Abstract
Background:
Fructus Gardeniae, commonly used traditional Chinese medicine (TCM) called Zhizi in chinese, is derived from the dried fruit of Gardenia jasminoides Ellis of the Madder Family. To our knowledge, previously reported analytical methods were not developing for simultaneous determination of geniposide, chlorogenic acid, crocin1, and rutin in Fructus Gardeniae and its processed products of chaozheng pin (CZP) extracts.
Materials and Methods:
In this study, a HPLC method was developed for simultaneous determination four major active components in Fructus Gardeniae and its processed products.
Results:
The contents of geniposide, chlorogenic acid, crocin1, and rutin in Fructus Gardeniae samples analyzed were 2.492 - 4.242%, 0.162 - 0.407%, 0.417 -0.837%, and 0.116 - 0.251%, respectively.
Conclusion:
The developed method can be applied to the intrinsic quality control of Fructus Gardeniae.
Keywords: Fructus gardeniae, high performance liquid Chromatography with diode array detector, simultaneous determination, quality control
INTRODUCTION
Traditional Chinese medicine (TCM) has a robust history with roots dating back thousands of years for the medicinal practice in China and some East Asian countries.[1–3] Fructus Gardeniae, a commonly used TCM called Zhizi in chinese, is derived from the dried fruit of Gardenia jasminoides Ellis of the Madder Family.[4–7] The crude Fructus Gardeniae and its processed products of chaozheng pin (CZP) have been used clinically over 2000 years for purging heat to clear away disturbing in the mind, reducing fever to induce urination, and cooling blood to remove pathogenic heat.[8,9]
In the last decades, Fructus Gardeniae has been extensively investigated in phytochemistry, and the results indicated that geniposide, chlorogenic acid, crocin1, and rutin were the main active components in Fructus Gardeniae.[10] To our knowledge, previously reported analytical methods were not developing for simultaneous determination of geniposide, chlorogenic acid, crocin1, and rutin in Fructus Gardeniae and CZP extracts. In this study, a HPLC method was developed for simultaneous determination four major active components in Fructus Gardeniae distributed in south of China. Figure 1 illustrates the structures of the four major active components their contents are considerable in Fructus Gardeniae.
Figure 1.
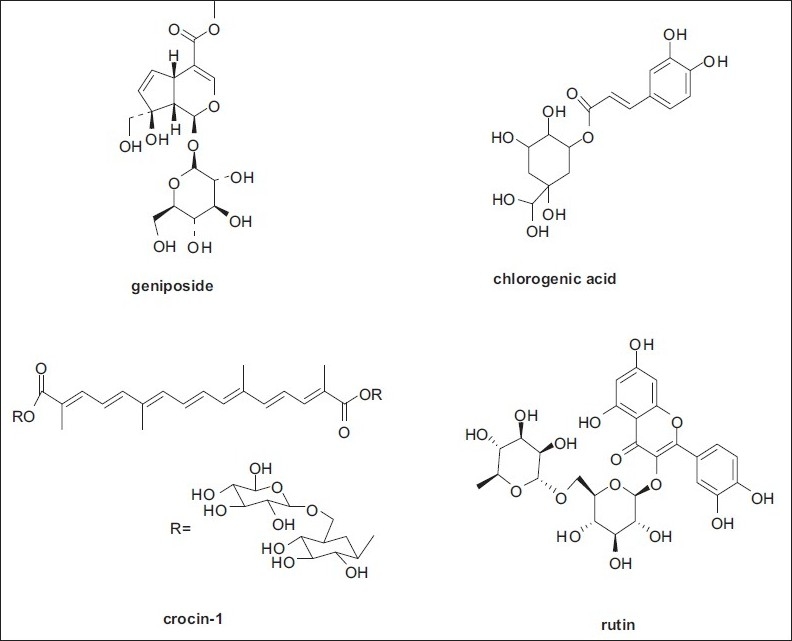
The chemical structures of four active components in Fructus Gardeniae
MATERIALS AND METHODS
Materials and reagents
Fructus Gardeniae was collected from four suppliers (Henan, Jiangxi, Zhejiang in China). Reference standards of geniposide, chlorogenic acid, crocin1, and rutin were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). The purity for each standard compound was greater than 98% by HPLC analysis. All reagents with high grade were obtained from others. Milli-Q water (Millipore, Bedford, MA, USA) was used throughout the study.
