Abstract
Recently, exercise has been recommended as a part of lifestyle modification for all hypertensive patients; however, the precise mechanisms of its effects on hypertension are largely unknown. Therefore, this study aimed to investigate the mechanisms within the brain that can influence exercise-induced effects in an animal model of human essential hypertension. Young normotensive WKY and SHR rats were given moderate-intensity exercise for 16 weeks. Blood pressure was measured bi-weekly by tail-cuff method. Animals were then euthanized; paraventricular nucleus (PVN) and rostral ventrolateral medulla (RVLM), important cardiovascular regulatory centers in the brain, were collected and analyzed by Real-time RT-PCR, western blot, EIA, and fluorescent microscopy. Exercise of 16 weeks duration attenuated systolic, diastolic, and mean arterial pressure in SHR. Sedentary SHR exhibited increased proinflammatory cytokines (PICs) and decreased anti-inflammatory IL-10 levels in the PVN and RVLM. Furthermore, SHRsed rats exhibited elevated levels of ACE, AT1R, and decreased levels of ACE2 and receptor Mas in the PVN and RVLM. Chronic exercise not only prevented the increase in PICs (TNF-α, IL-1β), ACE, and AT1R protein expression in the brain of SHR, but also dramatically upregulated IL-10, ACE2, and Mas receptor expression in SHR. In addition, these changes were associated with reduced plasma AngII levels, reduced neuronal activity, reduced NADPH-oxidase subunit gp91phox and inducible NO synthase (iNOS) in trained SHRs indicating reduced oxidative stress. These results suggest that chronic exercise not only attenuates PICs and the vasoconstrictor axis of the RAS but also improves the anti-inflammatory defense mechanisms and vasoprotective axis of the RAS in the brain, which, at least in part, explains the blood pressure-lowering effects of exercise in hypertension.
Keywords: Exercise, cytokines, angiotensin, hypertension, oxidative stress, brain
2. Introduction
Hypertension is the most common chronic disease in the United States, currently affecting more than 33% of US adults[36]. Uncontrolled hypertension may lead to coronary heart disease, heart failure, chronic renal failure, and stroke. Though, the brain has typically been considered as a target for late stage hypertensive disease, a growing body of evidence has implicated brain in the initiation of all forms of hypertension including essential hypertension[25]. One of the important hallmarks of hypertension is chronic low-grade inflammation. Past few years of research has implicated brain cytokines in the pathogenesis of hypertension. It is apparent from these studies that pro-inflammatory cytokines (PICs) such as tumor necrosis factor (TNF)-α and interleukin (IL)-1 act as neuromodulators and play a pivotal role in sympathetic regulation of blood pressure (BP)[53]. In addition, recent discoveries indicate that besides elevated levels of circulating and brain PICs[48, 53], anti-inflammatory cytokines (AICs) such as IL-10 has a significant impact on sympathetic outflow, arterial pressure and cardiac remodeling in experimental models of hypertension[53]. In the brain, paraventricular nucleus (PVN) and rostral ventrolateral medulla (RVLM) are known to be the most important cardiovascular regulatory centers playing role in sympathetic regulation of BP. Studies over the last several decades have also established that an over-activity of the brain/central renin-angiotensin system (RAS) plays a vital role in development and maintenance of genetic hypertension [62]. Interestingly, it is becoming clear from previous studies that cytokines and RAS interacts with each other, possibly via production of reactive oxygen species (ROS), and thereby regulate BP[4, 43, 53, 66].
Although, several currently available anti-hypertensive medications targeting RAS and sympathetic nervous system have been found to be effective in reducing BP, still more than 50% of those diagnosed with hypertension fail to respond to these anti-hypertensive regimens. Moreover, recent epidemiological studies suggest a strong relationship between sedentary behavior and hypertension and therefore, physical activity has been recommended as a first line intervention for preventing and treating patients with hypertension[7]. Several experimental, clinical, and epidemiological studies have clearly shown that physical activity reduces BP in animals and humans[3, 58]. Recent studies have explored the possible mechanisms underlying the exercise-induced attenuation of BP; however, the exact mechanisms of exercise-induced effects in hypertension are still poorly understood. Few studies on obese individuals[13, 38, 65] and diabetic patients[19] have documented the reduction in inflammatory markers by exercise. However, until now, no studies have examined the effect of chronic exercise on brain pro- and anti-inflammatory cytokines in hypertension. In addition, effects of physical activity on sympathetic activity and vasodilatory or vascoconstrictory components of central RAS in setting of hypertension has never been investigated yet.
Therefore, this study was undertaken to gain more insight into the effects of regular long-term exercise within the brain (PVN and RVLM) of hypertensive animals. We hypothesize that 1) chronic, regular moderate intensity exercise training would attenuate blood pressure in spontaneously hypertensive rats (SHRs) 2) regular exercise would improve the balance between central anti- and pro-inflammatory cytokines in SHRs; 3) chronic exercise would modulate components of RAS and reduce oxidative stress in the brain of SHRs; and 4) exercise would attenuate neuronal excitation in the brain of SHRs. Understanding of mechanisms of exercise-induced benefits in hypertension may lead us to develop most efficient exercise regimen for hypertensive patients.
3. Materials and Methods
3.1 Animals
Seven-week–old male normotensive Wistar-Kyoto (WK) and spontaneously hypertensive (SHR) rats were used in this study. Animals were housed in a temperature- (25±1oC) and light-controlled (12:12 hour light:dark cycle) room with free access to food and water. All of the procedures in this study were approved by the Louisiana State University Institutional Animal Care and Use Committee and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
3.2 Experimental protocol
WK and SHR rats were randomly assigned either to the sedentary group (SHRsed, n=20; WKsed, n=20) or to the exercise group (SHRex, n=20; WKex, n=20). Exercise groups were subjected to moderate-intensity exercise for 16 weeks. Body weight was recorded 24 hours after the last exercise session, animals were then euthanized, and brain tissue was harvested. PVN and RVLM tissues were punched from the excised brain for later analyses. We performed the following experimental procedures: real time RT-PCR, Western blot analysis, immunofluorescence, EIA, and statistical analysis.
3.3 Exercise protocol
Exercise groups were subjected to moderate-intensity exercise on a motor-driven treadmill continuously for a period of 16 weeks (5 days per week; 60 min per day at 18 m/min, 0o inclination) which includes an acclimation period of 2 weeks. After acclimation, training intensity was set at approximately 60% of maximal aerobic velocity (MAV), which corresponds to moderate intensity exercise (18-20m/min). This training intensity was maintained throughout the study period. The MAV was evaluated from an incremental exercise test as reported previously [6, 58]. The rats in sedentary groups were kept in the treadmill for the same duration as exercising rats except that the treadmill was not turned on.
3.4 Assessment of efficacy of the exercise protocol
Citrate synthase (CS), a respiratory enzyme which has been shown to undergo adaptive increases due to exercise in skeletal muscle fibers, was used as a marker of training efficacy. Soleus muscles from both legs of each animal were collected and stored at –80 °C for determination of CS activity (n=8 per group), a measure of muscle oxidative capacity, to determine the efficacy of the training protocol [47]. CS activity was measured from whole muscle homogenate by using commerically available citrate synthase activity assay kit (Sigma Aldrich). Briefly, muscle tissue from each animal were homogenized in an extraction buffer (50 mM Tris·HCl and 1 mM EDTA, pH 7.4). After centrifugation at 13,000 rpm, for 1 min, at 4° C, aliquots of supernatants were used for the measurement of the enzyme activity. The activity of CS was expressed as nanomoles per minute per milligram of protein. Protein content of muscle homogenate was determined as described by Bradford using bovine serum albumin as a standard.
3.5 Blood pressure measurements
Systolic, diastolic, and mean arterial blood pressure (BP) were measured noninvasively using a Coda 6 Blood Pressure System (Kent Scientific, Torrington, CT), as described previously[3]. BP was measured at baseline (7 weeks of age) and then every two weeks until the end of the study period. BP was measured on three consecutive days, and values were averaged from at least six consecutive cycles.
3.6 Real-time RT-PCR Analysis
Semi-quantitative real-time RT-PCR (n=9 per group) was used to determine the mRNA levels of RAS components viz. angiotensin converting enzyme (ACE), ACE2 AT1R, and receptor Mas; PICs viz. tumor necrosis factor-alpha (TNF-α), and interleukin (IL)-1β; oxidative stress markers viz. gp91phox, and iNOS in the PVN and RVLM by using specific primers. Rat primers used are listed in the Table2. Briefly, the rats were euthanized, the brains were quickly removed, immediately frozen on dry ice, and blocked in the coronal plane. Brains were then sectioned at 100 um thickness, and the PVN and RVLM were punched from each brain according to the methods described by Palkovits and Brownstein [15]. Total RNA isolation, cDNA synthesis and RT-PCR were performed as previously described [57]. Gene expression was measured by the ΔΔCT method and was normalized to GAPDH mRNA levels. The data are presented as the fold change of the gene of interest relative to that of control animals.
