Abstract
Background:
Glycyrrhizin, obtained from Abrus precatorius (Indian liquorice), is a phytoconstituent of importance for pharmaceutical and food industries.
Materials and Methods:
High producing and fast growing cell lines of A. precatorius were developed by transformation with Agrobacterium tumefaciens for glycyrrhizin production. Its maximum transformation efficiency of 85% was obtained by infecting leaves with A. tumefaciens MTCC-431 supplemented with 50 μM acetosyringone. Thorough culture growth kinetics with sugar consumption profiles was established.
Results:
A twofold increase in glycyrrhizin productivity was obtained in transformed A. precatorius cell suspension cultures over the untransformed cultures. The fungal elicitors prepared from Aspergillus niger and Rhizopus stolonifer were tested at different concentrations to enhance glycyrrhizin production in transformed cell suspension cultures of A. precatorius. Maximum enhancement of 4.9- and 3.8-fold in glycyrrhizin contents, were obtained with A. niger (7.5% v/v) and R. stolonifer (5.0% v/v), respectively, on the 5th day after elicitor treatment.
Conclusion:
This study indicates the prospective of the amalgamation of elicitation methodology with transformed cell cultures for the large-scale production of glycyrrhizin.
Keywords: Abrus precatorius, Agrobacterium, fungal elicitor, glycyrrhizin, transformed cell cultures
INTRODUCTION
Plant cell cultures remain a favored methodology for biotechnological production of nature-based therapeutics. Transformed cultures, obtained from A. tumefaciens mediated infection, are one of the important tools for the enhanced production of secondary metabolites. Untransformed cultures generally require exogenously added phytohormones and have a tendency to loose their biosynthetic capability after a short period. Furthermore, Agrobacterium transformed cultures have been comparatively fast growing and genetically more stable and hence more suitable for large-scale production of plant-based products.[1,2] Transgenic cell and organ cultures have proved valuable in the study of different aspects of secondary metabolism as these are more stable genetically and biochemically over long periods in cultures. Crown galls are significant steps for the metabolic manipulation of plant secondary compounds and applicable for large-scale production of phytopharmaceuticals from cell cultures. Crown galls are developed by integration and expression of oncogenes of Agrobacterium Ti-plasmid into plant genome. Transgenic calli induced by Agrobacteria of several plant species have been shown even to synthesize secondary metabolites such as pharmaceuticals in higher amounts than the untransformed tissue does.[3] Elicitors are compounds that stimulate plant cell secondary metabolism and are usually derived from components of fungal or plant cell walls. Various biotic and abiotic elicitors like bacterial and fungal dried cell powders (DCPs),[4] jasmonates,[5] salicylates,[6] chitosan and gibberlic acid,[7] yeast extract,[8] etc. have been investigated to enhance the yield of plant-based secondary metabolites.
Elicitation has been found as an effective strategy for the induction and enhancement of secondary metabolites in plant cell cultures as well as at a commercial scale. Plant defense reactions can be triggered by elicitors present in the cell walls of microorganisms or in their extracellular media. Thus, the fungal elicitors are significant in triggering the phytoalexin production in the cultured cells. For example, the elicitor preparation from Fusarium conglutinans enhanced thiophene production in Tagetes spp., Rhizoctonia solani increased solavetivone production in Hyoscyamus muticus,[9,10] and enhanced the production of shikonin in suspension cultures of Lithospermum erythrorhizon by Rhizopus oryzae and Aspergillus niger.[11,12] Increased levels of ajmalicine and catharanthine in Catharanthus roseus and capsaicin production in Capsicum annuum by fungal elicitors have also been reported.[13,14] This study is to elaborate the role of elicitation strategy with the transformed A. precatorius cell cultures for further improvement in glycyrrhizin accumulation. The in vitro cell culture of Abrus precatorius was established and the effect of different elicitors was studied in our previous report which showed a positive result regarding the production of glycyrrhizin.[15] In this report, the influence of fungal elicitors on the transgenic cell suspension cultures of A. precatorius was studied.