Preparation of sample solutions
The powder of Fructus Gardeniae samples quantitatively (0.1 g) transferred into dark brown calibrated flasks and extracted with 30 ml of 50% methanol in an ultrasonic bath for 30 min and cooled at room temperature; 50% methanol was added to compensate for the lost weight. The solution was filtered through a 0.45 μm membrane filter before subjecting 20 μl to HPLC analysis.
Preparation of standard solutions and calibration curve
Standard stock solutions of rutin (0.004838 mg/ml), geniposide (1.54 mg/ml), chlorogenic acid (0.18 mg/ml), crocin1 (0.114mg/ml) were prepared in acetonitrile. Working standard solutions containing each of the four compounds were prepared by diluting the stock solution with acetonitrile to the proper volumes within the ranges, respectively. The standard stock and working solutions were all prepared in calibrated flasks and stored at 4°C. All calibration curves were constructed from peak areas of the reference standards versus their concentrations. The solutions were filtered through a 0.45 μm membrane prior to injection.
Chromatographis analysis
Analyses were performed using HPLC system Agilent 1200 (Agilent Technologies, Palo Alto, CA, USA) with diode array detector. Detection wavelengths were set at 254 nm for rutin, 240 nm for geniposide, 330 nm for chlorogenic acid, and 440 nm for crocin1. An Agilent Zorbax Extend C18 (250 mm × 4.6 mm, 5 μm) was used with a flow rate of 1.0 ml/min. The injection volume was 20 μl and the column temperature was maintained at 25°C. Mobile phase was composed of (A) aqueous phosphoric acid (0.4%,v/v) and (B) acetonitrile using a gradient elution of 0~45 min, 5%~30% B; 45~60 min, 30%~50% B.
RESULTS AND DISCUSSION
Optimization of HPLC chromatography conditions
The aim of this study was to develop a HPLC method using DAD detection for simultaneous determination of geniposide, chlorogenic acid, crocin1, and rutin in Fructus Gardeniae and its CZP. Different mobile phase compositions were tested: (1) water-methanol; (2) water-acetonitrile; (3) aqueous phosphoric acid (0.1%, v/v)-acetonitrile; (4) aqueous ammonium acetate (0.5%, v/v)-acetonitrile; aqueous phosphoric acid (0.4%, v/v)-acetonitrile. As a result, the combination of aqueous phosphoric acid (0.4%, v/v)-acetonitrile for mobile phase was the best for separation. Furthermore, other chromatographic variables were also optimized, including analytical columns (Hanbon Hedera ODS-2, Hanbon Lichrospher C 18 and Agilent Zorbax Extend C 18 ), the column temperatures (20°C, 25°C, and 30°C) and the flow rates (0.8 ml/min and 1.0 ml/min). Eventually, the optimal separation was achieved on an Agilent Zorbax Extend C 18 column (250 mm × 4.6 mm, 5 μm) at a column temperature of 25°C with a flow rate of 1.0 ml/min. Figure 2 showed the typical separation of a standard mixture (a) and crude and processed Fructus Gardeniae extracts (b, c) obtained under the above optimized HPLC conditions.
Figure 2.
