Abstract
Background:
The present investigation evaluates the hepatoprotective and in vitro antioxidant effect of methanolic extract and its isolated constituent, dehydroabietylamine, in Carthamus tinctorious L, var Annigeri-2-, an oil yielding crop.
Materials and Methods:
The hepatoprotective effects were estimated for the parameters viz, total bilirubin, total protein, serum alanine amino transaminase (ALT) and serum aspartate aminotransferase (AST) and alkaline phosphatase (ALP) and along with the pathological findings of hepatotoxicity. The in vitro antioxidant activity was evaluated by using free radical scavenging assays: DPPH, nitric oxide radical scavenging, hydroxyl radical, reducing power, ferrous ion chelating ability and total antioxidant capacity.
Results:
Both the methanolic extract (at 150 and 300 mg/kg bw) and dehydroabietylamine (at 50 mg/kg bw) showed significant liver protection against CCl4 -induced liver damage that was comparable with the standard drug, silymarin (100 mg/kg bw), in reducing the elevated serum enzyme markers. The liver sections of the animals treated with dehydroabietylamine elicit a significant liver protection compared with the methanolic extract against CCl4 -induced liver damage. Further, both the methanolic extract and dehydroabietylamine exhibited a considerable and dose-dependent scavenging activity of DPPH, nitric oxide and hydroxyl radical. Similarly, in the reducing power assay, the results were very persuasive. In addition, the Fe2+ chelating activity and the total antioxidant assay established the antioxidant property of the methanolic extract and its isolated constituent. Among the two experimental samples, dehydroabietylamine proved to be more effective for the said parameters.
Conclusion:
The potent antioxidant and its correlative hepatoprotective activity of the methanolic extract and isolated constituent dehydroabietylamine is therefore attributed to its antioxidant and free radical scavenging activities.
Keywords: Antioxidants, carbon tetrachloride, Carthamus tinctorious L, dehydroabietylamine, hepatoprotection, var Annigeri-2
INTRODUCTION
The liver is the largest internal organ in the human body, and is the premier chemical factory necessary for survival. It is the first stop for all nutrients, toxins and drugs absorbed by the digestive tract. It also plays a major role in metabolism and has a number of functions in the body, including glycogen storage, decomposition of red blood cells, plasma protein synthesis and detoxification. It produces bile, an alkaline compound that aids in digestion, via the emulsification of lipids. It also performs and regulates a wide variety of high-volume biochemical reactions requiring very specialized tissues.[1,2] Hepatitis is an inflammation and/or necrosis of liver cells. This may be due to chemical and biological contamination of food and water or due to deterioration in environmental conditions, eating fine and with less fiber contents are some of the important factors attributed for the rising liver dysfunction. Liver infection (viral hepatitis) and dysfunction result in jaundice.[3] Further, the involvement of free radicals such as superoxide anions and hydroxyl radicals and other reactive oxygen species like hydrogen peroxide in various diseases has been established. The metabolism of certain pesticides, drugs, alcohol, cigarette smoke and various other pollutants generate a number of reactive oxygen species and free radicals in the biological system. These radicals cause the depletion of antioxidant enzymes and induce lipid peroxidation in the liver.[4]
Carthamus tinctorius L (Asteraceae) is an annual oil-yielding herb, commonly known as “safflower.” Traditionally, this crop is cultivated in Northwest India, Iran and Northern Africa and Eastern and Northern America for its seeds, which are used for the extraction of edible oil and also for its flower, the latter used for coloring and flavoring the food stuffs. Ayurveda suggests that the leaves are laxative, appetizer and diuretic, and are useful in urorrhea and ophthalmopathy. The flowers are bitter, liver tonic, diuretic, laxative, expectorant, sedative and emmenagogue.[5] Jamaicans and Philippinos use flowers for the treatment of jaundice, and Unani view flowers as hepatotonic.[6] Phytochemical studies of the species revealed the presence of sesquiterpene glycosides,[7] aromatic acids and serotonins,[8] flavonoids[9,10] and sterols and triterpenes.[11] The pharmacological properties of safflower have been evaluated for antitumor, sedative,[12] antimicrobial,[13] antiinflammatory and analgesic effects.[14] Previously, Lee et al.[15] evaluated the DPPH scavenging radical assay of the leaves of this species. It has been hypothesized that one of the principle causes of CCl4-induced liver injury is lipid peroxidation, induced by free radical derivatives of CCl4. Thus, antioxidant activity, or the inhibition of the generation of free radicals, is important in the protection against CCl4-induced liver injury.[16–18] The present investigation reports the hepatoprotective activity of Carthamus tinctorius, methanolic leaf extract and its isolated diterpeniod, dehydroabietylamine, using a CCl4-induced hepatic injury model in rats, and explores the plausible underlying antioxidant mechanism.
MATERIALS AND METHODS
Plant material
The leaves of C. tinctorius L, var Annigeri-2- were collected during March–April 2006 from the All India Co-Ordinated Research Project (Safflower), Agriculture Research Station, Annigeri, Gadag District of Karnataka State, India.
Preparation of the extract and isolation of the constituents
Fresh leaves were washed in running tap water, shade dried at laboratory condition, powdered mechanically and extracted with methanol using a soxhlet apparatus. The extract was concentrated by a rotary vacuum evaporator and the residue obtained was dried and weighed. The concentrated methanolic extract was partitioned between 90% ethanol and petroleum ether. The methanolic and fractionates were subjected to preliminary phytochemical analysis.[19] Further, the alcohol fraction was separated out and the petroleum ether fraction was chromatographed over 60-120 mesh size silica gel column (Merck, Mumbai, India) and eluted with the solvent system, petroleum ether and ethyl acetate (8:2). The elutents were collected at intervals of 2 min and were monitored by thin layer chromatography (TLC) using the same solvent system. The fractions with similar TLC patterns with a single spot and Rf value were combined and the solvent was removed over a water bath. The structure of the isolated yellow viscous compound was determined by IR, Mass and 1H and 13C-NMR spectral analyses. The methanolic extract and the isolated constituent were used for the present investigations.