MATERIALS AND METHODS
Plant materials
The seeds of A. precatorius were collected from the medicinal plant garden of Department of Pharmaceutical Sciences, Dr. H. S. Gour University, Sagar, M.P., India. Seeds were sterilized and germinated by following the protocol described in our previous publication.[15]
Initiation of A. precatorius cell cultures
Different explants from aseptically germinated seeds viz. leaves, epicotyles, and petioles were tested for culture initiation by variation in plant growth regulators (PGR) and Agrobacterium-mediated transformation. Nontransformed callus cultures were initiated by placing explants on solidified MS medium supplemented separately with the hormones: 1 mg/l naphthalene acetic acid (NAA); 1 mg/l kinetin (Kn); 0.5–2.0 mg/l 2,4-dichlorophenoxy acetic acid (2,4-D); and their combinations (data not shown).
For transformation experiments, leaves were excised from 30-day-old in vitro germinated plantlets of A. precatorius. A. tumefaciens strains (MTCC 431, MTCC 609, MTCC 2250, and MTCC 2251) were used to establish transformed callus cultures. These strains were procured from Microbial Type Culture Collection (MTCC), Institute of Microbial Technology (IMTECH), Chandigarh, India. A minimum of 30 explants were used for each experiment. All explants cultured on sterilized petriplates comprising MS medium solidified with 1.0% agar and supplemented with 30 g/l sucrose. The pH was adjusted to 5.7 ± 0.2. The medium was autoclaved under 15 psig pressure at 121 °C for 20 min.
The explants were cocultivated with Agrobacterium strains for infection to induce transformed callus. For this purpose, Agrobacterial colonies were cultured for 48 h on solid nutrient agar medium at 28 ± 2°C. Ten loopful bacteria were then allowed to grow in nutrient broth culture for 24 h at 28 ± 2 °C. Inoculum (2% v/v) of this culture was reinoculated in nutrient broth medium and grown till an OD600 of 1.0 was achieved. The agrobacterial suspension was centrifuged at 6000rpm for 10 min. The supernatant was discarded and the pellet was resuspended in 5 ml of the fresh liquid nutrient broth medium. This concentrated culture was used for infection of plant materials. The explants were kept in sterile petriplates; explant margins were incised manually with sterile scalpel, dipped in A. tumefaciens, and incubated for 5–20 min. In another experiment, 2 ml of acetosyringone solution at different concentrations (10, 25, and 50 mM) were also added along with suspension of Agrobacterium strain to be tested. Nutrient agar medium without bacteria applied to the explants served as a control. Excess Agrobacteria were then removed by blotting the infected explants on sterile filter papers. The infected explants (with or without acetosyringone) were then co-cultivated at 27 ± 2 °C for 48 h on solidified MS media in 12/12 h light/dark regime. The transformed cultures were then transferred to fresh MS medium containing varying concentration of acetosyringone for better transformation efficiency and 500 mg/l cefotaxime to check the overgrowth of bacteria. Axenic cultures were obtained by subsequent subcultures to fresh MS medium containing the same antibiotic (subsequently with decreased concentration) every 4 days. The transformed cultures were checked for Agrobacterium contamination by culturing samples on nutrient agar medium after every subculture.
Selection of high yielding cell lines
Transformed cell cultures of A. precatorius, induced by A. tumefaciens mediated transformation, were subjected for determination of growth index and glycyrrhizin content. On this basis, the fast growing and high glycyrrhizin yielding cell line was selected for further studies.
Establishment of growth and production profiles in cell suspension cultures of A. precatorius
To establish the transformed suspension cultures in 250 ml Erlenmeyer flasks, friable fraction of the transformed callus equivalent to 2 g/l cells on cell dry weight (DW) basis were transferred to 55 ml of phytohormone free liquid MS media with 30 g/l sucrose. The flasks were incubated on a rotary shaker at 110 ± 10 rpm and 25 ± 2 °C with a photoperiod of 12/12 h light/dark cycle. Cells from the 20-day-old suspension culture were used for further experiments. To establish growth and production kinetics, an individual flask was harvested in duplicate at an interval of 3 days and analyzed for cell dry weight (DW), glycyrrhizin content, and residual sucrose concentration. For DW estimation, cells were harvested and collected by centrifugation at 3000 rpm for 15 min and washed with distilled water. Cells were allowed to dry at 45 ± 2 °C until constant weight was achieved. Residual sucrose concentration was analyzed by the phenol-sulfuric acid method.[16]
Extraction and quantitative analysis of glycyrrhizin
The extraction and analysis of glycyrrhizin was carried out as described by Karwasara et al.[15] Statistical comparison was made using analysis of variance (ANOVA) followed by the Dunnett multiple comparison test and the level of significance used as P ≤ 0.05 using the GraphPad InStat software.