Table 2. Rat primers used for real-time RT-PCR.
| Gene | Sense | Antisense |
|---|---|---|
| GAPDH | agacagccgcatcttcttgt | cttgccgtgggtagagtcat |
| TNF-α | gtcgtagcaaaccaccaagc | tgtgggtgaggagcacatag |
| IL-1β | gcaatggtcgggacatagtt | agacctgacttggcagaga |
| IL-10 | gggaagcaactgaaacttcg | atcatggaaggagcaacctg |
| ACE | ttgacgtgagcaacttccag | cagatcaggctccagtgaca |
| ACE2 | acccttcttacatcagccctactg | tgtccaaaacctaccccacatat |
| AT1R | cacgagacacagcctttc | ttaaaatcgaccgtgtgcag |
| Mas | tccaccaagacctgctagga | tcttgtgctggaccacttca |
| gp91phox | cggaatctcctctccttcct | gcattcacacaccactccac |
| iNOS | ccttgttcagctacgccttc | ggtatgcccgagttctttca |
3.7 Western Blot Analysis
The tissue homogenate from the PVN and RVLM were subjected to western blot analysis (n=6 per group) for determination of protein levels of tyrosine hydroxylase (TH), 67-kDa isoform of glutamate decarboxylase (GAD67), RAS components (ACE, ACE2 AT1R, and Mas), PICs (TNF-α, IL-1β), oxidative stress markers (gp91phox, iNOS), and GAPDH. The PVN and RVLM tissues were collected as described under the section ‘real-time RT-PCR analysis’.The tissues were then homogenized in 100 ul of RIPA lysis buffer (Cell Signaling Technology, Inc., MA) containing protease inhibitor cocktail. The protein was extracted from the homogenates, and the protein concentration in the lysate was measured using a Bradford assay using BSA standards. Protein extracts (30 μg) were combined with an equal volume of × Laemmli loading buffer, boiled for 5 minutes and electrophoresed on 10-15% SDS-polyacrylamide gels. The proteins were then electroblotted onto polyvinylidene fluoride membranes (Immobilon-P, Millipore). Non-specific binding was blocked by incubating the membranes in 1% casein in phosphate-buffered saline-Tween for 1 h at room temperature (RT). Blots were then incubated overnight at 4°C with the primary antibodies. Specific antibodies used included TNF-α, IL-1β, gp91phox, iNOS, GAPDH, AT1R, ACE, and ACE2, GAD67, at 1:1000 dilution; TH and receptor Mas, at 1:200 dilution; and IL-10, at 1;500 dilutions. Antibodies were commercially obtained: TNF-α, AT1R, TH, GAD67 (Abcam Inc, MA); IL-1β, iNOS, GAPDH, ACE, and ACE2 (Santa Cruz Biotechnology, Santa Cruz, CA); IL-10 (Abbiotec, CA); receptor Mas (Alomone Labs Ltd., Jerusalem, Israel), and gp91phox (BD biosciences, USA). After washing with wash buffer (× TBS, 0.1% Tween-20) four times for 10 min each time at RT, blots were then incubated for 1 h with secondary antibody (1:10,000 dilution, Santa Cruz Biotechnology) labeled with horseradish peroxidase. Immunoreactive bands were visualized using enhanced chemiluminescence (ECL Plus, Amersham), band intensities were quantified using VersaDoc MP 5000 imaging system (Bio-Rad), and were normalized with GAPDH.
3.8 Immunofluorescence Staining
Immunofluorescence technique was used to determine the protein expression of PICs, RAS components and oxidative stress markers. The immunostaining protocol used was modified from Block et al [5]. Briefly, the rats (n=5 per group) were deeply anesthetized with carbon dioxide and perfused transcardially with PBS (pH 7.4), followed by 4% paraformaldehyde in PBS. The brain was then removed, postfixed for 2 h in 10% paraformaldehyde in PBS, and coronal sections (20 μm) were made in a cryostat. The sections were incubated in xylene solution for 15 minutes at RT, two times followed by dehydration in ethanol. The sections were then washed in PBS, three times, 5 minutes each. Antigen retrieval was then performed using citrate target retrieval solution (Biocare Medical, CA). Slides were then washed with PBS and the nonspecific staining was blocked with 2% normal donkey serum containing 1% bovine serum albumin (BSA) for 1 h at RT. Sequentially, the tissues were incubated with the primary antibodies TNF-α (Abcam Inc, MA), IL-10 (Abbiotec, CA), ACE (Santa Cruz, CA), ACE2 (Santa Cruz, CA), and AT1R (Abcam Inc, MA), at1:50 dilution for each, overnight at 4°C. The sections were then incubated either with Alexa 594-labeled anti-mouse secondary antibody (red fluorescence), Alexa 488-labeled anti-rabbit secondary antibody (green fluorescence), or Alexa 594-labeled anti-rabbit secondary antibody (red fluorescence) (Invitrogen, CA), at 1:500 dilution for 2 h at RT. The sections were rinsed 3 times in PBS and mounted in ProLong® Gold antifade reagent (Invitrogen). The stained sections were photographed with a confocal laser-scanning microscope.
3.9 Reverse-Phase High-Performance Liquid Chromatography
Plasma norepinephrine (NE) levels were measured using reverse-phase high-performance liquid chromatography (HPLC) with electrochemical detection (ECD) using an Eicom HTEC-500 system fitted with HPLC-ECD as described previously [3, 26].
3.10 Measurment of plasma angiotensin II levels
Plasma angiotensin II (AngII) levels were determined by using commercially available enzyme immunoassay (EIA) kit (Phoenix pharmaceuticals, Inc, CA).
3.11 Statistical Analysis
All data are presented as means±SE. Statistical analysis was done by either two-way ANOVA or one-way ANOVA with a Bonferroni post hoc test using Graph Pad Prism software (version 5.0). Blood pressure data were analyzed by repeated-measures ANOVA to examine with-in group changes over time. Results were considered significant when p<0.05.
4. Results
4.1 Assessment of training efficacy
Citrate synthase (CS) activity in soleus muscle was used as a marker of training efficacy. After the period of 16 weeks of exercise, the CS activity was significantly higher in SHR and WKY rats compared with their sedentary control groups indicating the efficacy of the exercise protocol (Fig1). CS activity was higher in WKY rats compared with SHR both in the exercise and the sedentary group.
Fig 1.
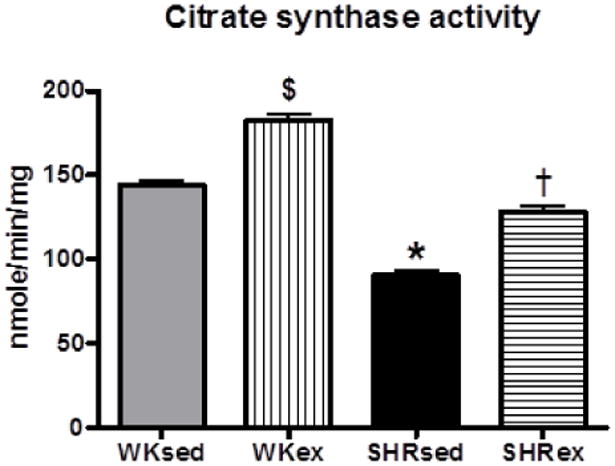
Citrate synthase activity (nmol/min1/mg of protein) in soleus muscle of sedentary or exercised SHR and WKY as measured by citrate synthase activity assay kit. After the period of 16 weeks of exercise, the activity of citrate synthase in the soleus muscle was significantly higher in SHR as well as in WKY rats compared with their sedentary control groups indicating the efficacy of the exercise protocol. $p<0.05 WKsed vs WKex; *p<0.05 WKsed vs SHRsed; †p<0.05 SHRsed vs SHRex
4.2 Body Weight
Body weight (BW) did not differ among groups at the start of the experiment. At the end of the study period, BW was not significantly different between the WKsed and SHRsed groups. Chronic exercise resulted in reduction in BW in both WK and SHR rats (Table1).
Table 1.