Chemicals
All the chemicals and reagents used in the study were of analytical grade. 1, 1-diphenyl, 2-picryl hydrazyl (DPPH), trichloro acetic acid and ferric chloride were purchased from Sigma Chemical Co. (St Louis, MO, USA). Ascorbic acid, potassium chloride, potassium ferric cyanide, hydrogen peroxide, 2-deoxy-2-ribose, ammonium molybdate, Folin Ciocalteu reagent (FCR), sulfuric acid, sodium nitroprusside, sulfanilic acid, naphthylethylene diamine dihydrochloride, phenanthroline, ferrozine, methanol, carbon tetrachloride (CCl 4 ) and Tween-80 were procured from Sd Fine Chem. Ltd., (Bosar, India). Silymarin tablets (Micro Labs Pvt. Ltd., USA) and the diagnostic reagent kit (ERBA Diagnostics Mannheim Germany and manufactured by: Transasia Bio-Medicals Ltd., Himachal Pradesh, India) were used in the present investigations. All solvents and chemicals used were of analytical grade and all the solutions were freshly prepared.
Experimental models
Animals used
Studies were carried out using albino rats (Wistar strain) of either sex weighing 150–200 g. The animals were maintained under standard laboratory conditions (temperature 27±2°C, relative humidity 55±10% and12-h light and dark cycles). The animals were fed with the standard dry pellet diet (Hindustan Lever, Kolkota, India) and water ad libitum. The animals were adapted to laboratory conditions for 7 days prior to the experiments.
Acute toxicity study
All procedures described were reviewed and approved by the institutional Animal Ethical Committee (Reg. No. 144/1999/CPCSEA/NCP/IAEC/CLEAR/P.COL./06/06/2007-08) according to the prescribed guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.[20] The acute toxicity studies were carried out as suggested by Ghosh.[21] Swiss albino mice of either sex weighing between 25 and 30 g were divided into different groups of six animals each. The control group received 1% Tween-80 (2 ml/kg). The other groups received both methanolic leaf extract and its isolated constituent dehydroabietylamine in the following doses: 100, 200, 300, 400 up to 3000 mg/kg. Immediately after dosing, the animals were observed continuously for the first 4 h for any behavioral changes. They were then kept under observation up to 24 h after drug administration to find out the mortality, if any. The LD50 was found to be 3000 mg/K bw and 500 mg/K bw, respectively, for methanolic extract and dehydroabietylamine. One-tenth of the LD50 dose was selected as the therapeutic dose for the evaluation of hepatoprotective activity.
Hepatoprotective activity
Six groups each comprising of six Wistar albino rats weighing in the range of 150–180 g were selected. The animals were divided into six groups of six animals each. The animals in group I served as normal control and received the vehicle (1 ml/kg/day of 1% Tween-80) for 13 days. Group II animals received 0.1 ml/kg bw of CCl4 intraperitonially for 13 days. Group III animals received 100 mg/kg/bw of standard drug silimyrin and 0.1 ml/kg bw of CCl4 orally for 13 days, and served as standard control.[22] Group IV and V animals received 0.1 ml/kg bw of CCl4 intraperitonially along with 150 mg/kg/bw and 300 mg/kg/bw methanolic leaf extract of C. tinctorius, respectively, for 13 days orally. Group VI animals received 0.1 ml/kg bw of CCl4 intraperitonially along with 50 mg/kg/bw isolated constituent dehydroabietylamine. Animals of all the groups were sacrificed under light ether anesthesia on the 14th day. Blood sample of each animal was collected separately by carotid bleeding into sterilized dry centrifuge tubes and allowed to coagulate for 30 min at 37°C. The clear serum obtained after centrifugation was used for the estimation of total bilirubin,[23] total protein,[24] serum alanine amino transaminase (ALT) and serum aspartate aminotransferase (AST)[25] and alkaline phosphatase (ALP).[26]
Histopathological study
The liver samples were excised from the experimental animals of each group and washed with normal saline. These were fixed in 10% buffered neutral formalin for 48 h and then washed with water to remove fixative. The tissues were fixed in bovine solution for 6 h and processed for microtome sections (5-μ thickness). Staining was performed with Harris hematoxylin and examined under a light microscope for the pathological findings of hepatotoxicity.[27]
Antioxidant activity
The total phenolic assay was carried out using the Folin Ciocalteu reagent method. The in vitro antioxidant activity of the methanolic extract and its isolated constituent dehydroabietylamine was evaluated by using free radical scavenging assays: DPPH assay,[28] nitric oxide radical scavenging activity,[29] hydroxyl radical scavenging assay,[30] reducing power assay,[31] ferrous ion chelating ability[32] and total antioxidant capacity.[33] The experiment was performed in triplicate and the results of the mean values were represented graphically.
Statistical analysis
The results were expressed as mean ± SE. Statistical analysis was performed with one-way analysis of variance (ANOVA) followed by Turkey's post-test. P< 0.01 was considered as statistically significant.
