Abstract
Background:
The current study was carried out to determine whether the aqueous-ethanolic extract or the butanolic fraction of Nigella sativa (NS) seeds could prevent or reduce calculi aggregation in experimental calcium oxalate nephrolithiasis in Wistar rats.
Materials and Methods:
Male Wistar rats were randomly divided into 5 groups: group A received tap drinking water for 28 days. Groups B, C, D and E received 1% ethylene glycol for induction of calcium oxalate (CaOx) calculus formation for 28 days. Rats in groups C, D and E also received aqueous-ethanolic extract of NS, N-butanol fraction and N-butanol phase remnant of NS, respectively, in drinking water at a dose of 250 mg/kg for 28 days. Urine concentration of oxalate, citrate, and calcium on days 0, 14, and 28, and also serum concentration of magnesium and calcium on days 0 and 28, were measured. On day 29, kidneys were removed for histopathologic study and examined for counting the calcium oxalate deposits in 10 microscopic fields.
Result:
Treatment of rats with N-butanol fraction and N-butanol phase remnant of NS significantly reduced the number and size of kidney calcium oxalate deposits compared with ethylene glycol group. Urinary concentration of oxalate in all experimental groups increased compared with control group on days 14 and 28, whereas the urine citrate concentration was lower in all experimental groups compared with control group on days 14 and 28.
Conclusion:
N-butanol fraction and N-butanol phase remnant of NS showed a beneficial effect on calcium oxalate deposition in the rat kidney. Therefore, the butanolic fraction of NS may be suggested for prevention of calcium oxalate calculi in humans.
Keywords: Calcium oxalate, ethylene glycol, kidney calculi, N-butanol fraction, Nigella sativa
INTRODUCTION
Urinary calculi are highly prevalent and affect the population in all countries.[1–3] Calcium oxalate (CaOx) and calcium phosphate stones are the highest common calculi which form approximately 80% of stones in the urinary system.[2,4–6] Urinary stones may cause infection, hemorrhage, obstruction, and hydronephrosis in the urinary tract. Kidney stones are also a major risk of chronic kidney diseases.[7] Extracorporeal shock wave lithotripsy (ESWL), percutaneous lithotomy (PNL), transureteral lithotripsy using laser (TUL), electrohydrolic, ultrasound, Swiss LithoClast, and even laparoscopy are widely used to remove the calculi. These invasive procedures employed for removing urinary stones are expensive and may also cause serious complications.[1,2] Therefore, it is worthwhile to look for alternative treatments by using medicinal plants or phytotherapy to replace these conventional methods. Medicinal plants are used worldwide and there is also an increasing interest for research in this area to provide a scientific explanation for their beneficial effects.[8–14]
Nigella sativa L. (NS) or black seeds have been used in traditional medicine for centuries.[15,16] Black seeds have been reported to be analgesic, antidiabetic, anticancer, anticonvulsant, anti-inflammatory, and antioxidant; it also lowers serum cholesterol and triglyceride, balances enzyme activity, increases glutathione in the kidney, and repairs kidney tissue of the nephrotoxicity.[6,17–23] We have previously reported the preventive effect of ethanolic extract of black seeds on kidney stone formation.[24] For further investigation in this line, we decided to investigate the effect of aqueous–ethanolic extract of NS and its N-butanol fractions on ethylene-glycol (EG)-induced CaOx kidney calculi in rats.
MATERIALS AND METHODS
Preparation of extract
The NS seeds were purchased from a local herb store in Mashhad, Iran; and approved by herbarium of Ferdowsi University (Mashhad, Iran). The black seeds were powdered and 500 g of powder were macerated in a 50% aqueous–ethanol solvent and kept at 40°C in an incubator for 72 h while it was mixed every 6 h during the incubation time. The mixture was filtered through a filter paper and the solvent was removed and the concentrated extract was used in this experiment. For preparation of N-butanol fraction, 10 g of concentrated extract was repeatedly dissolved in 50 mL N-butanol in a decanter funnel to separate the intermediate compounds. The solvent was removed and concentrated fraction was used. The remnant part on the decanter funnel that composed of polar and nonpolar particles were concentrated and used as N-butanol phase remnant in this study.
