 | 51.1 ± 5.1 | 28.0 ± 2.6 | 23.6 ± 2.4 |
| 50% | 73.2 ± 9.3 | 59.0 ± 5.6 | 34.6 ± 5.2 | 27.9 ± 2.8 |
| 75% | 83.2 ± 10.2 | 67.4 ± 6.5 | 40.8 ± 6.7 | 33.0 ± 3.3 |
| 100% | 109.1 ± 11.4 | 91.3 ± 11.3 | 51.5 ± 8.9 | 42.8 ± 3.9 |
The table lists the latencies (in milliseconds ± SEM) of the ascending limb of the ERG b-wave at different positions. In the rod response, the starting points are significantly different between Prkca−/− and Prkca+/+ (P = 0.0001, t-test). However, there is no significant difference between the latencies of the normalized ascending limb (P = 0.71; repeated-measures ANOVA). In contrast, the latencies at the start of the rod-cone response b-wave (a-wave trough) are not significantly different (P = 0.056, t-test), but the latencies at the different positions of the ascending limb are (P = 0.013, repeated-measures ANOVA).
−2.4 log cds · m−2.
0.48 log cds · m−2.
The OPs (low-amplitude oscillating waves on the rising phase of the b-wave) reflect neuronal synaptic activity in inhibitory feedback pathways initiated by the amacrine cells in the inner retina.43 As the OPs are not independent of bipolar cell function, changes in them may be in part secondary to changes in bipolar cell activity. However, a change in OP may also be related to PKCα activity in amacrine cells, as it can be found at least in some of them. Highlighting the OP by setting the high-pass recording filter to 100 Hz showed that the amplitudes in the Prkca−/− mice were lower and the implicit times were longer than in the control mice (Fig. 3C).
The Scotopic a-Wave.
ERG a-wave analysis was performed in five knockout and four control mice. In figure 3D, the averaged data at one flash energy (2.1 log cds · m−2) are shown. The mean values of log Rmax and log S derived from the ensemble fit (all stimulus strengths integrated) were almost identical (log Rmax, 2.27 ± 0.86 and 2.27 ± 0.95 μV, and log S, 2.04 ± 0.68 and 2.02 ± 0.87 s−2 [td · s−1], Prkca−/− and Prkca+/+ mice, respectively). These data clearly indicate that deletion of PKCα had no effect on the a-wave parameter log S, reflecting normal rod phototransduction, and on RmP3, reflecting unchanged rod disc area. An unchanged a-wave is consistent with the absence of a role of PKCα in the activation of phototransduction.
Alterations in the Prkca−/− c-Wave
The c-wave arises from hyperpolarization of the RPE apical membrane due to a decrease in the subretinal K+ concentration resulting from reduction in the dark current of photoreceptors.44 Figure 4A displays a typical c-wave recording in a control mouse. The top trace displays the original recording, with a long stimulus duration (200 ms), evoking the b- and c-wave. The second recording shows the b-wave evoked by a 1-ms stimulus evoking a b- but no c-wave. To isolate the c-wave, the second trace was subtracted from the first, rendering a c-wave without a b-wave (third curve). In contrast to wild-type recordings, the c-wave recording in the knockout mouse (Fig. 4B, top curve) showed the presence of a fusion-wave containing parts of b- and c-waves caused in part by a prolonged b-wave. Judging from the c-wave parameter, the fusion between the b- and c-wave was also due to a considerably shortened implicit time of the c-wave (Fig. 4B, bottom trace). The implicit time was determined by either the peak of the wave or, in the case of a plateau, by the center of the plateau. The difference in amplitudes did not reach statistical significance. Since the RPE expresses PKCα, and PKCα is known to regulate K+ channels in the RPE,17 the changes in the c-wave in Prkca−/− mice most likely result from an altered regulation of apical K+ conductance in the RPE.
The Cone Pathway Response: Photopic ERG
The photopic ERG was generated in the presence of a rod-desensitizing adapting field (background light, 1.5 log cds · m−2). Under these conditions, the responses of the control and knockout mice were essentially identical (Fig. 3E). The rise in cone b-wave amplitude during light adaptation45 showed no significant difference between control and knockout mice (mean amplitude gain, 58.3 and 53.0 μV, respectively; P = 0.72, repeated-measures ANOVA). These results suggest that deletion of PKCα does not affect the cone pathway.