RESULTS
The results of the preliminary phytochemical investigation of the methanolic extract and fractionates are presented in Table 1. The compound isolated from the petroleum ether fraction of C. tinctorius is a light yellow viscous solid with a melting point of 113°C. The yield of the compound was approximately 100–150 mg from 2 kg of the plant material. The spectral analyses of IR, 1H NMR and Mass were used for the structural elucidation of the isolated compound. The IR (γ = cm-1 ): spectra exhibited absorption peaks at 3441/cm due to -NH2 stretching frequency and at 1610/cm attributed to C=C, as shown in [Figure 1a]. The 1H NMR spectrum clearly indicated the presence of three aromatic protons appearing between δ 6.8 and 7.3 ppm. Among them, one appeared as a singlet at 6.8 δ, a doublet appeared at 7 δ and another doublet at 7.2 δ. These peaks confirmed the presence of the aromatic ring bearing three protons, as shown in Figure 1b. Similarly, protons of the alicyclic ring system, methyl, isopropyl and methyl amine appeared in between 0.9 and 3 ppm, respectively. The mass spectrum revealed the molecular ion peak at m/z: (m+1): 286 [Figure 1c] and the molecular formula of the compound was C20H31N. Based on the above spectral data, the compound is identified as dehydroabietylamine and the structure is shown in Figure 1d.
Table 1.
Results of qualitative tests for phytoconstituents
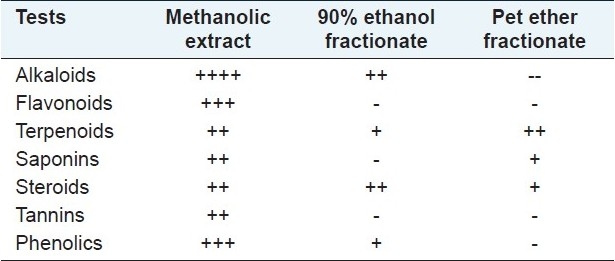
Figure 1a.
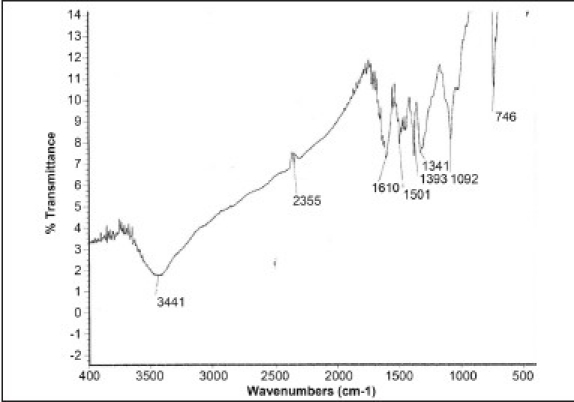
IR Spectra (3441 Cm-1 for NH2 and 1610 Cm-1 for C=C)
Figure 1b.
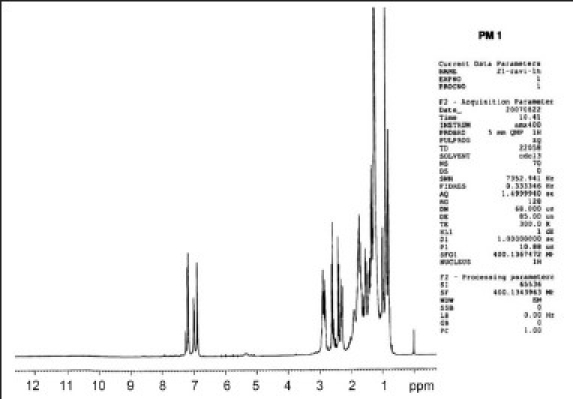
1H NMR Spectra (singlet at 6.8 δ, a doublet appeared at 7 δ and another doublet at 7.2 δ)
Figure 1c.
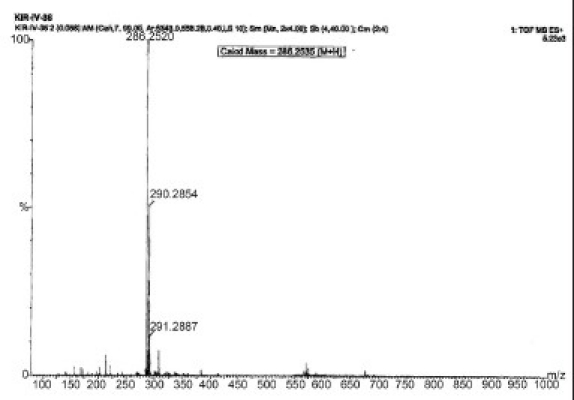
Mass Spectra, m/z: (m+1): 286
Figure 1d.
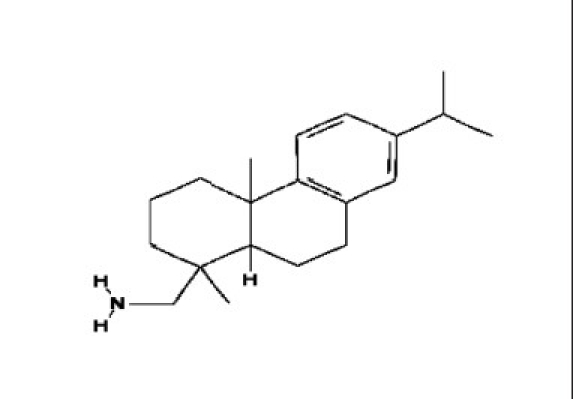
Structure of Dehydroabietylamine
Hepatoprotective activity
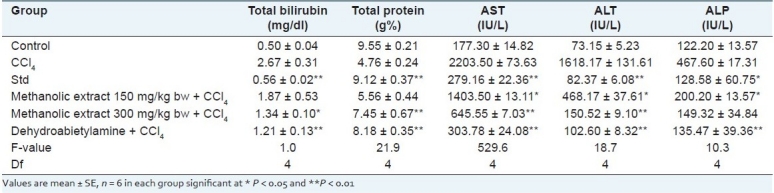
The results of CCl4-induced hepatotoxicity are shown in Table 2. Animals treated with a single intraperitoneal dose of CCl4 developed significant liver damage in inducer control, as evident from a significant (P < 0.01) increase in the serum activities of total bilirubin, AST, ALT and ALP concentrations and a decrease in the total protein compared with normal control rats, indicating acute hepatocellular damage and biliary obstruction. The restoration level of elevated serum enzyme markers and increase in protein level was observed in silymarin-treated animals [Table 2]. The elevated levels of serum markers were reduced in the animal groups treated with methanolic extracts and dehydroabeitylamine. Among the two doses of methanolic extract, 150 mg showed a significant hepatoprotection for total protein (5.56 g/L) only, while at 300 mg, the restoration was significant for enzyme markers, viz. AST, ALT, ALP (645.55 IU/L, 150.52 IU/L and 149.32 IU/L) and for the nonenzyme marker, viz. bilirubin, in addition to total protein (1.34 mg/dl and 7.45 g/L, respectively). However, dehydroabeitylamine (303.78 IU/L, 102.60 IU/L, 135.47 IU/L, 1.21 mg/dl and 8.18 g/L) exhibited significant restoration of serum markers for all the parameters studied, indicating its effectiveness [Table 2].