Experimental protocol
Male Wistar rats, weighing 200 ± 20 g were randomly divided into 5 groups (A, B, C, D, E). All groups were kept at 25°C ± 2°C with standard diet and treated for 28 days. The animal procedures were complied with the international guidelines and the Guide for Care and Use of Laboratory Animals and approved by Mashhad University of Medical Sciences, Mashhad, Iran.
Rats in group A as intact control animals received tap drinking water and group B (EG controls) received 1% EG (Merck KGaA, Darmstadt, Germany) in drinking water for 28 days. In addition to 1% EG, rats in group C were treated with 250 mg/kg of the aqueous-ethanolic extract of NS and groups D and E were treated with the equivalent of 250 mg/kg of the N-butanol phase and N-butanol phase remnant respectively added in drinking water for 28 days. Twenty-four hour urine samples were collected on the 1st, 14th, and 28th days of the study while each rat was kept in a separate metabolic cage. Blood was collected from the orbital cavernous sinus on the first and the 28th days of the study.
Serum concentration of calcium and magnesium were measured by enzymatic methods and xylidyl blue reaction, respectively. Urine oxalate and citrate were measured by enzymatic methods (Darman Kaw, Tehran, Iran) and using autoanalyzer.
Histopathology study
At the end of the experiment (day 29), all rats were decapitated by guillotine. Their kidneys were removed, weighed, and kept in formalin 10% for histopathology studies. Five micron sections were prepared from both kidneys, and the slides were stained with hematoxylin-eosin method. The slides were examined under light microscope and CaOx deposits were determined. Aggregations of CaOx deposits in renal tubules and calyces were counted in 10 microscope fields.
Statistical analysis
Data were expressed as mean ± SEM and were analyzed by analysis of variance (one-way ANOVA) test to find the differences among all groups, and Tukey test for comparison between each two groups. P value less than 0.05 was considered as significant.
RESULTS
No deposits of CaOx or other pathological defects were found in different segments of the nephrons of the rats in group A (intact control group) [Figure 1]. But CaOx crystals in the proximal tubules, loops of Henle, distal tubules, collecting ducts, and calyces of the rat kidney in group B were plenty [Figures 2 and 3]. The mean number of CaOx deposits in 10 microscopic fields of the kidney in group B was 26.85 ±4.27 [Figure 4], which was significantly more than group A (P < 0.001).
Figure 1.
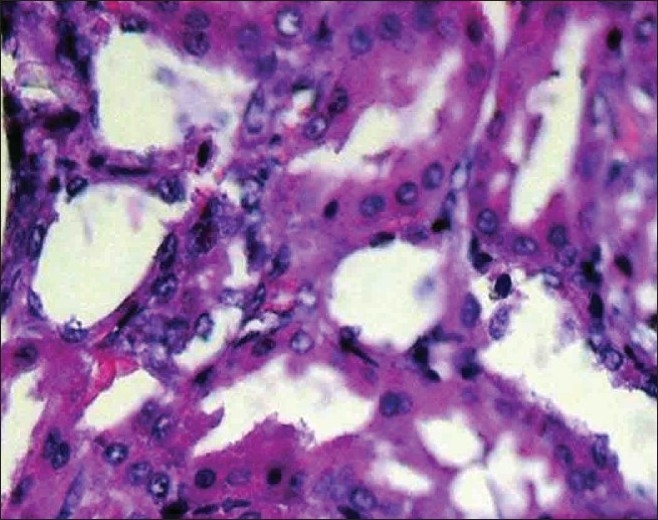
Normal tubules and collecting ducts (H and E, ×400)
Figure 2.
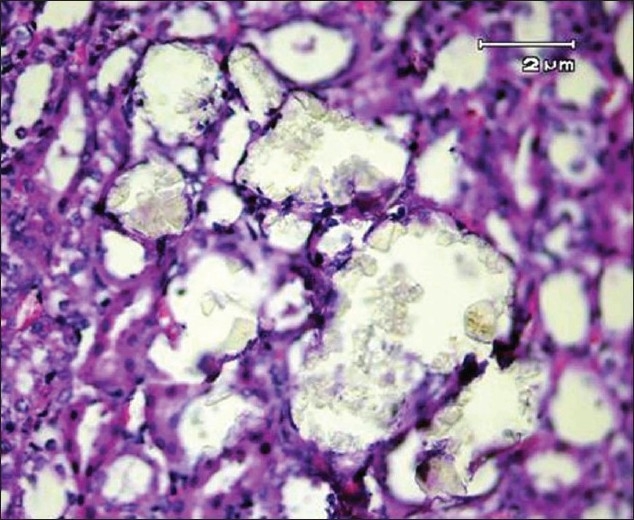
Calcium oxalate crystals in renal tubules of an ethylene glycol-treated rat (H and E, ×400)
Figure 3.