Genetic confirmation of transformed cultures
Transformed callus cell lines induced by infecting leaves of A. precatorius with A. tumefaciens strains MTCC 431 were selected. Total genomic DNA was isolated using the AuPrep Kit (Life Technologies Pvt. Ltd., India) from the transformed cell line and untransformed leaves of in vitro grown A. precatorius. Integration of the opine synthase gene regions of the pTi plasmid was confirmed by PCR analysis of the genes in this region. All primers were obtained from Microsynth (Switzerland). The primers designed to amplify the opine synthase gene were 5*-CAGAATGGAATTAGCCGGACTA-3* and the reverse primer 5*-CGTATTAATCCCGTAGGTTTGTTT-3*. The cycling conditions for amplification of gene sequences from MTCC 431 strain consisted of a 1-min denaturation at 94 °C, a 2-min annealing at 55 °C followed by a 3-min extension at 72 °C and a final extension at 72 °C for 7 min. The amplicons were analyzed by electrophoresis on 1% agarose gel (w/v).
Initiation and preparation of the fungal elicitors
The fungal cultures (A. niger and R. stolonifer)used for the elicitation were prepared as described in Karwasara et al .[15]
Addition of elicitors
Dried cell powders (DCPs) and culture filtrates (CFs) of A. niger and R. stolonifer were tested at 0.1%, 0.25%, and 0.5% (w/v) and 5%, 7.5%, and 10% (v/v) concentrations, respectively. These elicitor preparations were added on 6th, 8th, 10th, and 12th day of suspension cultures. The individual flasks, in triplicate, were harvested on 15th day and were analyzed for biomass and glycyrrhizin contents.
RESULTS AND DISCUSSION
Transformed cell cultures
Transformed callus cultures were developed by Agrobacterium -mediated transformation of various explants. The type of explant and A. tumefaciens strain used showed a great influence on tumor induction. A. tumefaciens infectivity was determined in terms of percentage efficiency to induce tumor cells from the explants. The time of exposure of A. tumefaciens, co-cultivation period, and the type of explant played an important role in the transformation protocol. Infection time of 10 min and a co-cultivation period of 48 h were found to be optimum for induction of transformed callus cultures. Out of four strains tested, A. tumefaciens MTCC 2250 strain did not show any transformation irrespective of the explant type tested, while MTCC 2251 strain failed to induce transgenic calli from epicotyle explants. The leaves were found to be easier to transform in comparison to petioles and epicotyles for MTCC 431 strain. This may be due to their higher regeneration capacity and better wounding surface as compared to the other two explants. Higher transformation efficiency of leaves was observed with MTCC 431 strain (70%), while epicotyle portions were better transformed with MTCC 609 strain (55%). MTCC 2251 strain exhibited the lowest infectivity of 10% and 25% for petiole and leaf explants, respectively. A. tumefaciens MTCC 609 also resulted in callus induction when the infection period was increased by 10 min, with only the leaf and epicotyl explants. This might be due to variation in virulence of different strains of Agrobacterium. A petiole explant was difficult for the Agrobacterium transformation. The % transformation efficiency, growth index and glycyrrhizin content of various transformed cell lines of A. precatorius are given in Table 1.
Table 1.
Transformed cell lines from different A. precatorius explants*
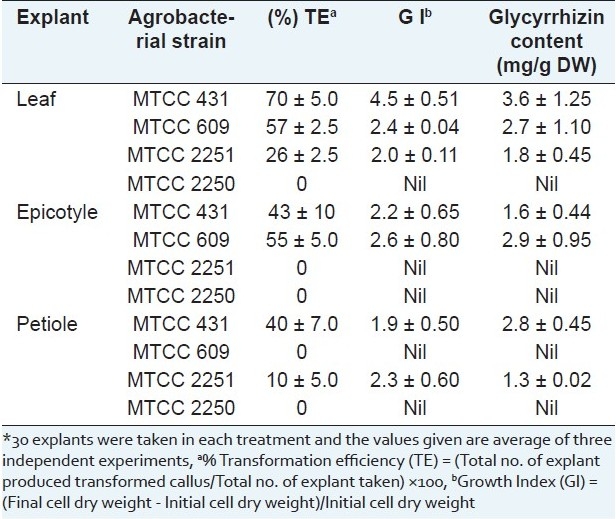
Acetosyringone or correlated compounds functioning as signal molecules have been reported to improve the Agrobacterium -mediated transformation in several plant species.[17,18] Figure 1 shows the effect of acetosyringone on the transformation efficiency of the leaf explant when treated with different Agrobacterial strains. A high concentration of acetosyringone (50 mM) improved the transformation efficiency to a maximum of 85%. A large variation in the glycyrrhizin content was found among the developed cell lines. This difference in glycyrrhizin accumulation pattern may be due to the fact that cell lines were initiated from different explants having variable glycyrrhizin contents. Somaclonal variations and differences in genotypes of seeds collected from wild could also contribute to these differences.[19] The transformed cell culture having higher glycyrrhizin contents over the untransformed cell culture, might be due to their rapid growth of the tumors. Sharma and Dixit[2] reported higher content of artemisinin in the A. tumefaciens transformed culture of Artemisia annua over the untransformed culture. Similarly higher content of polysaccharides in the A. rhizogenes mediating transformed culture of Echinacea purpurea over the untransformed culture was also reported.[20]
Figure 1.