Table 2.
Effect of methanolic extract and isolated constituent dehydroabietylamine on CCl4-induced hepatotoxicity
The histopathological studies of control, CCl4, silymarin and test sample-treated liver sections are shown in Figure 2a–f. Histological studies of control animals showed normal hepatocytes [Figure 2a], whereas the liver section of CCl4-treated animals showed intense centrilobular, necrosis, vacuolization and macrovesicular fatty changes [Figure 2b]. The liver section of standard drug and silymarin-treated animals showed normal hepatocytes [Figure 2c], which is comparable with the liver section of normal animals. The animals treated with different doses of the methanolic showed recovering of hepatocyte, especially in the 300 mg/kg bw-treated group, with minimal inflammation and near-normal architecture possessing higher hepatoprotective activity [Figure 2e] as compared with the methanolic extract treated at 150 mg/kg bw, which shows a moderate number of recovered hepatocytes with a small amount of necrosis, vacuolization and macrovesicular fatty changes [Figure 2d]. However, the liver sections of the animals treated with dehydroabeitylamine exhibited significant liver protection against CCl4, as evident by the presence of normal hepatic cords, absence of necrosis and fatty infiltration, supplementing the protective effect of dehydroabeitylamine [Figure 2f], which is comparable to the standard drug and control animals liver sections.
Figure 2.
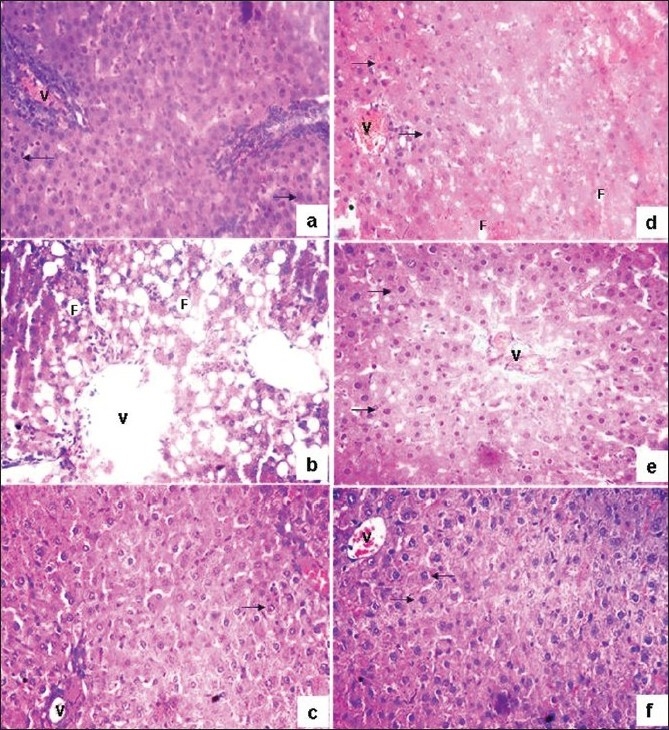
(a) Liver tissue of the control animal showing normal histology (b) Treated with CCl4 showing necrosis, central vein (V) and fatty vacuole (F) (c) Silymarin treated showing normal hepatocytes (arrow mark) with central vein (d) With methanolic extract @150 mg showing absence of necrosis with few fatty vacuoles with normal arrangement of hepatocytes (e) With methanolic extract @ 300 mg showing absence of necrosis with few fatty vacuoles with normal arrangement of hepatocytes (f) With dehydroabietylamine showing normal arrangement of hepatocytes, absence of necrosis and fatty vacuoles
Antioxidant activity
The result of the antioxidant activity, viz total antioxidant capacity, DPPH, nitric oxide, hydroxy radical scavenging activities, reducing power assay and Fe2+ chelating activity of the methanolic extract and its isolated constituent, dehydroabietylamine, are shown in Figures 3 and 4.
Figure 3.
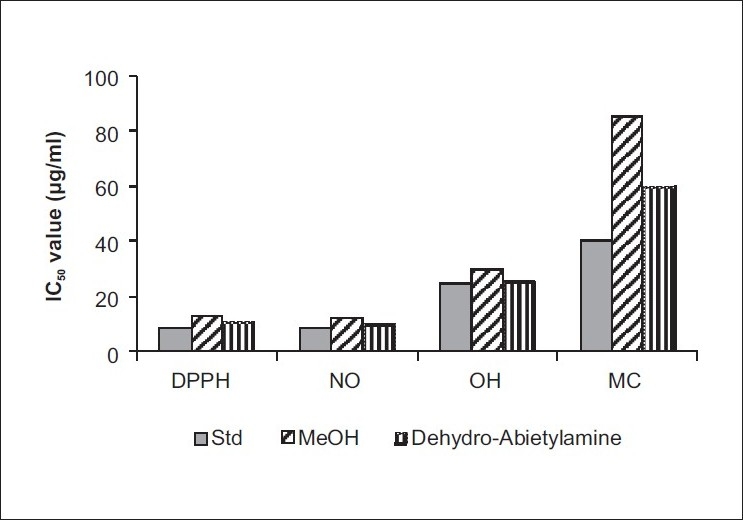
The IC50 values of in vitro antioxidant ability of Methanolic extract and Dehydroabietylamine (DPPH: DPPH radical scavenging activity, NO: Nitric oxide scavenging activity, OH: Hydroxyl ion scavenging activity, MC: Metal chelating activity)
Figure 4.