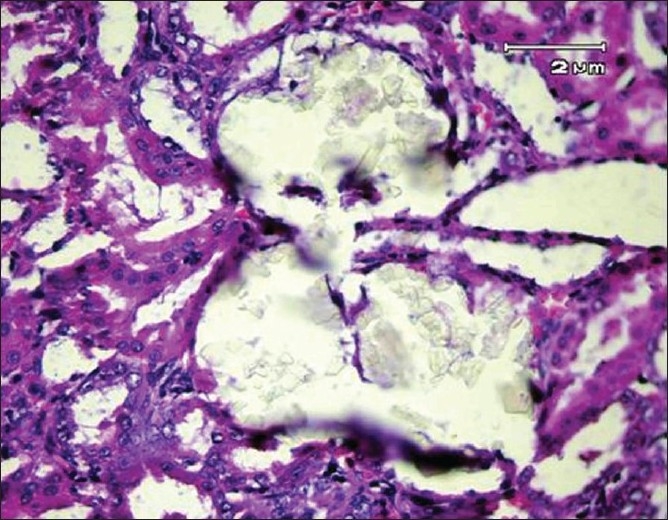
Secondary renal tubular dilation with epithelial damage and leukocyte reaction (H and E, ×400)
Figure 4.
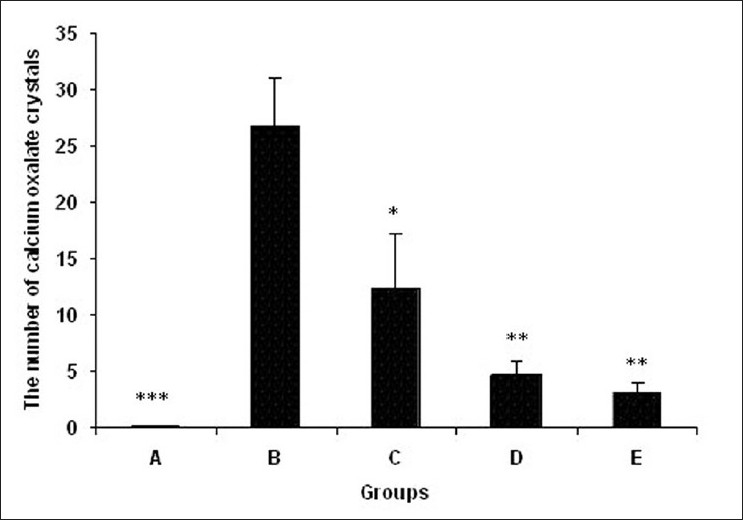
The number of calcium oxalate crystals in 10 microscopic fields in experimental groups. (A) Control group (n=7), (B) ethylene glycol group (n=8), (C) group treated with aqueous–ethanolic extract of NS (250 mg/kg, n=7), (D) group treated with remnant phase of N-butanol (250 mg/kg, n=7), (E) group treated with N-butanol fraction of NS (250 mg/kg, n=8). Data were expressed as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 vs group B
In group C, crystals were thin and tiny compared with group B, and the number of CaOx deposits in 10 microscopic fields of the kidney was 12.5 ± 4.78, which was significantly lower than group B (Figure 4, P < 0.05).
In the kidney specimens of groups D and E, deposits were composed of tiny and thin CaOx crystals developed in different parts of the nephron tubules. The number of deposits in 10 microscopic fields of kidney slices in group D was 4.66 ± 1.35 and in group E was 3.12 ± 0.89 that were both significantly lower than group B (Figure 4, P < 0.01).
Urine oxalate concentration in all experimental groups increased in comparison with group A on days 14 and 28 (Figure 5, P < 0.05).
Figure 5.