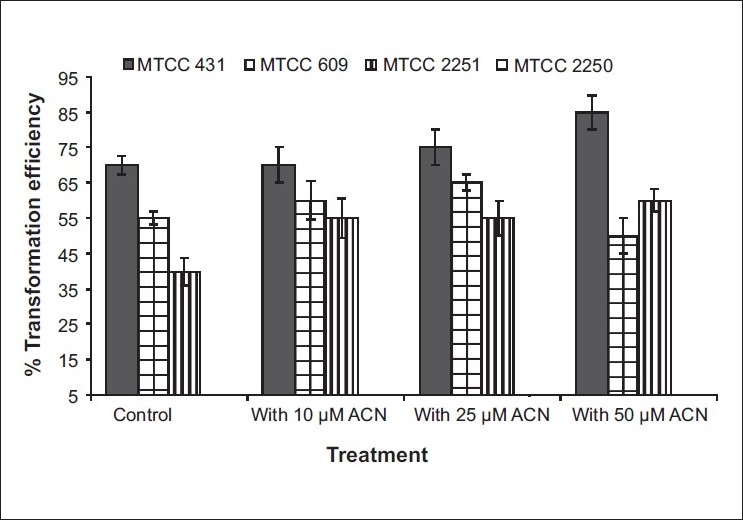
Effect of acetosyringone on the (%) transformation efficiency of A. precatorius* leaf explants. (*30 explants were used in each treatment. No transformation was found with MTCC 2250 strain with any treatment. Average values are given; error bars are represented as vertical lines)
On the basis of higher glycyrrhizin content and growth index, transformed cell lines induced from A. tumefaciens MTCC 431 infected leaf explants were chosen for further study. Excised tumor clumps, cultured on MS solidified medium devoid of growth regulators, grew rapidly. The transformed leaf calli were used for establishment of the transformed suspension cultures. Transformed cell cultures developed by infection of leaves of in vitro germinated A. precatorius with MTCC 431 strain was selected as high glycyrrhizin yielding cell line (3.6 mg/g DW). This tumor cell line had friable consistency and cream to brownish in color. In the case of transformed cell suspension cultures, maximal dry cell weight (19.61 ± 2.72 g/l) occurred at 15th day of cultivation with 30 g/l sucrose in MS medium which was more than twofold increase over the untransformed culture [Figure 2]. This was associated with increase in glycyrrhizin accumulation up to 16.4 mg/l, which indicated combined growth associated product formation. This phenomenon was also observed in cell suspension cultures of Podophyllum hexandrum[21] and Azadirachta indica.[22] After the linear growth phase up to 15 days, a similar decline phase was also observed. The highest accumulation of biomass and product was correlated well with about 80% consumption of initial sugar. It has been found that transformed cell suspension cultures derived from this tumorous calli can accumulate glycyrrhizin (16.42 ± 1.41 mg/l DW). This was two times higher than the untransformed cell suspension cultures. The untransformed cell suspensions where growth hormones were crucial for their growth as well as glycyrrhizin production, the transformed cultures were able to grow in hormone autotrophic medium.
Figure 2.