Absorbance of methanolic extract and dehydroabietylamine isolated from C. tinctorious in reducing power assay
The total phenolic content of the methanolic leaf extract of C. tinctorious was found to be 0.760 μg gallic acid equivalent/mg. Both the extract and dehydroabietylamine exhibited a considerable dose-dependent inhibition of DPPH activity, with a 50% inhibition at a concentration of 12.6 μg/ml and 11.46 μg/ml, respectively, while a similar activity was observed at 9.04 μg/ml for standard, ascorbic acid [Figure 3]. The scavenging of nitric oxide by the methanolic extract and its isolated constituent dehydroabietylamine was found to increase in a dose-dependent manner [Figure 3]. The IC50 values for the methanolic extract and dehydroabietylamine were 12.0 μg/ml and 11.48 μg/ml, respectively, whereas the standard showed IC50 at 9.16 μg/ml.
For quenching of hydroxyl radical, a higher dose of extracts was required when compared with the DPPH and nitric oxide radical scavenging. Methanolic extract and its isolated constituent dehydroabietylamine have recorded IC50 values at 30.0 μg/ml and 25.51 μg/ml, respectively, while it was 24.53 μg/ml for the standard. In regard to the reductive capabilities of the methanolic extract and its isolated constituent, dehydroabietylamine, both the tested samples showed very potent reducing power in a dose-dependent manner [Figure 4]. Even though the tested samples recorded a slightly lesser reductive activity than the standard, ascorbic acid, it is evident from the figure that they were able to reduce the Fe3+ ions considerably. Among the dehydroabietylamine and methanolic leaf extracts of the plant, dehydroabietylamine showed the highest ferrous iron chelating ability [Figure 3] compared with the methanolic extract of the plant. The IC50 values of ascorbic acid, dehydroabietylamine and methanolic extract were 40 μg/ml, 60 μg/ml and 90 μg/ml, respectively. The total antioxidant capacity of the methanolic extract and its isolated constituent dehydroabietylamine calculated on the formation of the phosphomolybdenium complex was 645.0 nmol ascorbic acid/g of sample and 675.0 nmol ascorbic acid/g for methanolic extract and dehydroabietylamine, respectively, thus establishing the antioxidant property.
DISCUSSION
Preliminary phytochemical analysis of the methanolic extract of C. tinctorious revealed the presence of alkaloids, triterpenoids, tannins, phenolic compounds and flavonoids. It is not possible to attribute with certainty the hepatoprotective effect to one or several active principles among those detected in the methanolic extract of the leaves of C. tinctorious. However, flavonoids,[34] triterpenoids,[35] saponins[36] and alkaloids[37] are known to possess a hepatoprotective activity in animals.
The present investigation documents the first report of the isolation of dehydroabietylamine from the petroleum ether fractionate portioned from the methanolic extract of the leaves of C. tinctorious. The structure of this isolated compound was determined by IR, Mass 1H and 13C NMR spectral analyses. Previously, Mitova et al.[11] isolated dehydroabietylic acid from the aerial part of C. lanatus and dehydroabietylamine from the Rosin plant.[38,39] In the present investigation, we have isolated dehydroabietylamine from the leaves of C. tinctorius. The derivatives of dehydroabietylamine have been reported for a wide range of biological properties, including antibacterial and antifungal activities.[40] Further, antiinflammatory activities for derivatives of dehydroabietylamine at N position were also evaluated.[41,42]
Hepatoprotective activity
CCl4-induced hepatic injury is a commonly used model for studying the hepatoprotective effects of drugs or medicinal plant extracts, and the extent of hepatic damage is assessed by the level of released cytoplasmic alkaline phosphatase and transaminases in circulation. Further, the extent of hepatic damage is assessed by histopathological evaluation.[37] The results of the present study undertaken to evaluate the hepatoprotective activity of the methanolic crude extract and its isolated active constituent dehydroabietylamine in CCl4 -induced liver damage of rats showed that both the animals treated with the methanolic extract at 300/kg bw and the constituent dehydroabietylamine significantly reduced the toxic effect of CCl4, similar to the standard silymarin in the levels of liver function serum markers, viz AST, ALT and ALP, total bilirubin and increase in the protein synthesis. The percentage of protection was greater in dehydroabietylamine at 50 mg/kg bw and in the methanolic extract at 300 mg/kg bw, which is comparable to the reference drug silymarin (100 mg/kg bw). The results showed that pretreatment with the methanolic extract and dehydroabietylamine restored the biochemical parameters, thereby indicating their protection against the injurious effects of carbon tetrachloride, which may be due to the inhibitory effects on cytochrome P450 resulting in the hindrance of the formation of hepatotoxic free radicals.[28,43]
Further, histopathological examination of the liver section of the rats treated with toxicant showed intense centrilobular necrosis and vacuolization. However, the rats treated with silymarin, methanolic extract (at two doses) and the dehydroabietylamine along with toxicant showed signs of protection against these toxicants to a considerable extent, as evident from the formation of normal hepatic cards and absence of necrosis and vacuoles. Thus, the histological study supports the hepatoprotective activity of the constituent dehydroabietylamine from the toxic effect of CCl4 -induced liver damage, which was comparable to silymarin.