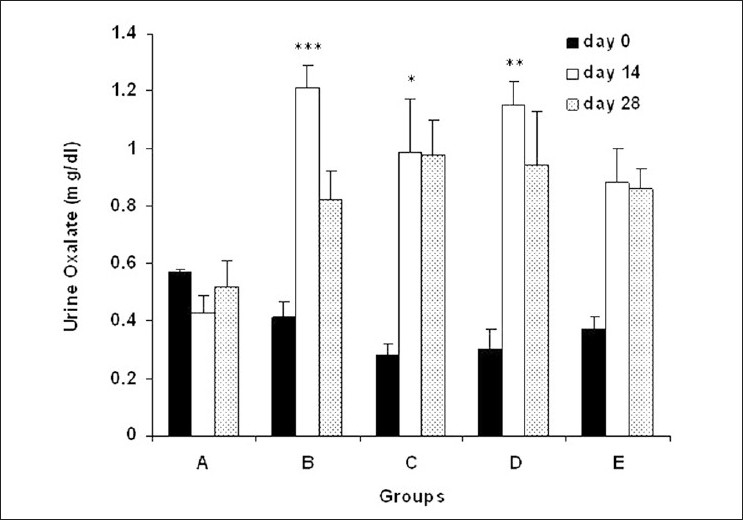
Urine oxalate concentration in experimental groups. (A) Control group (n=7), (B)ethylene glycol group (n=8), (C) group treated with aqueous–ethanolic extract of NS (250 mg/kg, n=7), (D) group treated with remnant phase of N-butanol (250 mg/kg, n=7), (E) group treated with N-butanol fraction of NS (250 mg/kg, n=8). Data were expressed as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 vs. group A
Urine citrate concentration was lower in all experimental groups compared with group A on day 14 and 28. However, urine citrate concentration was only in group E significantly lower than group A on day 28 (Figure 6, P < 0.05).
Figure 6.
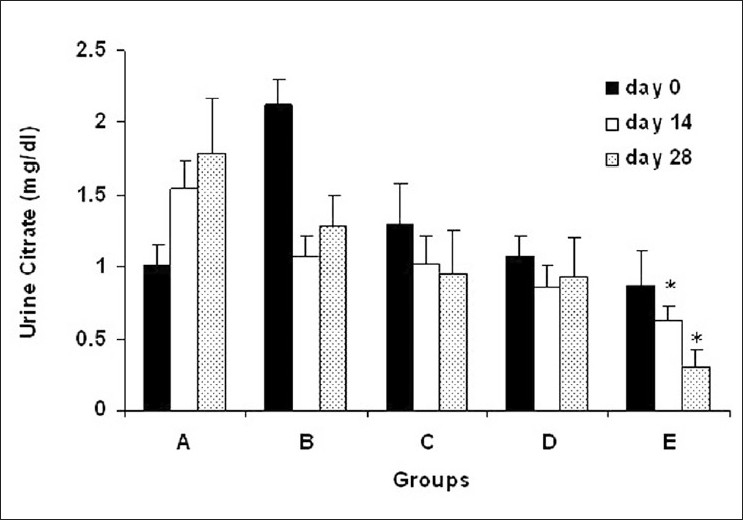
Urine citrate concentration in experimental groups. (A) Control group (n=7), (B)ethylene glycol group (n=8), (C) group treated with aqueous–ethanolic extract of NS (250 mg/kg, n=7), (D) group treated with remnant phase of N-butanol (250 mg/kg, n=7), (E) group treated with N-butanol fraction of NS (250 mg/kg, n=8). Data were expressed as mean ± SEM, *P < 0.05 vs. group A
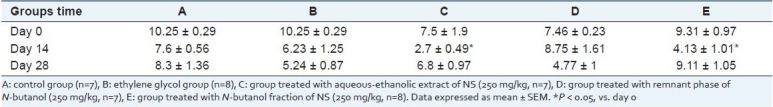
Urine calcium concentration in groups C and E on day 14 was significantly lower than day 0 in these groups (Table 1, P < 0.05).
Table 1.
Urine calcium concentration (mg/dL) in experimental groups
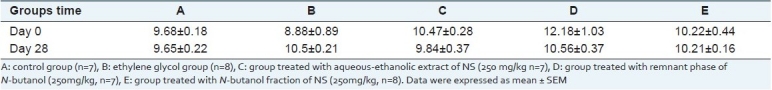
Serum magnesium concentration in group E on day 28 was significantly lower than day 0 in this group (Table 2, P < 0.05). There was no significant difference in serum calcium concentration among experimental groups in this study [Table 3].
Table 2.
Serum magnesium concentration (mg/dL) in experimental groups

Table 3.