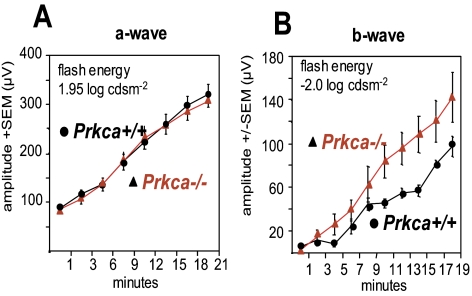
Dark Adaptation
The rod-dominated b-wave of knockout mice evoked by a −2.0 log cds · m−2 stimulus seemed to recover faster from bleaching than in the control mice (Fig. 5B), but this difference did not reach statistical significance (ANOVA, P = 0.06). However, the response to the 0.48 log cds · m−2 stimulus (combined rod–cone response) after 20 minutes of dark adaptation was more distinctive as the b-wave amplitude of the knockout mice at that time was significantly larger (t-test, P = 0.0016, data not shown). In contrast, the recovery of the a-wave amplitude was nearly indistinguishable between the control and knockout mice (Fig. 5A). These results suggest that the faster recovery of Prkca−/− mice is due to postsynaptic mechanisms.
Figure 5.
Recovery of the b- and a-wave amplitudes. (A) The averaged ERG responses in control (black) and Prkca−/− mice (red) after prolonged light exposure (5 minutes, 1.85 log cds · m−2). There was essentially no difference between the a-wave recoveries in the two groups (stimulus energy 2.0 log cds · m−2). (B) The recordings display the b-wave recovery in the dark over time (recorded every 2 minutes, from 1 to 19 minutes, stimulus energy −2.0 log cds · m−2). The b-wave of the Prkca−/− retina seemed to recover faster, but the difference did not reach statistical significance (ANOVA: P = 0.06). Error bars. SEM.
Discussion
Our ERG measurements in mice lacking PKCα revealed that this isoform plays an important role in shaping the rod bipolar cell response to light. Since PKCα was described in previous studies as only an enhancer of bipolar function, the data presented herein shed new light on the role of PKCα in rod bipolar cell function.
The Prkca−/− retinal structure as well as the function of photoreceptors appeared unaltered until the age of 12 months. However, knockout of the PKCα gene led to specific changes in retinal function. Specifically, the rise of the b-wave was slower and the decline was substantially more prolonged in the Prkca−/− mice than in the wild-type mice. Thus, PKCα shapes the bipolar light response by influencing the activation and the termination of the bipolar cell response. This function seems to be specific to the PKCα isoform, as PKCβ-1, also expressed in bipolar cells, is obviously unable to compensate for the lack of PKCα function.16
Regulation of ON-Bipolar Cell Activity
Role of PKCα in the Activation of Rod Bipolar Cells.
Until now, the mechanisms of the ON-bipolar response have not been thoroughly understood.12 However, there is evidence that the cation channel is the TRP channel (transient receptor potential-like cation channel) TRPM1 (TRP subfamily M, member 1).7,8 Light-dependent deactivation of the mGluR6 receptor activates synaptic cation channels via a reduction in GαO expression.9–11,46 Activation of this channel leads to an influx of extracellular Ca2+ into the cell.4 The later onset and the slower increase in the ERG b-wave in Prkca−/− mice, together with the fact that PKCα belongs to the group of Ca2+-dependent PKC isoforms, point to a role in the mGluR6-induced signal transduction pathway. In this role, PKCα would be a cofactor of cGMP, which is crucial for threshold modulation and response amplification.47
It may be that PKCα is part of the signal cascade of the ON-bipolar cell response by phosphorylation of the cation channel. This notion is in accordance with observations made in studies exploring the role of calcineurin in rod bipolar cells. It was concluded that phosphorylation is essential for maintaining the activity of the cation conductance in ON-bipolar cells.4
Proper Termination of Rod Bipolar Cell Activity by PKCα.
At low light intensities at which rod bipolar cells are active, the time of onset of the b-wave was prolonged and the rise of the b-wave was slower in the Prkca−/− mice. At higher stimulus intensities, the less steep slope of the b-wave prevailed. Thus, PKCα is necessary to accelerate the deactivation of the glutamate-driven signal transduction cascade in rod bipolar cells. The desensitization of ON-bipolar cells, which is essential for perceiving light signals on a steady background light, depends on the possible entry of Ca2+ into the cell through the cation channel.5 The time constant for the inactivation after delivery of a saturating light stimulus has been shown to be 60 to 70 ms in mouse retinal slice preparations.48 A prolongation of this inactivation is most probably due to the Ca2+-dependent inactivation mechanism. However, the mode of Ca2+ action on the cation channel is still unknown, and so the possible roles of PKCα in this mechanism remain elusive. Of interest, a second Ca2+-dependent inactivation pathway depressing the mGluR6 cascade with a time constant of approximately 2 minutes has been described,4,5 but, in salamander retinal slices. One can speculate that PKCα plays a role in the fast, but not in the slow, component of the Ca2+-dependent mechanism of mGluR6-pathway inhibition. However, the existence of such a slow component in the mouse retina has still to be established.