Effect of exercise training on body weight and plasma norepinephrine and plasma AngII levels in normotensive WK and SHR rats.
| Parameters | WKsed | WKex | SHRsed | SHRex |
|---|---|---|---|---|
| Number of animals (n) | 10 | 10 | 10 | 10 |
| Body weight (grams) | 377.3 ± 7.3 | 333.3 ± 8.4$ | 380.5 ± 3.5 | 327.6 ± 6.2† |
| Plasma Norepinephrine (pg/μl) | 16.50 ± 1.2 | 13.50 ± 1.1 | 69.56 ± 3.9* | 18.59 ± 0.5† |
| Plasma AngII (ng/ml) | 88.26 ± 3.6 | 90.83 ± 3.2 | 127.6 ± 5.4* | 94.30 ± 3.5† |
Data are mean±SE.
p<0.05 WKsed vs SHRsed;
p<0.05 SHRsed vs SHRex;
p<0.05 WKsed vs WKex.
4.3 Chronic exercise reduces blood pressure in SHRs
Systolic, diastolic, and mean arterial blood pressure (SBP, DBP, and MAP, respectively) were significantly higher in SHRsed than in WKsed rats at the beginning of the experiment (at age 7 weeks) and remained increased for the duration of the study (Fig 2). Chronic exercise resulted in significantly reduced SBP, DBP, and MAP in SHRex rats when compared with SHRsed rats; the significant difference in BP was observed beginning from 8 weeks of exercise. Exercise did not affect BP in WK rats (Fig 2).
Fig 2.
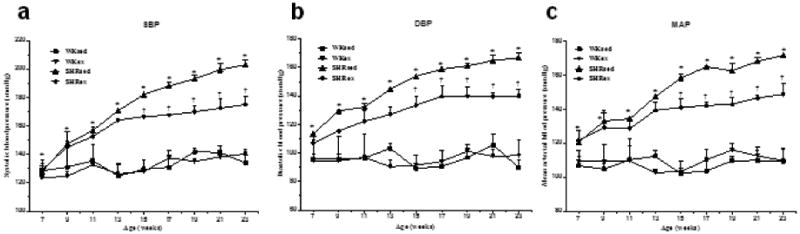
Effect of chronic exercise on time course of blood pressure (in millimeters of mercury) in WK rats and SHRs. A, systolic blood pressure (SBP); B, diastolic blood pressure (DBP); C, mean arterial pressure (MAP). Exercise significantly reduced SBP, DBP, and MAP in SHRex compared with SHRsed rats from 8 weeks of exercise (at 15 weeks of age). Values are mean±SE; n=10 in each group. *p0.05 WKsed vs SHRsed; †p0.05 SHRsed vs SHRex
4.4 Chronic exercise attenuates pro-inflammatory cytokines in the PVN and RVLM of SHRs
To investigate the influence of exercise on PICs within the brain of hypertensive rats, we examined the mRNA and protein levels of TNF-α, and IL-1β in the PVN and RVLM. We observed that SHRsed rats exhibited marked increases in TNF-α and IL-1β expression in the PVN (Fig 3a, 3b, and 6) as well as in the RVLM (Fig 3c, 3d) compared to WKsed. This upregulation of TNF-α and IL-1β was significantly attenuated by chronic exercise in SHRs. At the mRNA level, chronic exercise in SHR resulted in 7 fold decrease in TNF-α and 5 fold decrease in IL-1β expression in the PVN (Fig 3a), whereas these changes were 6 fold and 4 fold, respectively in the RVLM of SHRex rats (Fig 3c). Exercise did not change PIC levels in WK rats.
Fig 3.

Effects of exercise on TNF-α, IL-1β, and IL-10 expression in the PVN and RVLM of WK rats and SHRs. mRNA expression in the PVN (a); A representative Western blot (left panel, b) and densitometric analysis (right panel, b) of TNF-α and IL-10 protein expression in the PVN; mRNA expression (c) and densitometric analysis (d) of these cytokines in the RVLM. Values are mean±SE. *p<0.05 WKsed vs. SHRsed; †p<0.05 SHRsed vs SHRex. n=9/group for mRNA analysis and n=6/group for protein analysis
Fig 6.

A representative confocal photomicrographs (×20) showing the effects of exercise on protein expression of ACE, ACE2, and TNF-α in the PVN of WK rats and SHRs (n=5/group). Scale bar=100 μ m. 3V,third ventricle
4.5 Exercise improves balance between pro- and anti-inflammatory cytokines in the PVN and RVLM of SHRs
To investigate the influence of exercise on anti-inflammatory status within the PVN and RVLM, we determined the mRNA and protein levels of IL-10, a potent AIC. To further investigate whether chronic exercise has ability to improve the balance between PICs and AICs in the brain of hypertensive rats, we determined the alterations in ratio of TNF-α to IL-10 protein levels in these rats. A significant attenuation in the levels of IL-10 in the hypertensive sedentary rats compared with the WKsed rats was evident within the PVN (Fig 3a, 3b) as well as RVLM (Fig 3c, 3d, and 7). Moreover, SHRsed rats had higher TNF-α/IL-10 ratio in the PVN (Fig 3b) and RVLM (Fig 3d) compared to WKsed. These results provide further evidence that an imbalance between PICs and AICs plays role in pathogenesis of hypertension. Interestingly, exercise resulted in significantly increased levels of IL-10 in the brain of SHRs. We observed 93% and 85% increase in IL-10 mRNA levels in the PVN and RVLM of trained SHRs, respectively. These results were also accompanied by dramatic decrease in TNF-α/IL-10 ratio in SHRs indicating an improvement in balance between PICs and AIC by chronic exercise.
Fig 7.
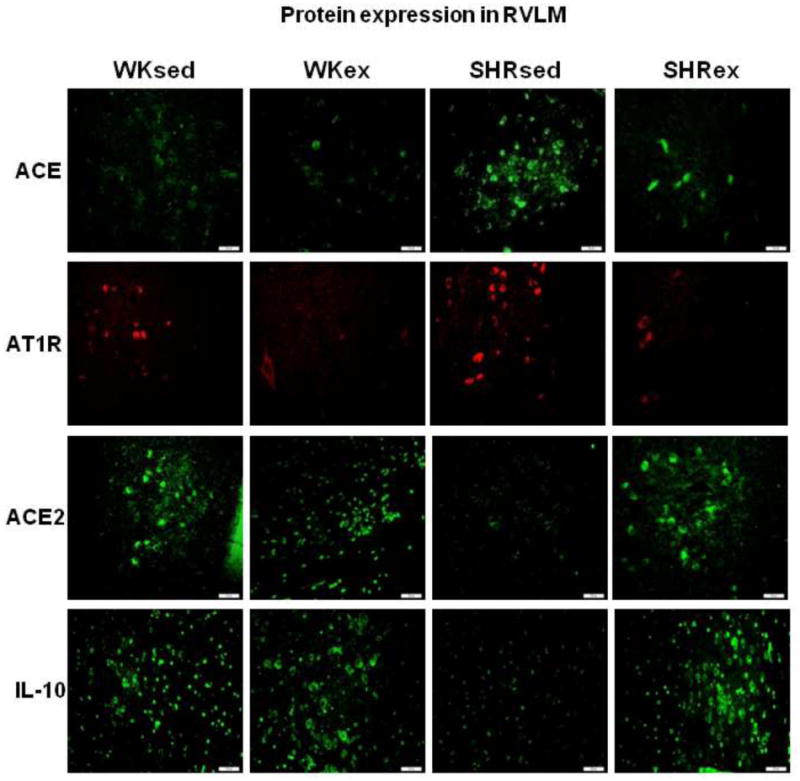
A representative confocal photomicrographs (×20) showing the effects of exercise on protein expression of ACE, AT1R, ACE2, and IL-10 in the RVLM of WK rats and SHRs (n=5/group). Scale bar=100 μm
4.6 Chronic exercise modulates RAS components in the PVN and RVLM of SHRs
To determine whether chronic exercise modulates vasoconstrictory and vasodilatory components of RAS, we examined the mRNA and protein levels of ACE, AT1R, ACE2, and Mas (receptor of Ang(1-7), an AngII metabolite with vasodilator properties) in the PVN and RVLM. At basal conditions, sedentary SHRs exhibited marked increase in ACE and AT1R in the PVN (Fig 4 and 6) and RVLM (Fig 5 and 7) compared to WKsed. In addition, ACE2 and Mas levels were significantly reduced in SHRsed compared to WKsed. These results indicate the existence of an imbalance between vasoconstrictor and vasodilatory axis of RAS in hypertensive rats. Interestingly, chronic exercise prevented the increase in ACE and AT1R expression in SHR. At the mRNA level, ACE expression in SHRex was lowered by 85% and 77% in the PVN and RVLM, respectively when compared to SHRsed (Fig 4a and 5a). Furthermore, expression of ACE2 and Mas, were dramatically upregulated in trained SHR. ACE2 expression was elevated by 9 fold in the PVN and 5 fold in the RVLM of SHRex compared to SHRsed. Similarly, Mas levels were increased by about 82% in the SHRex group. However, exercise did not change levels of these RAS components in WK rats. Additionally, plasma angiotensin II (AngII) levels were found to be significantly higher in SHRsed compared with WKsed rats, whereas, chronic exercise resulted in significantly decreased plasma AngII concentrations in SHRs but did not change plasma AngII level in WK rats (Table 1).