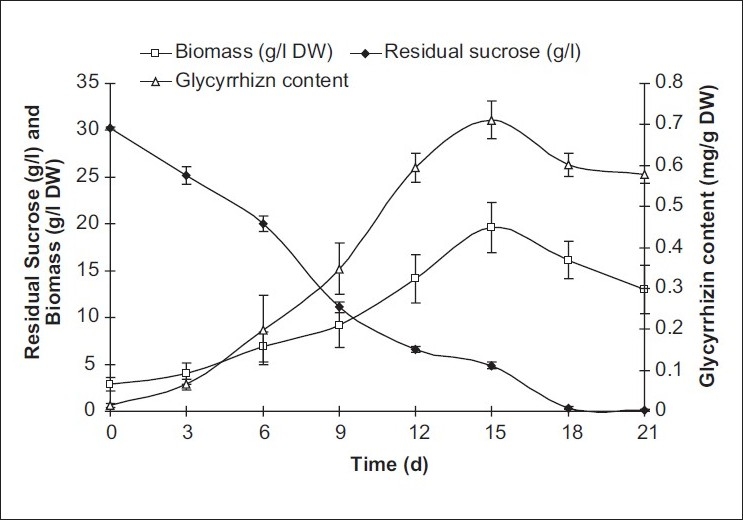
Substrate consumption profiles, biomass, and glycyrrhizin accumulation of A. precatorius transformed cell suspension cultures (average values are given; error bars are represented as vertical lines)
Molecular analysis of tumor tissue
Agrobacterium harbors a large tumor-inducing (Ti) plasmid that contains a region responsible for transfer of Ti plasmid (T-DNA) and another region encodes for virulence (vir) genes. The virulence genes mediate the transfer of T-DNA region into infected plant cells, where T-DNA integrate into nuclear DNA via illegitimate recombination and mediate the expression of the tumor growth inducing genes in plant cells. To detect the transformation and integration of Ti-plasmid into plant cells, PCR reaction was performed using Agrobacterial DNA (as a positive control), nontransformed DNA (as a negative control) obtained from callus cultures of A. precatorius, and primer pair specific to opine synthase gene. Gel electrophoresis of amplified opine synthase gene PCR products indicates the successful transformation of T-DNA from Agrobacterium plasmid to the transformed callus cell lines [Figure 3].
Figure 3.
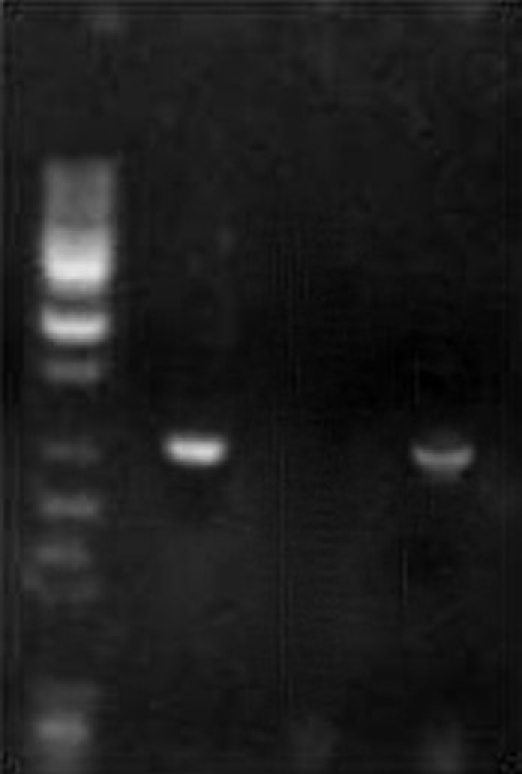
PCR analysis of transformed cultures of A. precatorius showing the presence or absence of the opine synthase gene; Lane I: 500-bp ladder, Lane II: positive control (pTi-431), Lane III: negative control (Abrus leaf), Lane IV: transformed cell culture
Influences of fungal elicitors on culture biomass and glycyrrhizin production
When checked for fungal elicitation at different incubation periods, mostly the elicitation was caused on the 5th day after elicitor treatment. Figures 4 and 5 depict the influence of CF and DCP of A. niger and R. stolonifer on glycyrrhizin accumulation in A. precatorius cell cultures, respectively. Studies were also conducted on 3rd, 5th, 7th, and 9th days after elicitor treatment. Highest elicitation of about 4.9-fold higher glycyrrhizin production over that of control culture was recorded with CF of A. niger after 5th day of treatment at 7.5% v/v elicitor concentration [Figure 4]. Biomass accumulation was increased 1.47-fold compared to the control culture on 5th day after treatment at 7.5% v/v concentration. CF of R. stolonifer at 5.0% (v/v) concentration caused about 3.8-fold higher glycyrrhizin production over the control culture on the 5th day after elicitor treatment [Figure 5]. Meanwhile biomass accumulation (11.658 g/l DW) was increased to a highest 1.74-fold compared to the control (5.586 g/l DW) with 7.5 % v/v CF of R. stolonifer on 3rd day after elicitor treatment.