Notably, the effect on b-wave termination became more pronounced at higher flash intensities, which indicates that at higher light intensities, PKCα activation leads to a more pronounced inhibition of the rod bipolar response, which in turn minimizes a rod input into photopic vision, in accordance with PKCα-dependent changes in the recovery of the scotopic b-wave after light adaption. The recovery of the scotopic b-wave after light adaptation was faster in the Prkca−/− mice. Thus, in these mice the switch between photopic and scotopic vision is shifted toward higher rod-driven input. The almost identical a-wave adaptation implied that this phenomenon reflects either again a role in the signal cascade leading to activation of bipolar cells or an additional role in the control of transmitter release by bipolar cells.
In summary, two major pathways seem to be of importance in bipolar cell activation and deactivation. One is the basic mechanism by which the ON-bipolar cell is depolarized,7,8 by the opening of TRPM1 channels caused by the reduction of GαO after deactivation of mGluR6. The other one is based on Ca2+-dependent phosphorylation and modulates the sensitivity and time dependence of the first pathway. The second pathway seems to involve dephosphorylation by calcineurin4 or phosphorylation by PKCα. An increased dephosphorylation leads to a slower rise in bipolar cell activation, prolonged bipolar cell shut off, and decreased sensitivity of the mGluR6 pathway. The mechanism of this effect is not clear, because the targets of Ca2+-dependent phosphorylation are not known.
Bipolar cells are known to receive an inhibitory input from horizontal cells by modulation of postsynaptic Cl− conductance through the likely activation of GABA receptors.49 Since we found localization of PKCα in horizontal cell terminals, the prolonged b-wave in the Prkca−/− mice may also have resulted from insufficient inhibitory input by horizontal cells, but this assumption cannot be proven at present. However, this hypothesis is in accordance with previous work suggesting a PKCα-dependent modulation of GABAergic input from horizontal cell currents.30–32
PKCα and the RPE
The generation of the c-wave is also changed in PKCα-deficient mice. Thus, PKCα may also play a role in the regulation of ion channels in the RPE. The c-wave results from a light-induced reduction of subretinal potassium concentration leading to hyperpolarization of the apical membrane of the RPE. PKCα may be a regulator of inward rectifier potassium channels in the RPE, which are believed to be involved in the generation of the c-wave. However, a precise interpretation requires a careful investigation of RPE physiology in PKCα-deficient mice by single-cell recording.
Footnotes
Supported by Deutsche Forschungsgemeinschaft (DFG) Grant Ru 457/7-1.
Disclosure: K. Ruether, None; A. Feigenspan, None; J. Pirngruber, None; M. Leitges, None; W. Baehr, None; O. Strauss, None
References
- 1. Vardi N, Dhingra A, Zhang L, Lyubarsky A, Wang TL, Morigiwa K. Neurochemical organization of the first visual synapse. Keio J Med. 2002;51:154–164 [DOI] [PubMed] [Google Scholar]
- 2. Nomura A, Shigemoto R, Nakamura Y, Okamoto N, Mizuno N, Nakanishi S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994;77:361–369 [DOI] [PubMed] [Google Scholar]
- 3. Vardi N, Duvoisin R, Wu G, Sterling P. Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J Comp Neurol. 2000;423:402–412 [DOI] [PubMed] [Google Scholar]
- 4. Snellman J, Nawy S. Regulation of the retinal bipolar cell mGluR6 pathway by calcineurin. J Neurophysiol. 2002;88:1088–1096 [DOI] [PubMed] [Google Scholar]