Fig 4.
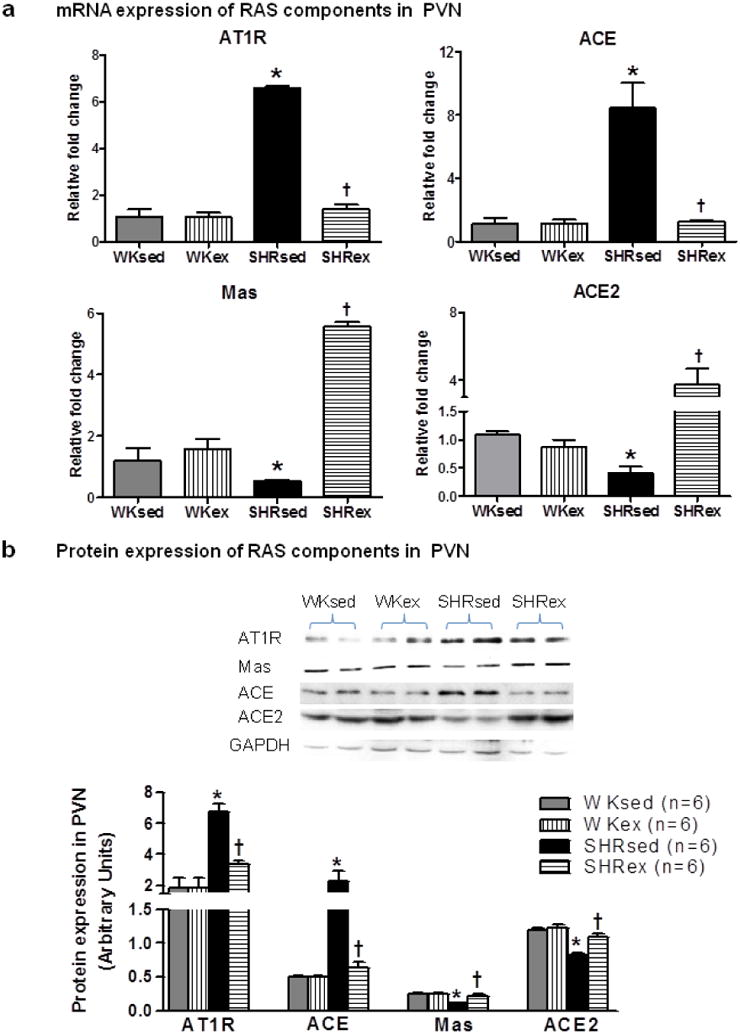
Effects of exercise on RAS components (AT1R, ACE, Mas, and ACE2) in the PVN of WK rats and SHRs. a, mRNA expression; b, densitometric analysis of protein expression and a representative Western blot. Values are mean±SE. *p<0.05 WKsed vs. SHRsed; †p<0.05 SHRsed vs SHRex. n=9/group for mRNA analysis and n=6/group for protein analysis
Fig 5.

Effects of exercise on RAS components (AT1R, ACE, Mas, and ACE2) in the RVLM of WK rats and SHRs. a, mRNA expression; b, densitometric analysis of protein expression and a representative Western blot. Values are mean±SE. *p<0.05 WKsed vs. SHRsed; †p<0.05 SHRsed vs SHRex. n=9/group for mRNA analysis and n=6/group for protein analysis
4.7 Chronic exercise reduces oxidative stress in the PVN and RVLM of SHR
Because Ang II, through activation of AT1R, regulates NAD(P)H oxidase and contributes to oxidative stress, the expression of gp91phox, a subunit of NAD(P)H oxidase, was analyzed in the brain of training and control groups. Expression of gp91phox was markedly higher in SHRsed when compared to WKsed rats; this expression was significantly reduced by chronic exercise (Fig 8). In trained SHRs, a diminished mRNA expression of gp91phox by 59% in the PVN and 77% in the RVLM was observed compared with the SHRsed group. The training-associated lower mRNA expression was linked to a significant reduction in protein expression as well (Fig 8).
Fig 8.
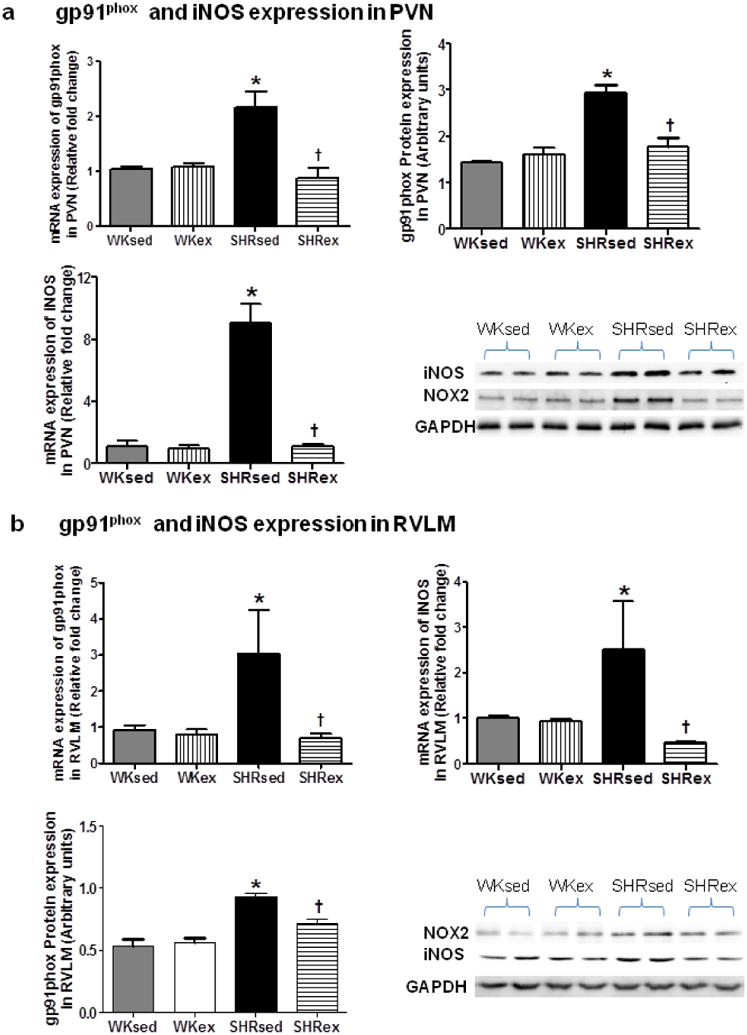
Effects of exercise on gp91phox and iNOS expression in the PVN and RVLM of WK rats and SHRs. mRNA and protein expression of gp91phox and iNOS in the PVN (a) and RVLM (b). Values are mean±SE. *p<0.05 WKsed vs. SHRsed; †p<0.05 SHRsed vs SHRex. N=9 per group for mRNA analysis and n=6 per group for protein analysis
Inducible nitric oxide synthase (iNOS) has been considered another marker of oxidative stress because of its ability to sequester excess superoxide leading to formation of more toxic reactive oxygen species, peroxynitrite. Therefore, we investigated whether exercise has any influence on iNOS levels within the brain. Our mRNA analysis demonstrated that SHRsed rats had marked increase in iNOS within the PVN and RVLM. Surprisingly, exercise in SHR caused an 8 and 5 fold decrease in iNOS expression in the PVN and RVLM, respectively (Fig 8). These results further confirm that exercise has ability to reduced oxidative stress in the brain of hypertensive rats.
4.8 Chronic exercise attenuates neuronal excitation in the brain of SHRs
To determine whether chronic exercise influences sympathoexcitation in the brain, we examined the protein expression of Fra-like (Fra-LI, fos familygene; indicating chronic neuronal excitation) by immunofluorescence staining. To further determine whether exercise-induced effects are mediated by alterations in neurotransmitter in the brain, we determined the levels of tyrosine hydroxylase (TH) and 67-kDa isoform of glutamate decarboxylase (GAD67) in the brain. TH is a rate-limiting enzyme in the synthesis of the catecholamines, Norepinephrine (NE) and Epinephrine. GAD is the rate-limiting enzyme in the synthesis of inhibitory neurotransmitter GABA. We observed that SHRsed rats exhibited increasd Fra-LI activity in the PVN neurons compared to WKsed (Fig 9a). Notably, this upregulation of Fra-LI activity was significantly attenuated by chronic exercise in SHR. However, Exercise did not change Fra-LI activity in WK rats.
Fig 9.