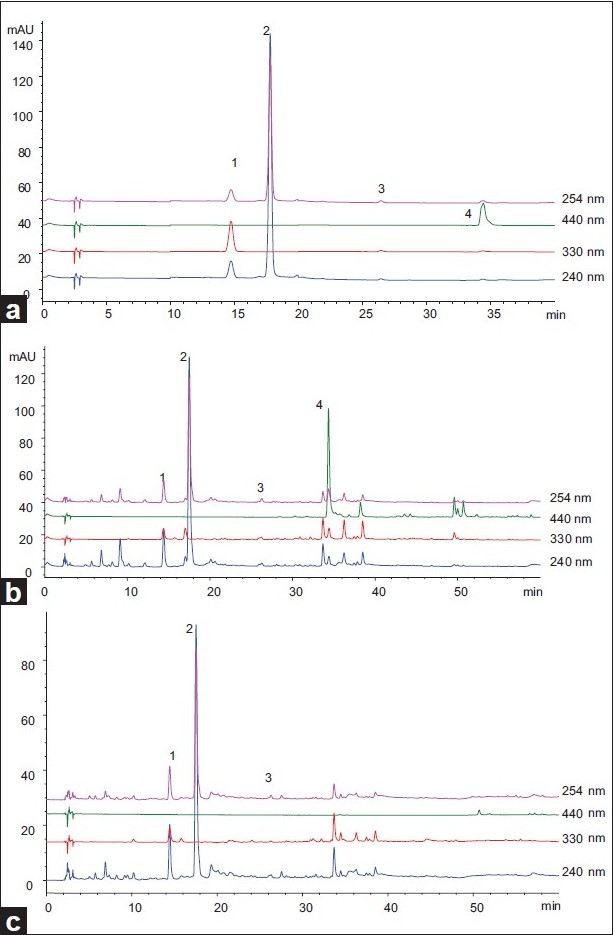
Typical chromatograms of reference compounds (a), crude drug (b), CZP (c), in different detection wavelengths. (1) chlorogenic acid; (2) Geniposide; (3) rutin; (4) crocin-1
Calibration curves, limits of detection and quantification
The working solutions containing all four reference compounds were prepared as described above to construct calibration curves. Each calibration curve contained five different concentrations and was performed in triplicate. An aliquot (20 μl) of each standard working solution was subjected to HPLC analysis. The regression equations were calculated in the form of y = ax + b, where y and x were peak area and concentration. The LOD was determined at a signal-to-noise ratio of 3, and the LOQ was determined as the lowest concentration in the linear range of each analyze. The regression equations (linear ranges) were y = 1075.6 x +5.7406 (1.32–2.464 mg/ml, geniposide), y = 395.4 x -2.1524 (0.144–0.324 mg/ml, chlorogenic acid), y = 5147.4 x + 5.3463 (0.0912–0.2052 mg/ml, crocin1), y = 28.815 x –0.4437 (0.0048–0.0109 mg/ml, rutin). All the marker substances showed good linearity (r2 ≥ 0.9999). The LOD and LOQ of the four analytes were 0.002–0.168 mg/ml and 0.0048–1.32 mg/ml, respectively.
Precision, repeatability and stability
The intra-day and inter-day precision was determined by analyzing calibration samples during a single day and on three different days, respectively. The intra-day variation was determined by analyzing the six replicates on the same day and inter-day variation was determined on three consecutive days. The relative standard deviation (RSD) was taken as a measure of precision, and overall intra-day and inter-day variations were less than 1.01%. To further evaluate the repeatability of the developed assay, Fructus Gardeniae was analyzed in six replicates as described above. The contents of four compounds in Fructus Gardeniae were calculated from the corresponding calibration curves. The relative standard deviations (R.S.D.s) were taken as measurements of repeatability. Stability was tested with Fructus Gardeniae at room temperature and analyzed at 0 h, 2 h, 4 h, 8 h, 12 h, 24 h, and 48 h within two days, respectively. The R.S.D.s of repeatability test and stability were not more than 3.01% for all analytes.
Accuracy
Accuracy was determined by the recovery test. An appropriate amount of Fructus Gardeniae powder was weighed and spiked with known amount of each standard compound. They were then treated and analyzed as described above. Each sample was analyzed in six replicates. The total amount of each analyte was calculated from the corresponding calibration curve.
Recovery (%) = (Amountdetermined –Amountoriginal)/Amountspiked × 100%.
Where Amountdetermined is the determined total of each analyte, Amountoriginal is the original amount of each analyte in Fructus Gardeniae measured and Amountspiked is the spiked amount of each analyte. The R.S.D. of recoveries of the analytes was less than 3.63%.