Antioxidant activity
The main characteristic of an antioxidant is its ability to trap free radicals. Highly reactive free radicals and oxygen species are present in biological systems from a wide variety of sources. These free radicals may oxidize nucleic acids, proteins, lipids or DNA and can lead to degenerative disease. Antioxidant compounds like phenolic acids, polyphenols, terpenoids and flavonoids scavenge free radicals such as peroxide, hydroperoxide or lipid peroxyl and thus inhibit the oxidative mechanisms that lead to degenerative diseases.[44]
In the present investigation, different antioxidant assays have been used to evaluate the antioxidant activity of the methanolic extract and its isolated constituent dehydroabietylamine of C. tinctorious. Despite the undefined chemical nature of FCR, the total phenol assay by FCR is convenient, simple and reproducible.[45] The total phenolic content of the methanolic leaf extract was 0.760 μg gallic acid equivalent/mg. Several investigators have reported the use of total phenol assay and antioxidant capacity assays (e.g., DPPH, reducing power assay, hydroxyl radical assay, etc.), and often found excellent linear correlations between the two assays.[32,46,47] Thus, the evident total phenolic concentration in the methanolic leaf extract could forecast its antioxidant property. DPPH assay is a widely used, technically simple and rapid method of antioxidant assay.[44] In the present study, it was demonstrated that the methanolic extract and its isolated constituent dehydroabietylamine on interaction with DPPH either transferred electron or hydrogen atom to DPPH, thereby neutralizing its free radical character, which gives rise to uncolored methanol solutions.[46,47] In the nitric oxide (NO) scavenging assay, among the two samples, dehydroabietylamine showed potent scavenging activity in a dose-dependent manner followed by the methanolic extract, with IC50 values of 11.48 μg /ml and 12.00 μg /ml, respectively. The scavenging activity of the two samples might be due to the inhibition of nitrite formation by competing with oxygen to react with nitric oxide directly and also by inhibition of its synthesis.[48] Hydroxyl radical (HO.-) is the most reactive free radical known, and can react with everything in living organisms. These short-lived species can hydroxylate DNA, proteins and lipids. In the present investigation, the methanolic extract and dehydroabietylamine showed significant hydroxyl radical scavenging activity by their ability to remove hydroxyl free radicals and preventing the degradation of 2-deoxy-2-ribose in the reaction mixture The potential scavenging abilities of the methanolic extract and dehydroabietylamine might be due to the active hydrogen donor ability of hydroxyl substitution or may be due to the metal chelating ability with high molecular weight and the proximity of many aromatic rings and hydroxyl groups by specific functional groups.[49] The reducing ability of a compound generally depends on the presence of reductants, which have exhibited antioxidative potential by breaking the free radical chain, by donating a hydrogen atom.[50] Kimura et al.[51] have reported that the reducing power of secondary metabolites from medicinal plants prevents liver injury by inhibiting formation of lipid peroxides. Reductones are believed not only to react directly with peroxides but also to prevent peroxide formation by reacting with certain precursors. Dehydroabietylamine has the highest reducing power when compared with the methanolic extract. Further, the reducing power of the methanolic extracts and dehydroabietylamine increased with higher concentrations of the extracts, showing their efficacy as excellent reductants, which resulted in reduction of the Fe3+ ferricyanide complex to the ferrous form.[32,46] Ferrous salts can react with hydrogen peroxide and form hydroxy radical via Fenton's reaction. The iron required for this reaction is obtained either from the pool of iron or the heme-containing proteins. [ 32 ] The hydroxyl radical (OH.) thus produced may attack the sugar of the DNA base, causing sugar fragmentation, base loss and DNA strand breakage.[52] In the present investigation, secondary antioxidant property was measured by the Fe2+ chelating activity, showing that the formation of the ferrozine and ferrous ion complex was interrupted in the presence of the methanolic extract and dehydroabietylamine by recording the highest ferrous iron chelating ability (IC50 60 μg/ml and 90 μg/ml) when compared with the standard (40 μg/ml). The ferrous ion chelating properties of the antioxidant extract may be attributed to their endogenous chelating agents, mainly tannins, terpenes and phenolics.[32,53] The phosphomolybdenum method is quantitative as the total antioxidant activity is expressed as the number of equivalents of ascorbic acid.[33] Among the two test samples, total antioxidant capacity was found to be high in dehydroabietylamine (675.0 nmol of ascorbic acid/g of sample) when compared with the methanolic extract (645.0 nmol of ascorbic acid/g of sample).[47]
CONCLUSION
Because the antioxidant activity or the inhibition of the generation of free radicals is important in the protection against CCl4-induced liver injury, the potent antioxidant and its correlative hepatoprotective activity of the methanolic extract and isolated constituent dehydroabietylamine is therefore attributed to its antioxidant and free radical scavenging activities.