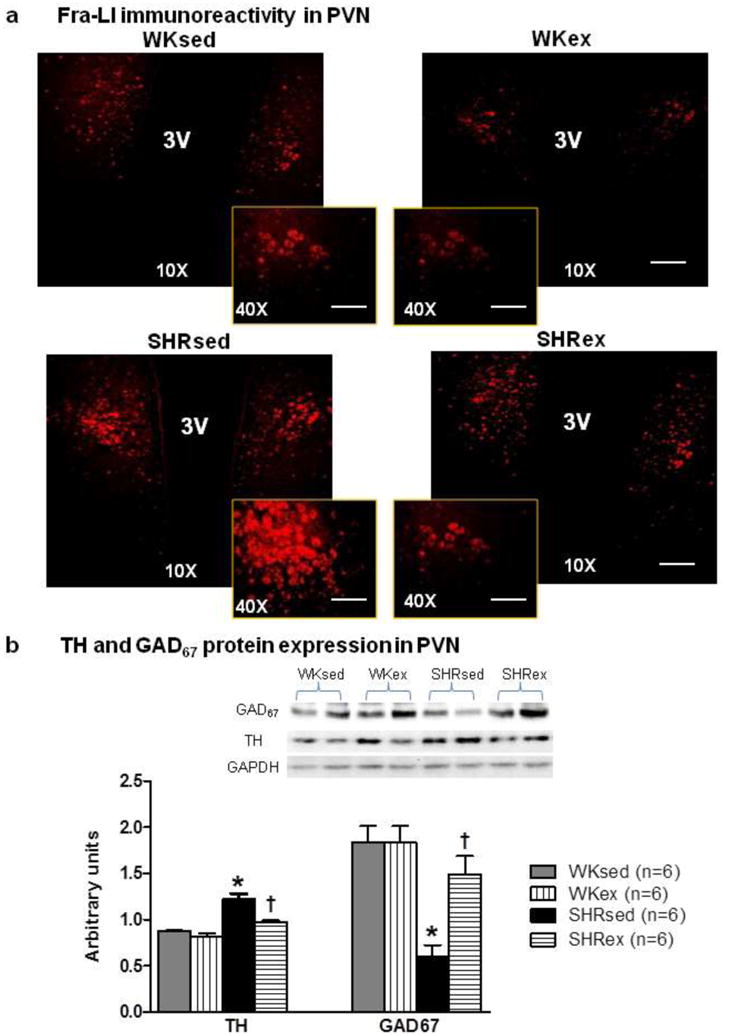
Effects of exercise on sympathoexcitation in the PVN of WK rats and SHRs. a, A representative confocal photomicrographs (×10, scale bar=200 μ m; and ×0, scale bar=50 μ m) from each group showing Fra-LI immunoreactivity in PVN neurons (n=5/group); b, Densitometric analysis (n=6/group) of protein expression of TH and GAD67 in the PVN accompanied with a representative Western blot. Values are mean±SE; *p<0.05 WKsed vs. SHRsed; †p<0.05 SHRsed vs SHRex. 3V,third ventricle
Furthermore, we found that sedentary SHRs exhibited higher levels of TH and significantly lower levels of GAD67 in the PVN when compared to WKsed rats (Fig 9b). Interestingly, exercise significantly reduces TH levels, whereas, GAD67 levels were upregulated in SHRex rats compared to SHRsed. However, in the RVLM, there was no significant difference of TH and GAD67 levels among all groups (Fig 10). Additionally, plasma NE levels were found to be significantly higher in SHRsed compared with WKsed rats, whereas, chronic exercise resulted in significantly decreased plasma NE concentrations in SHRs but did not change plasma NE level in WK rats (Table 1). Taken together, these results indicate that chronic exercise attenuates sympathoexcitation, possibly by altering neurotransmitter levels in the PVN of hypertensive rats.
Fig 10.

Densitometric analysis (n=6/group) of protein expression of TH and GAD67 in the RVLM of WK rats and SHRs. In the RVLM, there was no significant difference of TH and GAD67 levels among all groups. Values are mean±SE
4 Discussion
The present study sought to evaluate the impact of chronic moderate intensity exercise of 16 weeks duration on blood pressure, pro- and anti-inflammatory cytokines, RAS components, neuronal activity, and oxidative stress, within the brain of spontaneously hypertensive rats (SHRs), a genetic model of hypertension. Three major novel findings emerge from this study. First, chronic exercise improves balance between pro- and anti-inflammatory cytokines by attenuating PICs (TNF-α, IL-1β) and upregulating anti-inflammatory IL-10 expression in the PVN and RVLM of SHR. Second, effects of chronic exercise in hypertensive rats were modulated by both vasoconstrictor as well as the vasoprotective components of RAS in the PVN and RVLM. Finally, exercise attenuated oxidative stress in the PVN and RVLM of SHRs, possibly by reducing sympathoexcitation. These results suggest that chronic exercise not only attenuates PICs and the vasoconstrictor axis of the RAS but also attenuates sympathoexcitation, improves anti-inflammatory defense mechanisms and vasoprotective axis of the RAS in the brain, which, at least in part, explains the blood pressure-lowering effects of exercise in hypertension.
At the end of the study, we observed significant reductions in SBP, DBP, and MAP in trained SHRs compared with SHRsed rats and saw no comparable changes in trained WK rats. The pressure-lowering effect of ExT was significant starting from 8 weeks of regular exercise and continued until the end of the study, emphasizing the importance of long-term exercise in patients with hypertension. Additionally, we observed that chronic exercise caused significant reduction in body weight (BW) both in WK as well as in SHR rats, whereas, reduction in BP was observed only in SHRs. This excludes the possibility that exercise-induced reduction in BP observed in this study was due to reduction in BW. Hypertension is characterized by chronic low-grade inflammation which is reflected by a two- to threefold increase in circulating levels of several PICs [48, 53]. Interestingly, recent discoveries indicate that anti-inflammatory cytokines (AICs) such as IL-10, exerts inhibitory effects on PICs and therefore, has a significant impact on sympathetic outflow, arterial pressure, and cardiac remodeling in experimental models of hypertension [53]. More importantly, several cross-sectional studies demonstrated an association between physical inactivity and low-grade systemic inflammation [1, 18]. Our current findings together with previous other studies clearly suggest that physical activity reduces BP in hypertensive humans and animals. These findings led us to explore the role of brain pro- and anti-inflammatory cytokines in pressure-lowering effects of exercise. Although, very few studies have shown that exercise reduces circulating levels of PICs [3, 39, 49], influence of exercise on brain inflammatory status has never been investigated. In this study, we found that regular exercise resulted in robust decrease in brain PICs (TNF-α and IL-1β) in hypertensive rats. More importantly, brain IL-10 levels were dramatically upregulated and TNF-α/IL-10 ratio was reduced in trained SHRs. Though not in the brain, a recent study reported similar improvement in plasma IL-10 levels by physical exercise of 8-weeks duration in rats with chronic heart failure (CHF) [46]. Similarly, Smith et al. (1999) showed that the ability of blood mononuclear cells to produce IL-10 increased by 36% in trained individuals at risk of ischemic heart disease [55]. Since, the two TNF-α receptors, TNFR1 and TNFR2, have been shown to differentially regulate cardiac and endothelial function in vitro and in vivo [17, 52], it may be interesting to investigate the role played by these receptors in exercise-induced effects on hypertension as well. Nevertheless, taken together, the results of this study provide evidence of a shift in the balance between PIC and AIC by physical training, favoring anti-inflammatory response.
IL-10 has been shown to inhibit the production of various PICs as well as chemokines from LPS-activated human monocytes [11]. Therefore, it is possible that exercise induced increase in IL-10 may be responsible for the observed decrease in TNF-α and IL-1β. However, the possibility of direct effects of exercise on production of PIC cannot be ignored. Schulz and Heusch (2009) have summarized in their recent article that TNF-α overexpression in mice leads to progressive cardiomycoyte hypertrophy, left ventricular dilation, and diastolic dysfunction; whereas, anti-TNF-α treatment preserves diastolic dysfunction [52]. Therefore, it can be speculated that exercise-induced reduction in TNF-α may be responsible for improved diastolic dysfunction in trained SHRs as reported previously [3]. Nonetheless, the ability of exercise to improve IL-10 levels in the PVN and RVLM is noteworthy, because overexpression of brain IL-10 has been shown to preserve cardiac function and prevent cardiac damage and hypertension [44, 45]. Therefore, based on the results of this study, exercise-induced improvement in overall immune condition of the brain in hypertensive rats, explains, at least in part, the underlying mechanisms of exercise-mediated reduction in BP.