Sample analysis
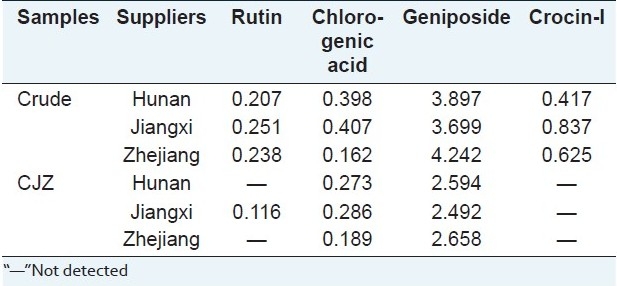
The contents of geniposide, chlorogenic acid, crocin1, and rutin in Fructus Gardeniae samples analyzed were listed in Table 1. The results showed that the content of each compound in crude drug, CZP varied significantly. For instance, for Fructus Gardeniae collected from Zhejiang, the content of chlorogenic acid was lower in crude drug (0.16%), but higher in CZP (0.286%), crocin1 was hardly detected in CZP, but higher in crude drug (0.625%), the contents of geniposide in crude drug (4.242%) were higher than those in CZP (2.658%). The variations might result from different processing procedures for Fructus Gardeniae. It could also be seen that the total contents of four compounds varied slightly in the same type of samples from different suppliers which might be due to the differences in soils and climates in each region. Thus, it is necessary to control the main active components in Fructus Gardeniae by good agricultural practice (GAP) and the norm of Chinese medicinal materials processing.
Table 1.
The contents (%) of different compounds in Fructus Gardeniae from different (n=5)
CONCLUSION
An HPLC-DAD method has been developed to simultaneously determine geniposide, chlorogenic acid, crocin1, and rutin in crude and processed Fructus Gardeniae samples. This newly established method is validated as simple, precise, and accurate. It can be used as a valid analytical method for intrinsic quality control of Fructus Gardeniae.
ACKNOWLEDGEMENT
The authors are grateful to the financial support of the fund of Chinese Pharmacopeia Commission research item (YZ-308). The authors would also like to express special thanks to Miss. Shengbo Wang and Mr. Jiao Kun for their technical support.
Footnotes
Source of Support: Chinese Pharmacopeia Commission research item (YZ-308)
Conflict of Interest: None declared.
REFERENCES
- 1.Wen XD, Qi LW, Chen J, Song Y, Yi L, Yang XW, et al. Analysis of interaction property of bioactive components in Danggui Buxue Decoction with protein by micro dialysis coupled with HPLC-DAD-MS. J Chromatogr B. 2007;852:598–604. doi: 10.1016/j.jchromb.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 2.Qian ZM, Qin SJ, Yi L, Li P, Wen XD. Binding study of Flos Lonicerae japonicae with bovine serum albumin using centrifugal ultrafiltration and liquid chromatography. Biomed Chromatogr. 2008;22:202–6. doi: 10.1002/bmc.916. [DOI] [PubMed] [Google Scholar]
- 3.Du WF, Cai H, Wang MY, Ding X, Yang H, Cai BC. Simultaneous determination of six active components in crude and processed Fructus Corni by high performance liquid chromatograp-hy. J Pharm Biomed Anal. 2008;48:194–7. doi: 10.1016/j.jpba.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Vol. 1. Beijing: Chemical Industry Press; 2005. Chinese Pharmacopoeia Commission, Pharmacopoeia of The Peoples Republic of China; p. 20. [Google Scholar]
- 5.Gong QF. Beijing: Chinese Publishing House of Traditional Chinese Medicine; 2003. Chinese Materia Medica Preparation. [Google Scholar]
- 6.Li BH. A survey of the research on Fructus Gardenia. Li Shizhen Med Mater Med Res. 2004;15:370–1. [Google Scholar]
- 7.Ma T, Han MC, Su J, Wang BQ. Advances in determining method of geniposide in Fructus Gardeniae and relative preparations. Inf Tradit Chin Med. 2005;12:49–51. [Google Scholar]
- 8.Yang Y, Lv JF. Advances of study on Fructus Gardeniae and compound preparations. Li Shizhen Med Mater Med Res. 2004;15:48–9. [Google Scholar]
- 9.Du SY, Hong YL, Yang G, Wu Q. Study of the process for extraction of Gardenia. Chin J New Drugs. 2004;13:715–7. [Google Scholar]
- 10.Oshima T, Sagara K, Yoshida T. Determination of geniposide, gardeniside, geniposidic acid and genipin-1-β-gentiobioside in Gardenia jasminoides by high-performance liquid chromatography. J Chromatogr. 1988;455:410–4. doi: 10.1016/s0021-9673(01)82148-8. [DOI] [PubMed] [Google Scholar]