Figure 4.
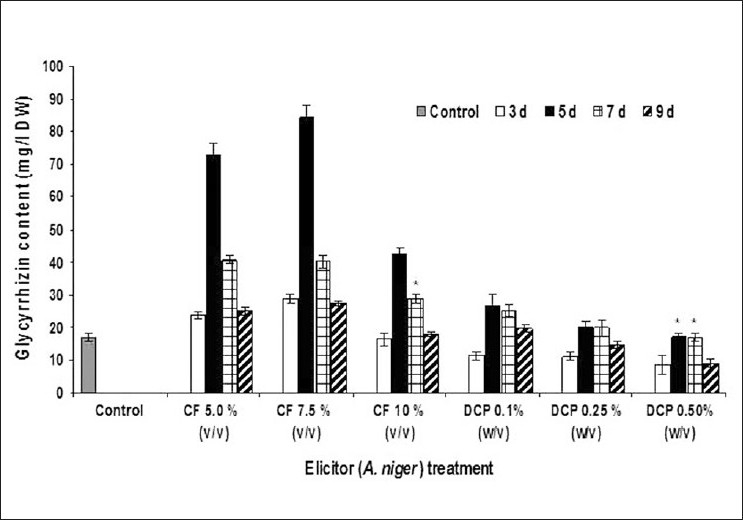
Effect of Aspergillus niger culture filtrate (CF) and dried cell powder (DCP) on the glycyrrhizin accumulation in the transformed cell suspension culture of A. precatorius. *Values were significant at P ≤ 0.05. The data shown are mean of three replicates and ±S.D. values presented as error bars.
Figure 5.
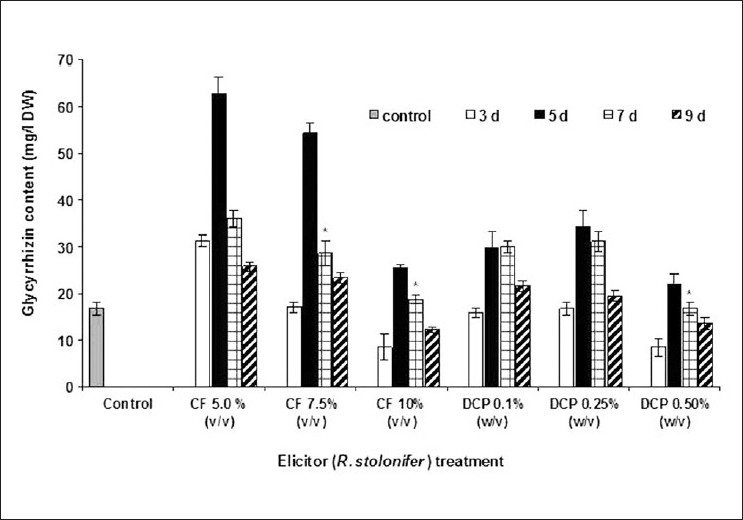
Effect of Rhizopus stolonifer culture filtrate (CF) and dried cell powder (DCP) on the glycyrrhizin accumulation in the transformed cell suspension culture of A. precatorius. *Values were significant at P ≤ 0.05. The data shown are mean of three replicates and ±S.D. values presented as error bars
Unlike fungal CFs, treatment with DCP of both fungi resulted in marginal elicitation, not appreciably higher at any of the treatment. The DCP of R. stolonifer caused a 2.1-fold increase in the glycyrrhizin production compared over the control with 0.25% (w/v) concentration, on 5th day after elicitor treatment [Figure 5]. There was a maximum 1.58-fold increase in biomass accumulation (10.586 g/l DW) over the control (6.709 g/l DW) at 0.25%w/v concentration, on 5th day after treatment. Whereas the DCP of A. niger could not cause any marked elicitation i.e. this could be only about 1.65-fold increase in glycyrrhizin production over the control cultures at 5.0% (w/v) concentration, on 5th day after elicitor treatment [Figure 5]. While there was a marginal 1.07-fold increases in the biomass accumulation that was seen at 0.1% w/v treatment with DCP of A. niger and 5th day after treatment over the control culture. It is widely accepted that microbial foray on intact plants will trigger plant defense mechanisms such as synthesis of secondary metabolites. Fungal elicitation increased oleandrin production in the A. tumefaciens transformed Nerium oleander cell suspension culture.[23] A large and rapid increase in ajmalicine in the cultures of C. roseus has been reported when fungal elicitors were added.[24,25] This might be possible that the species-specific action of elicitor's cell debris on plant cells of A. precatorius stimulates the enzyme system of glycyrrhizin synthesis by recognizing the cell debris as an invading pathogen upon contact with the cell debris. This may affect the intermediate formation of glycyrrhizin biosynthesis. Thus, we propose that the higher concentrations of glycyrrhizin accumulated in the cells (threefold higher than those of control cultures) upon elicitation with fungal CF elicitors is because of the result of the stimulation of the enzyme system of glycyrrhizin synthesis by the fungal cell debris. Similarly treatment of the C. roseus suspension cell culture with the fungal CF resulted in two- to fivefold increase in the biosynthesis of indole alkaloids.[26]