Hypertension is also characterized by an overactivation of central/brain RAS. Besides, classical pathway of RAS (ACE, AngII, and AT1R), newly discovered RAS components such as ACE2, Ang1-7, and receptor Mas have been shown to play an important role in BP regulation, by counteracting the classical pathway. Research over the past decade has suggested that the balance between ACE and ACE2, particularly within the brain, is an important factor determining the outcome of hypertension [9]. We and others have previously shown that PICs, particularly TNF-α, mediates AngII-induced hypertension,cardiac hypertrophy [57], endothelial and cardiac dysfunction [31], and modulates RAS components in the PVN in rats with heart failure (HF) [27]. Interestingly, findings of this study revealed that chronic exercise not only reduced ACE and AT1R levels, but also dramatically upregulated expression levels of ACE2 and Mas receptor within the PVN and RVLM of SHRs. These findings provide evidence that effects of chronic exercise in hypertension are modulated by both vasodilatory and vasoconstrictor arms of central RAS. These results extended the observations of previous studies showing that physical activity reduces plasma levels of AngII and AT1R in rabbits with CHF [16, 35], and increases Mas receptor expression in the left ventricle of SHR [14]. Our results were also in agreement with a recent report that demonstrated normalization of ACE and ACE2 levels by exercise in the RVLM of rabbits with CHF[29]. Besides RVLM, they have demonstrated similar changes in hypothalamus, cerebellum, and NTS suggesting the involvement of brain regions other than RVLM and PVN in exercise-induced effects, at least, in animals with HF. In addition to central RAS, we observed almost complete normalization of plasma AngII levels in trained SHRs (Table 1). In a recent publication Zamo et al (2011) reported that low-intensity swimming exercise of 8 weeks duration caused marked differences in systemic and cardiac RAS in young as well as adult SHRs, however, the effects were more pronounced in young rats (60% and 39% reduction in plasma AngII in young and adult SHRs, respectively) [63]. The difference in degree of improvement in plasma AngII levels could be attributed to the longer duration of exercise protocol used in the present study. Because role of AngII is well established in regulation of renal excretion of water and electrolyte, exercise-induced increase in urinary sodium excretion could also attribute to pressure-lowering effects of exercise. For instance, Ciampone et al (2011) have recently reported an association between reduced BP, increased natriuresis, and improvement in renal RAS components[8]. It is also important to discuss that adipocytes are known to play an important role in cytokine production and a recent study reported increased ACE expression by adipocyte-derived lipid mediators in macrophages [32]. Although, we observed that exercise significantly reduced body weight in SHR as well as in WK rats, the role of adipose tissue in exercise-induced reduction in ACE in SHRs is not clear. A recently published report from our lab demonstrated that overexpression of ACE2 within the PVN by bilateral microinjection of an adenovirus encoding human ACE2 reduces BP in AngII-induced hypertensive rats [56]. The results of this study also revealed that attenuation of PICs in the PVN in combination with the shift of the RAS towards the anti-hypertensive axis (ACE2/Ang-(1-7)/Mas) may be responsible for the overall beneficial effects of ACE2 overexpression. Our current findings together with the previous reports from our lab clearly suggest that exercise has capability to not only improve the systemic RAS but also central RAS, which, at least in part, explains the pressure-lowering effects of chronic exercise in hypertension.
Besides PICs and RAS, sympathetic nervous system plays an important role in cardiovascular regulation of BP [12, 22]. Hypertension is often found to be associated with increased levels of excitatory neurotransmitter, norepinephrine (NE) [3] and deficit in inhibitory GABAergic system in the cardiovascular regulatory regions of the brain[24]. In this study, we demonstrated that SHRsed had significantly reduced levels of GAD67, a 67-kDa isoform of GAD, and increased tyrosine hydroxylase (TH) when compared to WKsed. Concomittantly, when compared to WKsed, SHRsed rats exhibited increased circulating plasma levels of NE (an indirect marker of sympathetic activity) as well as increased expression of Fra-LI in the PVN (indicative of increased neuronal activity). These results provide further evidence that neurotransmitters mechanisms within the cardiovascular regulatory centers in the brain contribute to sympathoexcitation and plays an important role in the pathogenesis of essential hypertension. More importantly, exercise caused reduction in Fra-LI staining and prevented the increase in TH and decrease in GAD67 in the PVN of SHRs, suggesting exercise-induced reduction in sympathoexcitation in hypertensive rats. Taken together, this study provide sufficient evidence that chronic exercise may cause alteration in excitatory and inhibitory neurotransmitter in the brain leading to reduced sympathoexcitation in hypertensive rats. It is now well established that PICs and RAS modulate sympathetic neuronal outflow in the CNS leading to elevated resting BP in conscious animal [27, 37, 50, 60, 64]. In addition, it has been suggested that TNF-induced imbalance in neurotransmitters in the PVN and RVLM, possibly via oxidative stress, contributes to sympathoexcitation [21, 28]. Therefore, current findings taken together with previous studies raise the possibility that improved balance between PIC and AIC in trained hypertensive rats either alone or in combination with improved RAS components may have contributed to exercise-induced attenuation in sympathoexcitation observed in this study.
Research over past several decades has established that cytokines and RAS alter neuronal activity via induction of oxidative stress [40, 66]. Of particular importance, NADPH oxidase (NOX)derived reactive oxygen species (ROS) act as potent intra- and inter-cellular second messengers in signaling pathways causing hypertension [41, 43, 54]. Of various isoforms of NOX, role of NOX2 (also known as gp91phox) in AngII-induced hypertension and endothelial dysfunction is well established [42]. Given the role of AngII-induced ROS generation in the brain in hypertension, it is interesting to investigate whether exercise has ability to attenuate ROS generation within the brain of hypertensive rats. Our data illustrated that moderate-intensity exercise reduces brain oxidative stress in hypertensive rats as indicated by reduced levels of gp91phox and iNOS within the brain of SHRs. In accordance with these findings, previous studies have shown that exercise causes reductions in various subunits of NADPH oxidase in isolated porcine aortic endothelial cells[51], thoracic aorta of SHR[20], aging arteries of rat[34], and human mammary arteries[2]. However, our results are first to provide evidence that exercise can attenuate oxidative stress in the PVN and RVLM neurons of hypertensive rats. In light of our findings that exercise causes reduction in ACE and AT1R levels and because RAS is a potent mediator of activation of NADPH oxidase [41], it is plausible to suggest that exercise-induced reduction in ACE and AT1R might be responsible for attenuation of gp91phox. In addition, catecholamines and cytokines have also known to promote ROS formation from NADPH oxidases [23]; therefore, role of exercise-induced improvement in neurotransmitters and cytokine levels in attenuation of oxidative stress cannot be ignored. Recently, it has been reported that inhibition of the cannabinoid receptor CB1 (CB1-R), which is mainly localized in the central nervous system, positively affects BP, endothelial function, and reduces aortic ROS production and NADPH oxidase activity [59]. These findings together with our current findings indicate that there exists a cross-talk between these various pathways within the brain that can influence exercise-induced attenuation of oxidative stress and BP. Paradoxically, however, exercise has been shown to induce oxidative stress in some cases. However, exercise-induced oxidative stress has been seen mainly after vigorous exercise and is more frequent in long-distance runners and/or long bursts of severe and unaccustomed exercise [10].On the other hand, regular and moderate intensity exercise seems more effective in reducing oxidative stress in hypertensive rats as evident from our findings. This can be further explained by a recent report of Craenenbroeck et al (2010) where they have demonstrated that acute exercise-induced functional changes in circulating angiogenic cells (CAC, known to contribute to endothelial repair) declined with exercise training in CHF patients, suggesting that repetitive exercise bouts progressively lead to functional endothelial repair [61].
In summary, the present study shows that chronic exercise not only attenuates PICs and the vasoconstrictor axis of the RAS but also improves the anti-inflammatory defense mechanisms and vasoprotective axis of the RAS in the brain. Also, exercise alters the adrenergic and GABAergic system and reduces oxidative stress in the brain of hypertensive rats. These results provide mechanistic evidence that unlike currently available pharmacological anti-hypertensive therapies, regular moderate intensity exercise has ability to favorably affect multiple pathways involved in pathogenesis of hypertension. The results of this study provide greater insight into the mechanisms by which exercise exerts beneficial effects in hypertensions and therefore, may lead us to design an exercise regimen resulting in maximum cardio-protective benefits in hypertensive patients.
5 Perspectives
The present study provides insights into the mechanisms within the brain that can influence exercise-mediated effects in SHR. Our data demonstrated that chronic moderate-intensity exercise attenuates sympathoexcitation, modulates RAS components, improves the balance between PIC and AIC, and reduces oxidative stress in the PVN as well as RVLM of hypertensive rats. Since, RAS is thought to be a driving force in increased sympathetic activation and reduced oxidative stress; our findings suggest that exercise-induced reduction in BP could be mediated, at least in part by, improvement in central vasodilatory RAS components. In addition, increased IL-10 levels could be responsible for additional benefits. This is the first evidence to our knowledge showing the effectiveness of exercise in ameliorating the hypertensive components within the brain of SHR. These results further support the hypothesis that exercise can affect cardiovascular regulation by specifically impacting regions in the central nervous system [33].