ACKNOWLEDGMENT
The authors express their gratitude to Prof. B. R. Siddaramappa, Principal, and Prof. V. R. Padmanabhan, Head, Department of Zoology, Sahyadri Science College (Autonomous), Shivamogga, for providing lab facility and encouragement. Thanks are also due to Dr. K. M. Mahadevan, PG Department of Chemistry, Kuvempu University, for rendering help in structural elucidation, and Dr. Manjuanth, PG Department of Biotechnology and Bioinformatics, Kuvempu University, for his valuable suggestions and support during the experimentation.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Anthea M, Hopkins J, William C, McLaughlin, Johnson S, Warner MQ, et al. Englewood Cliffs, New Jersey: Prentice Hall; 1993. Human Biology and Health. [Google Scholar]
- 2.Absar AQ, Prakah T, Patil T, Viswanath Swamy AH, Veeran GV, Prabhu K, et al. Hepatoprotective and antioxidant activities of flowers of Calotropis procera (Ait) R.Br in CCl4 induced hepatic damage. Indian J Exp Biol. 2007;45:304–10. [PubMed] [Google Scholar]
- 3.Sinha RK, Sinha S. Jaipur: Surabhi Publishers; 2001. Ethnobiology; Role of Indigenous and Ethnic Societies in Biodiversit conservation, Human Health Protection and Sustainable Development; p. 181. [Google Scholar]
- 4.Koul IB, Kapil A. Evaluation of the liver protective potential of piperine: An active principle of black and long peppers. Planta Med. 1999;59:413–7. doi: 10.1055/s-2006-959721. [DOI] [PubMed] [Google Scholar]
- 5.Varier PS. Vol. 1. New Delhi: Orient Longman Private Ltd; 2007. Indian Medicinal Plants-a compendium of 500 species; pp. 390–3. [Google Scholar]
- 6.Duke JA, Duke PK, duCellie JL. USA: Taylor and Francis Group: CRC Press; 2008. Duke's handbook of medicinal plants of the Bible; pp. 80–3. [Google Scholar]
- 7.Feliciano AS, Medarde M, Del Rey B, Del Corral JM, Barrero AF. Eudesmane glycosides from Carthamus lanatus. Phytochemistry. 1990;29:3207–11. [Google Scholar]
- 8.Lahloub M, Amor M, El-Khajaat S, Haraz F. A new serotonin derivative from seeds of Carthamus lanatus L. Mans. J Pharm Sci. 1993;9:234–43. [Google Scholar]
- 9.El-Shaer N, Shaaban E, Abou-Karam M, El-Din A. Flavonoids from Carthamus lanatus. Alex J Pharm Sci. 1998;12:23–6. [Google Scholar]
- 10.Novruzov EN, Shamsizade LA. Anthocyans of Carthamus species. Chem Nat Comp. 1998;34:514–5. [Google Scholar]
- 11.Mitova M, Taskova R, Popov S, Berger RG, Krings U, Handjieva N. GC/MS analysis of some bioactive constituents from Carthamus lanatus L. Z Naturforsch (C) 2003;58c:697–703. doi: 10.1515/znc-2003-9-1018. [DOI] [PubMed] [Google Scholar]
- 12.Benedi J, Iglesias I, Manzanares J, Zaragoza F. Preliminary pharmacological studies of Carthamus lanatus L. Z Naturforsch (C) 1986;20:25–30. [Google Scholar]
- 13.Taskovaa R, Mitova M, Bozhanka M, Duddeckc H. Bioactive Phenolics from Carthamus lanatus L. Z Naturforsch(C) 2003;58c:704–7. doi: 10.1515/znc-2003-9-1019. [DOI] [PubMed] [Google Scholar]
- 14.Bocheva A, Mikhova B, Taskova R, Mitova M, Duddeck H. Antiinflammatory and analgesic effects of Carthamus lanatus aerial parts. Fitoterapia. 2003;74:559–63. doi: 10.1016/s0367-326x(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 15.Lee JY, Chang EJ, Kim HJ, Park JH, Choi SW. Antioxidative flavonoids from leaves of Carthamus tinctorius. Pharm Res. 2002;25:313–9. doi: 10.1007/BF02976632. [DOI] [PubMed] [Google Scholar]
- 16.Castro JA, De-Ferrya GC, De-Castro CR, De-Fenos OM, Sasamelt H, Giltelte JR. Prevention of CCl4 necrosis by inhibitors of drug metabolism. Further studies on metabolism of their action. Biochem Pharmacol. 1974;23:295–302. doi: 10.1016/0006-2952(74)90420-1. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi NN, Bhanudansh SK, Nadeem AL, Majid AH. Antioxidant and hepatoprotective activity of Cordia macleodii leaves. Saudi Pharma J. 2009;17:317–22. doi: 10.1016/j.jsps.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishna KL, Mruthunjaya K, Jagruti AP. Antioxidant and hepatoprotective potentials of stem methanolic extract of Justicia gendarussa Burm. Int J Pharmacol. 2010;6:72–80. [Google Scholar]
- 19.Kokate CK, Purohit AP, Gokhale SB. 2nd ed. Pune: Nirali Publication; 1996. Pharmacognosy; p. 107. [Google Scholar]
- 20.Paris: Published by Environment Directorate Organization for economic Co-operation and development; 2001. Jun, OECD Series on testing and assessment “Guidance Document on oral toxicity Testing”; pp. 1–25. [Google Scholar]
- 21.Ghosh MN. Calcutta: Scientific book Agency; 1984. Fundamentals of experimental pharmacology; pp. 156–7. [Google Scholar]
- 22.Kumar P, Deval Rao G, Lakshmayya, Ramachandra Setty S. Antioxidant and hepatoprotective activity of tubers of Momordica tuberosa Cogn. against CCl4 induced liver injury in rats. Indian J Expl Biol. 2008;46:510–3. [PubMed] [Google Scholar]
- 23.Malloy HT, Evelyn KA. The determination of bilirubin with the photoelectric colorimeter. J Biol Chem. 1936;119:481–90. [Google Scholar]
- 24.Kingsley GR. The determation of serum total protein, albumin and globulin by the Biuret reaction. J Biol Chem. 1939;214:197–200. [Google Scholar]