- 5. Nawy S. Regulation of the on bipolar cell mGluR6 pathway by Ca2+. J Neurosci. 2000;20:4471–4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghosh KK, Haverkamp S, Wassle H. Glutamate receptors in the rod pathway of the mammalian retina. J Neurosci. 2001;21:8636–8647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shen Y, Heimel JA, Kamermans M, Peachey NS, Gregg RG, Nawy S. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci. 2009;29:6088–6093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koike C, Obara T, Uriu Y, et al. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci U S A. 2010;107:332–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nawy S. The metabotropic receptor mGluR6 may signal through G(o), but not phosphodiesterase, in retinal bipolar cells. J Neurosci. 1999;19:2938–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dhingra A, Lyubarsky A, Jiang M, et al. The light response of ON bipolar neurons requires G[alpha]o. Neurosci. 2000;20:9053–9058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dhingra A, Jiang M, Wang TL, et al. N. Light response of retinal ON bipolar cells requires a specific splice variant of Galpha (o). J Neurosci. 2002;22:4878–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Snellman J, Kaur T, Shen Y, Nawy S. Regulation of ON bipolar cell activity. Prog Retin Eye Res. 2008;27:450–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greferath U, Grünert U, Wässle H. Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J Comp Neurol. 1990;301:433–442 [DOI] [PubMed] [Google Scholar]
- 14. Wang SW, Kim BS, Ding K, et al. Requirement for math5 in the development of retinal ganglion cells. Genes Dev. 2001;15:24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu DY, Zheng JQ, McDonald MA, Chang B, Twiss JL. PKC isozymes in the enhanced regrowth of retinal neurites after optic nerve injury. Invest Ophthalmol Vis Sci. 2003;44:2783–2790 [DOI] [PubMed] [Google Scholar]
- 16. Fyk-Kolodziej B, Cai W, Pourcho RG. Distribution of protein kinase C isoforms in the cat retina. Vis Neurosci. 2002;19:549–562 [DOI] [PubMed] [Google Scholar]
- 17. Strauss O, Rosenthal R, Dey D, et al. Effects of protein kinase C on delayed rectifier K+ channel regulation by tyrosine kinase in rat retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2002;43:1645–1654 [PubMed] [Google Scholar]
- 18. Kolb H, Zhang L, Dekorver L. Differential staining of neurons in the human retina with antibodies to protein kinase C isozymes. Vis Neurosci. 1993;10:341–351 [DOI] [PubMed] [Google Scholar]
- 19. Fukuda K, Saito N, Yamamoto M, Tanaka C. Immunocytochemical localization of the alpha-, beta I-, beta II- and gamma-subspecies of protein kinase C in the monkey visual pathway. Brain Res. 1993;658:155–162 [DOI] [PubMed] [Google Scholar]
- 20. Grunert U, Martin PR, Wassle H. Immunocytochemical analysis of bipolar cells in the macaque monkey retina. J Comp Neurol. 1994;348:607–627 [DOI] [PubMed] [Google Scholar]
- 21. Koistinaho J, Sagar SM. Localization of protein kinase C subspecies in the rabbit retina. Neurosci Lett. 1994;177:15–18 [DOI] [PubMed] [Google Scholar]
- 22. Haverkamp S, Wassle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000;424:1–23 [PubMed] [Google Scholar]
- 23. Wassle H, Boycott BB. Functional architecture of the mammalian retina. Physiol Rev. 1991;71:447–380 [DOI] [PubMed] [Google Scholar]
- 24. Vaquero CF, Velasco A, de la Villa P. Quantitative measurement of protein kinase C immunoreactivity in rod bipolar cells of the goldfish retina. Brain Res. 1997;773:208–212 [DOI] [PubMed] [Google Scholar]
- 25. Berglund K, Midorikawa M, Tachibana M. Increase in the pool size releasable synaptic vesicles by the activation of protein kinase C in goldfish retinal bipolar cells. J Neurosci. 2002;22:4776–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tachibana M. Regulation of transmitter release from retinal bipolar cells. Prog Biophys Mol Biol. 1999;72:109–133 [DOI] [PubMed] [Google Scholar]
- 27. Job C, Lagnado L. Calcium and protein kinase C regulate the actin cytoskeleton in the synaptic terminal of retinal bipolar cells. J Cell Biol. 1998;143:1661–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Minami N, Berglund K, Sakaba T, Kohmoto H, Tachibana M. Potentiation of transmitter release by protein kinase C in goldfish retinal bipolar cells. J Physiol. 1998;512:219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Osborne NN, Broyden NJ, Barnett NL, Morris NJ. Protein kinase C (alpha and beta) immunoreactivity in rabbit and rat retina: effect of phorbol esters and transmitter agonists on immunoreactivity and the translocation of the enzyme from cytosolic to membrane compartments. J Neurochem. 1991;57:594–604 [DOI] [PubMed] [Google Scholar]