Serum calcium concentration (mg/dL) in experimental groups
DISCUSSION
Our data demonstrated that both N-butanol fraction and N-butanol phase remnant from 50% aqueous-ethanolic extract of NS seeds had a preventive effect on CaOx calculi formation in the rat kidney. Similarly, 50% aqueous–ethanolic extract of NS seeds showed such a preventive action on kidney CaOx stone formation.
This study showed that the preventive effects of N-butanol and N-butanol phase remnant fractions were more potent than 50% aqueous–ethanolic extract. In another report, we also demonstrated that ethanolic extract of NS at a dose of 250 mg/kg had both preventive and curative effects on kidney stone formation in rat, although its preventive action was stronger.[24]
In another report from our laboratory, it was also demonstrated that ethyl-acetate phase remnant from 50% aqueous–ethanolic extract of NS seeds had a complete preventive effect on EG-induced kidney stones in rat [unpublished data].
The basis for calcium stone formation is supersaturation of urine with stone-forming calcium salts. A number of dietary factors and metabolic abnormalities can change the composition or saturation of the urine so as to enhance stone-forming propensity. Among the metabolic conditions are hypercalciuria, hypocitraturia, and hyperoxaluria.[25] However, the role of other factors, such as inhibitors, infection, matrix formation, as well as urinary obstruction, should not be ignored.[26]
Hypercalciuria and hyperoxaluria are important factors in the process of CaOx stone formation; N-butanol fraction was able to reduce urine calcium on day 14 but increased urine oxalate at the same time. Thus, it seems that the preventive effect of N-butanol fraction on CaOx formation cannot be attributed to alteration of urine oxalate concentration. However, reduction of urine calcium concentration induced by NS extract and N-butanol phase remnant in groups C and E, respectively, can be suggested as part of reason associated with prevention of CaOx stone formation in these groups.
Although hyperoxaluria and hypocitraturia are known as risk factors of calculi formation, preventive action of 50% aqueous–ethanolic extract, N-butanol fraction, and N-butanol phase remnant on CaOx crystals formation cannot be attributed to changes in urine oxalate or citrate, since these groups showed a reduction in urine citrate but an increase in urine oxalate concentration.
Calcium oxalate crystals and high oxalate levels in nephrons can produce damages in the epithelial cells, and consequently, the cells may produce some products, as well as free radicals, which induce heterogenous crystal nucleation and cause aggregation of crystals.[27]
Black seeds cultivated in Khorasan Province contain tannin, flavonoids, and alkaloids, which constitute a portion of the aqueous–ethanolic extract of the seeds.[28] Nonpolar components, such as thymoquinone, fatty acids, vitamin E, and carotene are present in N-butanol fraction of NS. Thymoquinone is the major non-polar compound in this fraction. Thymoquinone has antioxidant effect and scavenges superoxide anions and free radicals, and on the other hand, it also inhibits 5-lipoxygenase and cycloxigenase pathways and so stops inflammatory response and its products formation.[29,30] Accordingly, preventive actions of NS fractions and extract on CaOx crystals aggregation are partly due to its antioxidant and anti-inflammatory effects.
In a recent report we demonstrated that thymoquinone has both preventive and also a disruptive effect on CaOx crystals induced by EG in rats.[31] Hereby, it may be suggested that the preventive effect of N-butanol fraction in the present study is at least partially due to the thymoquinone action.
N-butanol phase remnant fraction and aqueous-ethanolic extract have polar and intermediate compounds such as tannin, flavonoids and alkaloids. Flavonoids including quercetin, kaempferol and glyceride flavonoids also have anti-inflammatory and antioxidant effects.[32–35] Thus it may be suggested that the effects of NS seeds fractions in preventing of CaOx calculi formation in the present study are in part caused by anti-inflammatory and antioxidant action of NS seeds components. These compounds are able to inhibit the process of epithelial cell injury and inflammation induced by CaOx crystals.[30]
In summary, according to this study, 50% aqueous–ethanolic extract of NS seeds and its fractions showed a clear preventive effect on CaOx formation. This beneficial action may be attributed to antioxidant as well as anti-inflammatory actions of NS extract and its fragments in the rat kidney rather than alternation in serum or urine ions concentration.
ACKNOWLEDGMENT
The results presented in this work has been taken from the student's thesis. This study was supported by a grant from the Council of Research, Mashhad University of Medical Sciences.