In conclusion, this study represents a successful cell-culture-based approach for the production of glycyrrhizin from A. precatorius. The present plant cell cultures study was addressed through the transformation of Abrus giving transformed callus cell lines and elicitation, which resulted in significant improvement in glycyrrhizin production. Genetic confirmation of transformed cultures indicated that cells have integration of Ti-T-DNA. Acetosyringone has a significant effect on the agrobacterial transformation efficiency. The agrobacterium transformed cell culture and further elicited with A. niger CF at 7.5% (v/v) concentration resulted in a maximum synergistic promotion of glycyrrhizin accumulation, i.e. 4.9-fold higher compared to transformed control cultures. This study indicates the potential of these biotechnology-based methodologies for large-scale production of glycyrrhizin. Furthermore, in order to develop a process for commercial production of glycyrrhizin by plant cell cultures some additional yield enhancement strategies may be worked out like, optimization of medium composition, culture conditions and addition of precursors.
ACKNOWLEDGMENTS
The authors are thankful to Dr. Ashish Baldi, Department of Biochemical Engineering and Biotechnology, Indian Institute of Technology, Hauz Khas, New Delhi, India for his valuable and timely assistance. The author VSK wishes to acknowledge All India Council for Technical Education, New Delhi for providing junior research scholarship.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Yeoman MM, Yeoman CL. Manipulating secondary metabolism in cultured plant cells. New Phytol. 1996;134:553–69. doi: 10.1111/j.1469-8137.1996.tb04921.x. [DOI] [PubMed] [Google Scholar]
- 2.Sharma T, Dixit VK. Studies on Ti-mediated transformed cultures of Artemisia annua L. Indian J Pharm Sci. 2006;68:448–55. [Google Scholar]
- 3.Zhang Y, Song J, Zhao B, Liu H. Crown gall culture and production of tanshinone in Salvia miltiorrhiza. Chin J Biotechnol. 1995;11:137–41. [PubMed] [Google Scholar]
- 4.Savitha BC, Timmaraju R, Bhagyalaksami N, Ravishankar GA. Different biotic and abiotic elicitors influence betalin production in hairy root cultures of Beta vulgaris in shake flask and bioreactor. Process Biochem. 2006;41:50–60. [Google Scholar]
- 5.Walker TS, Bais HP, Vivanco JM. Jasmonic acid induced hypercin production in Hypericum perforatum L. (St. John wort) Phytochemistry. 2002;60:289–93. doi: 10.1016/s0031-9422(02)00074-2. [DOI] [PubMed] [Google Scholar]
- 6.Katz V, Fuchs A, Conrath U. Pretreatment with salicylic acid primes parsley cells for enhanced ion transport following elicitation. FEBS Lett. 2002;520:53–7. doi: 10.1016/s0014-5793(02)02759-x. [DOI] [PubMed] [Google Scholar]
- 7.Nge KL, New N, Chandrkrachang S, Stevens WF. Chitosan as a growth stimulator in orchid tissue culture. Plant Sci. 2006;170:1185–90. [Google Scholar]
- 8.Yan Q, Hu Z, Tan RX, Wu J. Efficient production and recovery of diterpenoid tanshinones in Salvia miltiorrhiza hairy root cultures with in situ adsorption, elicitation and semi-continuous operation. J Biotechnol. 2005;119:416–24. doi: 10.1016/j.jbiotec.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Mukundan U, Hjortso MA. Thiophene accumulation in hairy root cultures of Tagetes patula in response to fungal elicitors. Biotechnol Lett. 1990;12:609–14. [Google Scholar]
- 10.Ramakrishna SV, Reddy RG, Curtis WR, Humphry E. Stimulation of solavetivone synthesis in free and immobilized cells of Hyoscyamus muticus by Rhizoctonia solani fungal components. Biotechnol Lett. 1993;15:307–10. [Google Scholar]
- 11.Tabata M, Fujita Y. Orlando, FL: Academic Press; 1985. Production of shikonin by plant cell cultures. Biotechnology in Plant Science; p. 207. [Google Scholar]