- 25.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxaloacetic acid, glutamic pyruvic transaminase. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 26.Bessey OA, Lowery OH, Brock MJ. A method for the rapid determination of alkaline phosphatase with five cubic millimeters of serum. J Biol Chem. 1964;164:321–9. [PubMed] [Google Scholar]
- 27.Galighter AE, Kayloff EN. Philadelphia: Lea and Febiger; 1971. Essential of practical microtechnique; p. 77. [Google Scholar]
- 28.Jain A, Manish S, Lokesh D, Jain A, Rout SP, Gupta VB, et al. Antioxidant and hepatoprotective activity of ethanolic and aqueous extracts of Momordica dioica Roxb. leaves. J Ethnopharmacol. 2008;115:61–6. doi: 10.1016/j.jep.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Garrat DC. Vol. 3. Japan: Chapman and Hall; 1964. The Quantitative Analysis of Drugs; pp. 456–8. [Google Scholar]
- 30.Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: A simple ‘test tube’ assay for determination of rate constants for reaction of hydroxyl radicals. Anal Biochem. 1987;165:215–9. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- 31.Oyaizu M. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–15. [Google Scholar]
- 32.Velavan S, Nagulendran K, Mahesh R, Hazeena Begum V. In vitro antioxidant activity of Asparagus racemosus root. Pharmacog Mag. 2007;3:26–33. [Google Scholar]
- 33.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E1. Anal Biochem. 1999;269:337–41. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 34.Paya M, Ferrandiz ML, Sanz MJ, Alcaraz MJ. Effects of phenolic compounds on bromobenzene mediated hepatotoxicity in mice. Xenobiotica. 1993;23:327–33. doi: 10.3109/00498259309059386. [DOI] [PubMed] [Google Scholar]
- 35.Gao J, Tang X, Dou H, Fan Y, Zhao X, Xu Q. Hepatoprotective activity of Terminalia catappa L. leaves and its two triterpenoids. J Pharm Pharmacol. 2004;56:1449–55. doi: 10.1211/0022357044733. [DOI] [PubMed] [Google Scholar]
- 36.Tran QL, Adnyana IK, Tezuka Y, Nagaoka T, Tran QK, Kadota S. Triterpene saponins from Vietnamese ginseng (Panax vietnamensis) and their hepatocyte protective activity. J Nat Prod. 2001;64:456–61. doi: 10.1021/np000393f. [DOI] [PubMed] [Google Scholar]
- 37.Manjunatha BK, Mankani KL, Vidya SM, Krishna V, Manohara YN. Hepatoprotective activity of Butea superba against carbon tetrachloride induced hepatic damage in rodents. Pharmacog Mag. 2008;4:S41–5. [Google Scholar]
- 38.Richard BH, Waukegan Purification of Dehydroabietylamine, United States Patent Office, 2,813895, Patented. 1957 Nov 19; [Google Scholar]
- 39.Steck EA. Topical prophylaxis against, U.S Patent 4282253. schistosomiasis. 1981 [Google Scholar]
- 40.Goodson B, Ehrhardt A, Simon NG, Nuss J, Johnson K, Giedlin M, et al. Characterization of novel antimicrobial peptoids. Antimicrob Agents Chemother. 1999;43:1429–34. doi: 10.1128/aac.43.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkerson WW, Galbraith W, DeLucca I, Harris RR. Topical antiinflammatory dehydroabietylamine derivatives IV. Bioorg Med Chem Lett. 1993;13:2087–92. [Google Scholar]
- 42.Wilkerson WW, DeLucca I, Galbraith W, Gans K, Harris R, Jaffee B, et al. Antiinflammatory phospholipase: A2 inhibitors. Eur J Med Chem. 1991;26:667–76. [Google Scholar]
- 43.Surendra V, Prakash T, Uday RS, Divakar G, Snehal DF, Kotresha D. Hepatoprotective activity of aerial parts of Cynodon dactylon against CCl4-induced in rats. Pharmacog Mag. 2008;4:S195–201. [Google Scholar]
- 44.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. [Google Scholar]
- 45.Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays: Reviews. J Agric Food Chem. 2005;53:1841–56. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 46.Kamiloglu O, Ercisli S, Sengül M, Toplu C, Serçe S. Total phenolics and antioxidant activity of jujube (Zizyphus jujube Mill.) genotypes selected from Turkey. Afr J Biotechnol. 2008;8:303–7. [Google Scholar]
- 47.Gupta S, Prakash J. Studies on Indian green leafy vegetables for their antioxidant activity. Plant Foods Hum Nutr. 2009;64:39–45. doi: 10.1007/s11130-008-0096-6. [DOI] [PubMed] [Google Scholar]
- 48.Gupta M, Upal KM, Gomathi P. In vitro antioxidant and free radical scavenging activities of Galega purpurea root. Pharmacog Mag. 2007;3:218–24. [Google Scholar]
- 49.Aurand LW, Boone NH, Gidding GG. Superoxide and singlet oxygen in milk lipid peroxidation. J Dairy Sci. 1977;60:363–9. [Google Scholar]
- 50.Pin-Der-Duh Antioxidant activity of Burdock (Arctium lappa Linne): Its scavenging effect on free radical and active oxygen. J Am Oil Chem Soc. 1998;75:455–61. [Google Scholar]
- 51.Kimura Y, Okuda H, Mori K, Okuda T, Arichi S. Studies on the activities of tannins and related compounds from medicinal plants and drugs, IV: Effects of various extracts of Geranii herba and Geraniin on liver injury and lipid metabolism in rats fed peroxidized oil. Chem Pharm Bull. 1984;32:1866–71. doi: 10.1248/cpb.32.1866. [DOI] [PubMed] [Google Scholar]
- 52.Kaneko T, Tahara S, Matsuo M. Retarding effect of dietary restriction on the accumulation of 8-hydroxy-2-deoxyguanosine in organs of Fischer 344 rats during aging. J Free Radic Biol Med. 1997;23:76–81. doi: 10.1016/s0891-5849(96)00622-3. [DOI] [PubMed] [Google Scholar]
- 53.Cai Y, Qiong L, Mei S, Harold C. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–84. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]