Since, brain RAS has been implicated in the initiation of various forms of hypertension, therapeutically targeting the brain RAS could be one of the strategies to treat hypertension. However, systemically administered pharmacological therapies such as ACE inhibitors have very less access to central ACE compared with circulating ACE due to the presence of blood brain barrier. Therefore, the results of this study are important from clinical perspective, because it suggests that regular long-term exercise could be one of the non-pharmacological yet cost effective tools in shifting the balance between vasoconstrictor RAS components to vasodilator components towards the vasodilatory and hence protective effects in hypertensive rats.
Although much progress has been made in animal studies, there is a need for rigorous clinical intervention trials on exercise that are guided by this knowledge from animal studies. The extent and frequency of exercise that result in maximum functional benefits in hypertensive patients must be determined. In this regard, it is worth mentioning that Kemi et al., have made an attempt to address this question [30]. They demonstrated that cardiovascular adaptations to training are intensity-dependent. However, further studies in relation to the parameters studied in the present study are still warranted. Furthermore, here, we chose SHR rat model of hypertension to elucidate the mechanisms of the beneficial effects of exercise in hypertension. However, the validation of results of this study in other animal models of hypertension could certainly be an important perspective.
Acknowledgments
The authors thank Sherry Ring for sectioning the tissue samples.
Source of Funding: This work was supported by National Heart, Lung, and Blood Institute Grant HL-80544 to Joseph Francis.
Footnotes
Conflict of interest: none declared
References
- 1.Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. 2002;162:1286–1292. doi: 10.1001/archinte.162.11.1286. doi: ioi10476. [DOI] [PubMed] [Google Scholar]
- 2.Adams V, Linke A, Krankel N, Erbs S, Gielen S, Mobius-Winkler S, Gummert JF, Mohr FW, Schuler G, Hambrecht R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation. 2005;111:555–562. doi: 10.1161/01.CIR.0000154560.88933.7E. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal D, Haque M, Sriramula S, Mariappan N, Pariaut R, Francis J. Role of proinflammatory cytokines and redox homeostasis in exercise-induced delayed progression of hypertension in spontaneously hypertensive rats. Hypertension. 2009;54:1393–1400. doi: 10.1161/HYPERTENSIONAHA.109.135459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai Y, Jabbari B, Ye S, Campese VM, Vaziri ND. Regional expression of NAD(P)H oxidase and superoxide dismutase in the brain of rats with neurogenic hypertension. Am J Nephrol. 2009;29:483–492. doi: 10.1159/000178817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block CH, Santos RA, Brosnihan KB, Ferrario CM. Immunocytochemical localization of angiotensin-(1-7) in the rat forebrain. Peptides. 1988;9:1395–1401. doi: 10.1016/0196-9781(88)90208-2. [DOI] [PubMed] [Google Scholar]
- 6.Boissiere J, Eder V, Machet MC, Courteix D, Bonnet P. Moderate exercise training does not worsen left ventricle remodeling and function in untreated severe hypertensive rats. J Appl Physiol. 2008;104:321–327. doi: 10.1152/japplphysiol.00442.2007. [DOI] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 8.Ciampone S, Borges R, de Lima IP, Mesquita FF, Cambiucci EC, Gontijo JA. Long-term exercise attenuates blood pressure responsiveness and modulates kidney angiotensin II signalling and urinary sodium excretion in SHR. J Renin Angiotensin Aldosterone Syst. 2011 doi: 10.1177/1470320311408750. [DOI] [PubMed] [Google Scholar]
- 9.Danilczyk U, Penninger JM. Angiotensin-converting enzyme II in the heart and the kidney. Circ Res. 2006;98:463–471. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- 10.Das UN. Anti-inflammatory nature of exercise. Nutrition. 2004;20:323–326. doi: 10.1016/j.nut.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Das UN. Beneficial effect of eicosapentaenoic and docosahexaenoic acids in the management of systemic lupus erythematosus and its relationship to the cytokine network. Prostaglandins Leukot Essent Fatty Acids. 1994;51:207–213. doi: 10.1016/0952-3278(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 12.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 13.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 14.Filho AG, Ferreira AJ, Santos SH, Neves SR, Silva Camargos ER, Becker LK, Belchior HA, Dias-Peixoto MF, Pinheiro SV, Santos RA. Selective increase of angiotensin(1-7) and its receptor in hearts of spontaneously hypertensive rats subjected to physical training. Exp Physiol. 2008;93:589–598. doi: 10.1113/expphysiol.2007.014293. [DOI] [PubMed] [Google Scholar]
- 15.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Simvastatin therapy normalizes sympathetic neural control in experimental heart failure: roles of angiotensin II type 1 receptors and NAD(P)H oxidase. Circulation. 2005;112:1763–1770. doi: 10.1161/CIRCULATIONAHA.105.552174. [DOI] [PubMed] [Google Scholar]
- 16.Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation. 2007;115:3095–3102. doi: 10.1161/CIRCULATIONAHA.106.677989. [DOI] [PubMed] [Google Scholar]
- 17.Garlie JB, Hamid T, Gu Y, Ismahil MA, Chandrasekar B, Prabhu SD. Tumor necrosis factor receptor 2 signaling limits beta-adrenergic receptor-mediated cardiac hypertrophy in vivo. Basic Res Cardiol. 2011 doi: 10.1007/s00395-011-0196-6. [DOI] [PubMed] [Google Scholar]
- 18.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 19.Giannopoulou I, Fernhall B, Carhart R, Weinstock RS, Baynard T, Figueroa A, Kanaley JA. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54:866–875. doi: 10.1016/j.metabol.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 20.Graham DA, Rush JW. Exercise training improves aortic endothelium-dependent vasorelaxation and determinants of nitric oxide bioavailability in spontaneously hypertensive rats. J Appl Physiol. 2004;96:2088–2096. doi: 10.1152/japplphysiol.01252.2003. [DOI] [PubMed] [Google Scholar]
- 21.Guggilam A, Cardinale JP, Mariappan N, Sriramula S, Haque M, Francis J. Central TNF inhibition results in attenuated neurohumoral excitation in heart failure: a role for superoxide and nitric oxide. Basic Res Cardiol. 2011;106:273–286. doi: 10.1007/s00395-010-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 23.Heusch G, Schulz R. A radical view on the contractile machinery in human heart failure. J Am Coll Cardiol. 2011;57:310–312. doi: 10.1016/j.jacc.2010.06.057. [DOI] [PubMed] [Google Scholar]
- 24.Horn EM, Shonis CA, Holzwarth MA, Waldrop TG. Decrease in glutamic acid decarboxylase level in the hypothalamus of spontaneously hypertensive rats. J Hypertens. 1998;16:625–633. doi: 10.1097/00004872-199816050-00010. [DOI] [PubMed] [Google Scholar]
- 25.Jennings JR, Zanstra Y. Is the brain the essential in hypertension? Neuroimage. 2009;47:914–921. doi: 10.1016/j.neuroimage.2009.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang YM, He RL, Yang LM, Qin DN, Guggilam A, Elks C, Yan N, Guo Z, Francis J. Brain tumour necrosis factor-alpha modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovasc Res. 2009;83:737–746. doi: 10.1093/cvr/cvp160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang YM, Wang Y, Yang LM, Elks C, Cardinale J, Yu XJ, Zhao XF, Zhang J, Zhang LH, Yang ZM, Francis J. TNF-alpha in hypothalamic paraventricular nucleus contributes to sympathoexcitation in heart failure by modulating AT1 receptor and neurotransmitters. Tohoku J Exp Med. 222:251–263. doi: 10.1620/tjem.222.251. doi:JST.JSTAGE/tjem/222.251 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Kang YM, Zhang AQ, Zhao XF, Cardinale JP, Elks C, Cao XM, Zhang ZW, Francis J. Paraventricular nucleus corticotrophin releasing hormone contributes to sympathoexcitation via interaction with neurotransmitters in heart failure. Basic Res Cardiol. 2011;106:473–483. doi: 10.1007/s00395-011-0155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kar S, Gao L, Zucker IH. Exercise training normalizes ACE and ACE2 in the brain of rabbits with pacing-induced heart failure. J Appl Physiol. 2010;108:923–932. doi: 10.1152/japplphysiol.00840.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemi OJ, Haram PM, Loennechen JP, Osnes JB, Skomedal T, Wisloff U, Ellingsen O. Moderate vs. high exercise intensity: differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc Res. 2005;67:161–172. doi: 10.1016/j.cardiores.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Kleinbongard P, Heusch G, Schulz R. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther. 2010;127:295–314. doi: 10.1016/j.pharmthera.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Kohlstedt K, Trouvain C, Namgaladze D, Fleming I. Adipocyte-derived lipids increase angiotensin-converting enzyme (ACE) expression and modulate macrophage phenotype. Basic Res Cardiol. 2011;106:205–215. doi: 10.1007/s00395-010-0137-9. [DOI] [PubMed] [Google Scholar]