- 12.Fu XQ, Lu DW. Stimulation of shikonin production by combined fungal elicitation and in situ extraction in suspension cultures of Arnebia euchroma. Enzyme Microb Technol. 1999;24:243–6. [Google Scholar]
- 13.Asada M, Shuler ML. Stimulation of ajmalicine production and excretion from Catharanthus roseus: Effects of adsorption in situ, elicitors and alginate immobilization. Appl Microbiol Biotechnol. 1989;30:475–81. [Google Scholar]
- 14.Sudhaker TJ, Ravishankar GA, Venkataraman LV. Elicitation of capsaicin production in freely suspended cells and immobilized cell cultures of Capsicum frutescens Mill. Food Biotechnol. 1991;5:197–205. [Google Scholar]
- 15.Karwasara VS, Jain R, Tomar P, Dixit VK. Elicitation as yield enhancement strategy for glycyrrhizin production by cell cultures of Abrus precatorius Linn. In vitro Cell Dev Biol Plant. 2010;46:354–62. [Google Scholar]
- 16.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–6. [Google Scholar]
- 17.Satchel SE, Messens E, Van Montagu M, Zambryski P. Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium rhizogenes. Nature. 1985;318:624–9. [Google Scholar]
- 18.Karwasara VS, Dixit VK. Agrobacterium rhizogenes mediated genetic transformation of Abrus precatorius Linn. Pharmacogn Mag. 2009;5:336–42. [Google Scholar]
- 19.Phillips RL, Kaeppler SM, Olhoft P. Genetic instability of plant tissue cultures: Breakdown of normal controls. Proc Natl Acad Sci U S A. 1995;91:5222–6. doi: 10.1073/pnas.91.12.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang B, Zhang G, Zhua L, Chena L, Zhang Y. Genetic transformation of Echinacea purpurea with Agrobacterium rhizogenes and bioactive ingredient analysis in transformed cultures. Colloids Surf B Biointerfaces. 2006;53:101–4. doi: 10.1016/j.colsurfb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Chattopadhyay S, Srivastava AK, Bhojwani SS, Bisaria VS. Development of suspension culture of Podophyllum hexandrum for the production of podophyllotoxin. Biotechnol Lett. 2001;23:2063–6. [Google Scholar]
- 22.Prakash G, Emmannuel CJ, Srivastava AK. Variability of azadirachtin in Azadirachta indica (neem) and batch kinetics studies of cell suspension culture. Biotechnol Bioprocess Eng. 2005;10:198–204. [Google Scholar]
- 23.Ibrahim AK, Khalifa S, Khafagi I, Youssef D, Khan IM. Stimulation of oleandrin production by combined Agrobacterium tumefaciens mediated transformation and fungal elicitation in Nerium oleander cell cultures. Enzyme Microb Technol. 2007;41:331–6. [Google Scholar]
- 24.Zhao J, Zhu WH, Hu Q. Enhanced ajmalicine production in Catharanthus roseus cell cultures by combined elicitor treatment: From shake-flask to 20-l airlift bioreactor. Biotechnol Lett. 2000;22:509–14. [Google Scholar]
- 25.Namdeo A, Patil S, Fulzele DP. Influence of fungal elicitors on production of ajmalicine by cell cultures of Catharanthus roseus. Biotechnol Prog. 2002;18:159–62. doi: 10.1021/bp0101280. [DOI] [PubMed] [Google Scholar]
- 26.Zhao J, Zhu W, Hu Q. Selection of fungal elicitors to increase indole alkaloid accumulation in Catharanthus roseus suspension cell culture. Enzyme Microb Technol. 2001;28:666–72. doi: 10.1016/s0141-0229(01)00309-x. [DOI] [PubMed] [Google Scholar]