Footnotes
Source of Support: Grant from the Council of Research, Mashhad University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Ramello A, Vitale C, Marangella M. Epidemiology of nephrolithiasis. J Nephrol. 2000;13:S45–50. [PubMed] [Google Scholar]
- 2.Gault MH, Chafe L. Relationship of frequency, age, sex, stone weight and composition in 15,624 stones: Comparison of results for 1980 to 1983 and 1995 to 1998. J Urol. 2000;164:302–7. [PubMed] [Google Scholar]
- 3.El-Reshaid K, Mughal H, Kapoor M. Epidemiological profile, mineral metabolic pattern and crystallographic analysis of urolithiasis in Kuwait. Eur J Epidemiol. 1997;13:229–34. doi: 10.1023/a:1007346727944. [DOI] [PubMed] [Google Scholar]
- 4.Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest. 2005;115:2598–608. doi: 10.1172/JCI26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menon M, Resnick MI. Campbell's Urology. 8th ed. Philadelphia: WB Saunders; 2002. Urinary lithiasis: Etiology, diagnoses, and medical management; pp. 3229–305. [Google Scholar]
- 6.Al-Ghamdi MS. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J Ethnopharmacol. 2001;76:45–8. doi: 10.1016/s0378-8741(01)00216-1. [DOI] [PubMed] [Google Scholar]
- 7.Rule AD, Bergstralh EJ, Melton LJ, 3rd, Li X, Weaver AL, Lieske JC. Non-Calculus signs and symptoms of Hyperoxaluria and Hyperuricosuria in children: A single experience. Clin J Am Soc Nephrol. 2009;4:804–11. doi: 10.2215/CJN.05811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajzadeh MA, Khoei A, Hadjzadeh Z, Parizady M. Ethanolic extract of Nigella Sativa L seeds on ethylene glycol-induced kidney calculi in rats. Urol J. 2007;4:86–90. [PubMed] [Google Scholar]
- 9.Christina AJ, Packia Lakshmi M, Nagarajan M, Kurian S. Modulatory effect of Cyclea peltata Lam. on stone formation induced by ethylene glycol treatment in rats. Methods Find Exp Clin Pharmacol. 2002;24:77–9. doi: 10.1358/mf.2002.24.2.677130. [DOI] [PubMed] [Google Scholar]
- 10.Atmani F, Slimani Y, Mimouni M, Aziz M, Hacht B, Ziyyat A. Effect of aqueous extract from Herniaria hirsuta L. on experimentally nephrolithiasic rats. J Ethnopharmacol. 2004;95:87–93. doi: 10.1016/j.jep.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 11.Satish H, Raman D, Kshama D, Shivananda BG, Shridhar KA. Study the relative effect of spironolactone and different solvent extract of Tibulus terrestrison urolithiatic rats. Pharmacogn Mag. 2009;5:83–9. [Google Scholar]
- 12.Vyas BA, Vyas RB, Joshi SV, Santani DD. Antiurolithiatic activity of whole-plant hydroalcoholic extract of Pergularia daemia in rats. J Young Pharm. 2011;3:36–40. doi: 10.4103/0975-1483.76417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajzadeh MA, Behnam-Rassouli F, Khajavirad A, Mahmoudian A. The effects of N-butanol fraction and N-butanol phase remnant from 50% aqueous-ethanolic extract of Cyndon dactylon on calcium oxalate kidney stones in rat. Pharmacognosy Res. 2009;1:431–4. [Google Scholar]
- 14.Ghodasara J, Pawar A, Deshmukh C, Kuchekar B. Inhibitory effect of rutin and curcumin on experimentally-induced calcium oxalate urolithiasis in rats. Pharmacognosy Res. 2010;2:388–92. doi: 10.4103/0974-8490.75462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aghili-Khorasani MH. Nigella sativa. In: Makhzan-Al-Adviah, editor. Tehran, Iran: Islamic Publishing and Educational Organization; 1992. pp. 556–8. [Google Scholar]
- 16.Mir-Heidar H. 6th ed. Tehran Iran: Islamic Culture Press; 2004. Encyclopedia of Medicinal Plants of Iran; pp. 211–4. [Google Scholar]
- 17.Hosseinzadeh H, Parvardeh S. Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine. 2004;11:56–64. doi: 10.1078/0944-7113-00376. [DOI] [PubMed] [Google Scholar]