- 30. Karschin A, Wassle H. Voltage- and transmitter-gated currents in isolated rod bipolar cells of rat retina. J Neurophysiol. 1990;63:860–876 [DOI] [PubMed] [Google Scholar]
- 31. Gillette MA, Dacheux RF. Protein kinase modulation of GABAA currents in rabbit retinal rod bipolar cells. J Neurophysiol. 1996;76:3070–3086 [DOI] [PubMed] [Google Scholar]
- 32. Feigenspan A, Bormann J. Modulation of GABAC receptors in rat retinal bipolar cells by protein kinase C. J Physiol. 1994;481:325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoffpauir BK, Gleason EL. Activation of mGluR5 modulates GABA(A) receptor function in retinal amacrine cells. J Neurophysiol. 2002;88:1766–1776 [DOI] [PubMed] [Google Scholar]
- 34. Leitges M, Plomann M, Standaert ML, et al. Knockout of PKC alpha enhances insulin signaling through PI3K. Mol Endocrinol. 2002;16:847–858 [DOI] [PubMed] [Google Scholar]
- 35. Ruether K, van de Pol D, Jaissle G, Berger W, Tornow RP, Zrenner E. Retinoschisislike alterations in the mouse eye caused by gene targeting of the Norrie disease gene. Invest Ophthalmol Vis Sci. 1997;38:710–718 [PubMed] [Google Scholar]
- 36. Lamb TD, Pugh EN. A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol. 1992;499:719–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pugh EN, Lamb TD. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta. 1993;1141:111–149 [DOI] [PubMed] [Google Scholar]
- 38. Hood DC, Birch DG. Light adaptation of human rod receptors: the leading edge of the human a-wave and models of rod receptor activity. Vision Res. 1993;33:1605–1618 [DOI] [PubMed] [Google Scholar]
- 39. Hood DC, Birch DG. Rod phototransduction in retinitis pigmentosa: estimation and interpretation of parameters derived from the rod a-wave. Invest Ophthalmol Vis Sci. 1994;35:2948–2961 [PubMed] [Google Scholar]
- 40. Goto Y, Peachey NS, Ziroli NE, et al. Rod phototransduction in transgenic mice expressing a mutant opsin gene. J Opt Soc Am A. 1996;13:577–585 [DOI] [PubMed] [Google Scholar]
- 41. Birch DG. Visual adaptation. In: Kaufmann PL, Alm A. eds. Adler′s Physiology of the Eye. Tenth ed St. Louis: Mosby; 2003;586–600 [Google Scholar]
- 42. Guan J, Luo Y, Denker BM. Purkinje cell protein-2 (Pcp2) stimulates differentiation in PC12 cells by Gbetagamma-mediated activation of Ras and p38 MAPK. Biochem J. 2005;392:389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wachtmeister L. Oscillatory potentials in the retina: what do they reveal. Prog Retina Eye Res. 1998;17:485–521 [DOI] [PubMed] [Google Scholar]
- 44. Steinberg RH. Interactions between the retinal pigment epithelium and the neuronal retina. Doc Ophthalmol. 1985;60:327–346 [DOI] [PubMed] [Google Scholar]
- 45. Peachey NS, Goto Y, al-Ubaidi MR, Naash MI. Properties of the mouse cone-mediated electroretinogram during light adaptation. Neurosci Lett. 1993;162:9–11 [DOI] [PubMed] [Google Scholar]
- 46. Dhingra A, Faurobert E, Dascal N, Sterling P, Vardi N. A retinal-specific regulator of G-protein signaling interacts with Galpha (o) and accelerates an expressed metabotropic glutamate receptor 6 cascade. J Neurosci. 2004;24:5684–5693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nawy S, Jahr CE. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature. 1990;346(6281):269–271 [DOI] [PubMed] [Google Scholar]
- 48. Berntson A, Smith RG, Taylor WR. Postsynaptic calcium feedback between rods and rod bipolar cells in the mouse retina. Vis Neurosci. 2004;21:913–924 [DOI] [PubMed] [Google Scholar]
- 49. Duebel J, Haverkamp S, Schleich W, et al. Two-photon imaging reveals somatodendritic chloride gradient in retinal ON-type bipolar cells expressing the biosensor Clomeleon. Neuron. 2006;49:81–94 [DOI] [PubMed] [Google Scholar]