- 18.Hosseinzadeh H, Parvardeh S, Nassiri-Asl M, Mansouri MT. Intracerebroventricular administration of thymoquinone, the major constituent of Nigella sativa seeds, suppresses epileptic seizures in rats. Med Sci Monit. 2005;11:106–10. [PubMed] [Google Scholar]
- 19.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14:323–8. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 20.Sattar A, Zaman-Latif MS, Tayyib M. Estimation of serum lipids in albino rats fed on atherogenic supplemented palm oil diet and Nigella sativa. J Rawal Med Coll. 2002;6:48–51. [Google Scholar]
- 21.Badary OA, Abdel-Naim AB, Abdel-Wahab MH, Hamada FM. The influence of thymoquinone on doxorubicin-induced hyperlipidemic nephropathy in rats. Toxicology. 2000;143:219–26. doi: 10.1016/s0300-483x(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 22.El-Dakhakhny M, Mady N, Lembert N, Ammon HP. The hypoglycemic effect of nigella sativa oil is mediated by extrapancreatic actions. Planta Med. 2002;68:465–6. doi: 10.1055/s-2002-32084. [DOI] [PubMed] [Google Scholar]
- 23.Mojab F. Nigella sativa. In: Ghasemi-Dehkordy N, editor. Iranian Herbal Pharmacopia. Tehran, Iran: Council of Food and Drugs, Ministry of Health and Medical Education; 2002. pp. 466–70. [Google Scholar]
- 24.Hajzadeh MA, Khoei A, Hadjzadeh Z, Parizady M. Ethanolic extract of Nigella sativa L seeds on ethylene glycol-induced kidney calculi in rats. Urol J. 2007;4:86–90. [PubMed] [Google Scholar]
- 25.Park S, Pearle MS. Pathophysiology and management of calcium stones. Urol Clin North Am. 2007;34:323–34. doi: 10.1016/j.ucl.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Miller NL, Evan AP, Lingeman JE. Pathogenesis of renal calculi. Urol Clin North Am. 2007;34:295–313. doi: 10.1016/j.ucl.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Khan SR, Thamilselvan S. Nephrolithiasis: A consequence of renal epithelial cell exposure to oxalate and CaOx crystals. Mol Urol. 2000;4:305–12. [PubMed] [Google Scholar]
- 28.Bazzaz BS, Haririzadeh G, Imami SA, Rashed MH. Survey of Iranian plants for alkaloids, flavonoids, saponins, and tannins [Khorasan Province] Pharm Biol. 1997;35:17–30. [Google Scholar]
- 29.Houghton PJ, Zarka R, De las Heras B, Hoult JR. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;61:33–6. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- 30.El-Dakhakhny M, Madi NJ, Lambert N, Ammon HP. Nigella sativa oil, nigellone and derived thymoquinone inhibit synthesis of 5-lipoxygenase products in polymorphonuclear leukocytes from rats. J Ethnopharmacol. 2002;81:161–4. doi: 10.1016/s0378-8741(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 31.Hajzadeh MA, Mohammadian N, Rahmani Z, Behnam F. Effect of thymoquinone on ethylene glycol-induced kidney calculi in rats. Urol J. 2008;5:149–54. [PubMed] [Google Scholar]
- 32.Ahmed MS, El Tanbouly ND, Islam WT, Saleem AA, El Senousy AS. Antiinflammatory flavonoids from Opuntia dillenii (Ker-Gawl) Haw. flowers growing in Egypt. Phytother Res. 2005;19:807–9. doi: 10.1002/ptr.1708. [DOI] [PubMed] [Google Scholar]
- 33.Xu J, Li X, Zhang P, Li ZL, Wang Y. Antiinflammatory constituents from the roots of Smilax bockii warb. Arch Pharm Res. 2005;28:395–9. doi: 10.1007/BF02977667. [DOI] [PubMed] [Google Scholar]
- 34.Comalada M, Ballester I, Bailón E, Sierra S, Xaus J, Gálvez J, et al. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure-activity relationship. Biochem Pharmacol. 2006;72:1010–21. doi: 10.1016/j.bcp.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 35.Nair MP, Mahajan S, Reynolds JL, Aalinkeel R, Nair H, Schwartz SA. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) geneexpression in normal peripheral blood mononuclear cells via modulation of the NF-Kappa beta system. Clin Vaccine Immunol. 2006;13:319–28. doi: 10.1128/CVI.13.3.319-328.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