- 33.Kramer JM, Plowey ED, Beatty JA, Little HR, Waldrop TG. Hypothalamus, hypertension, and exercise. Brain Res Bull. 2000;53:77–85. doi: 10.1016/S0361-9230(00)00311-7. [DOI] [PubMed] [Google Scholar]
- 34.Li QX, Xiong ZY, Hu BP, Tian ZJ, Zhang HF, Gou WY, Wang HC, Gao F, Zhang QJ. Aging-associated insulin resistance predisposes to hypertension and its reversal by exercise: the role of vascular vasorelaxation to insulin. Basic Res Cardiol. 2009;104:269–284. doi: 10.1007/s00395-008-0754-8. [DOI] [PubMed] [Google Scholar]
- 35.Liu JL, Kulakofsky J, Zucker IH. Exercise training enhances baroreflex control of heart rate by a vagal mechanism in rabbits with heart failure. J Appl Physiol. 2002;92:2403–2408. doi: 10.1152/japplphysiol.00039.2002. [DOI] [PubMed] [Google Scholar]
- 36.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 37.Lu Y, Chen J, Yin X, Zhao H. Angiotensin II receptor 1 involved in the central pressor response induced by interleukin-1 beta in the paraventricular nucleus. Neurol Res. 2009;31:420–424. doi: 10.1179/17431320×353677. [DOI] [PubMed] [Google Scholar]
- 38.Marfella R, Esposito K, Siniscalchi M, Cacciapuoti F, Giugliano F, Labriola D, Ciotola M, Di Palo C, Misso L, Giugliano D. Effect of weight loss on cardiac synchronization and proinflammatory cytokines in premenopausal obese women. Diabetes Care. 2004;27:47–52. doi: 10.2337/diacare.27.1.47. [DOI] [PubMed] [Google Scholar]
- 39.Mattusch F, Dufaux B, Heine O, Mertens I, Rost R. Reduction of the plasma concentration of C-reactive protein following nine months of endurance training. Int J Sports Med. 2000;21:21–24. doi: 10.1055/s-2000-8852. [DOI] [PubMed] [Google Scholar]
- 40.Mayorov DN, Head GA, De Matteo R. Tempol attenuates excitatory actions of angiotensin II in the rostral ventrolateral medulla during emotional stress. Hypertension. 2004;44:101–106. doi: 10.1161/01.HYP.0000131290.12255.04. [DOI] [PubMed] [Google Scholar]
- 41.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 42.Murdoch CE, Alom-Ruiz SP, Wang M, Zhang M, Walker S, Yu B, Brewer A, Shah AM. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res Cardiol. 2011;106:527–538. doi: 10.1007/s00395-011-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation. 2009;119:978–986. doi: 10.1161/CIRCULATIONAHA.108.824730. [DOI] [PubMed] [Google Scholar]
- 44.Nomoto T, Okada T, Shimazaki K, Yoshioka T, Nonaka-Sarukawa M, Ito T, Takeuchi K, Katsura KI, Mizukami H, Kume A, Ookawara S, Ikeda U, Katayama Y, Ozawa K. Systemic delivery of IL-10 by an AAV vector prevents vascular remodeling and end-organ damage in stroke-prone spontaneously hypertensive rat. Gene Ther. 2009;16:383–391. doi: 10.1038/gt.2008.151. [DOI] [PubMed] [Google Scholar]
- 45.Nonaka-Sarukawa M, Okada T, Ito T, Yamamoto K, Yoshioka T, Nomoto T, Hojo Y, Shimpo M, Urabe M, Mizukami H, Kume A, Ikeda U, Shimada K, Ozawa K. Adeno-associated virus vector-mediated systemic interleukin-10 expression ameliorates hypertensive organ damage in Dahl salt-sensitive rats. J Gene Med. 2008;10:368–374. doi: 10.1002/jgm.1166. [DOI] [PubMed] [Google Scholar]
- 46.Nunes RB, Tonetto M, Machado N, Chazan M, Heck TG, Veiga AB, Dall'Ago P. Physical exercise improves plasmatic levels of IL-10, left ventricular end-diastolic pressure, and muscle lipid peroxidation in chronic heart failure rats. J Appl Physiol. 2008;104:1641–1647. doi: 10.1152/japplphysiol.00062.2008. [DOI] [PubMed] [Google Scholar]
- 47.Ogihara CA, Schoorlemmer GH, Levada AC, Pithon-Curi TC, Curi R, Lopes OU, Colombari E, Sato MA. Exercise changes regional vascular control by commissural NTS in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R291–297. doi: 10.1152/ajpregu.00055.2009. [DOI] [PubMed] [Google Scholar]
- 48.Peeters AC, Netea MG, Janssen MC, Kullberg BJ, Van der Meer JW, Thien T. Pro-inflammatory cytokines in patients with essential hypertension. Eur J Clin Invest. 2001;31:31–36. doi: 10.1046/j.1365-2362.2001.00743.x. [DOI] [PubMed] [Google Scholar]
- 49.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 50.Phillips MI, de Oliveira EM. Brain renin angiotensin in disease. J Mol Med. 2008;86:715–722. doi: 10.1007/s00109-008-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rush JW, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol. 2003;284:H1378–1387. doi: 10.1152/ajpheart.00190.2002. [DOI] [PubMed] [Google Scholar]
- 52.Schulz R, Heusch G. Tumor necrosis factor-alpha and its receptors 1 and 2: Yin and Yang in myocardial infarction? Circulation. 2009;119:1355–1357. doi: 10.1161/CIRCULATIONAHA.108.846105. [DOI] [PubMed] [Google Scholar]
- 53.Shi P, Raizada MK, Sumners C. Brain cytokines as neuromodulators in cardiovascular control. Clin Exp Pharmacol Physiol. 2010;37:e52–57. doi: 10.1111/j.1440-1681.2009.05234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sirker A, Zhang M, Shah AM. NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Res Cardiol. 2011;106:735–747. doi: 10.1007/s00395-011-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith JK, Dykes R, Douglas JE, Krishnaswamy G, Berk S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA. 1999;281:1722–1727. doi: 10.1001/jama.281.18.1722. [DOI] [PubMed] [Google Scholar]
- 56.Sriramula S, Cardinale JP, Lazartigues E, Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res. 2011;92:401–408. doi: 10.1093/cvr/cvr242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun MW, Qian FL, Wang J, Tao T, Guo J, Wang L, Lu AY, Chen H. Low-intensity voluntary running lowers blood pressure with simultaneous improvement in endothelium-dependent vasodilatation and insulin sensitivity in aged spontaneously hypertensive rats. Hypertens Res. 2008;31:543–552. doi: 10.1291/hypres.31.543. [DOI] [PubMed] [Google Scholar]
- 59.Tiyerili V, Zimmer S, Jung S, Wassmann K, Naehle CP, Lutjohann D, Zimmer A, Nickenig G, Wassmann S. CB1 receptor inhibition leads to decreased vascular AT1 receptor expression, inhibition of oxidative stress and improved endothelial function. Basic Res Cardiol. 2010;105:465–477. doi: 10.1007/s00395-010-0090-7. [DOI] [PubMed] [Google Scholar]
- 60.Ufnal M, Zera T, Szczepanska-Sadowska E. Blockade of angiotensin II AT1 receptors inhibits pressor action of centrally administered interleukin-1beta in Sprague Dawley rats. Neuropeptides. 2005;39:581–585. doi: 10.1016/j.npep.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Van Craenenbroeck EM, Hoymans VY, Beckers PJ, Possemiers NM, Wuyts K, Paelinck BP, Vrints CJ, Conraads VM. Exercise training improves function of circulating angiogenic cells in patients with chronic heart failure. Basic Res Cardiol. 2010;105:665–676. doi: 10.1007/s00395-010-0105-4. [DOI] [PubMed] [Google Scholar]
- 62.Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12:170–175. doi: 10.1007/s11906-010-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zamo FS, Barauna VG, Chiavegatto S, Irigoyen MC, Oliveira EM. The renin-angiotensin system is modulated by swimming training depending on the age of spontaneously hypertensive rats. Life Sci. 2011;89:93–99. doi: 10.1016/j.lfs.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Zhang ZH, Wei SG, Francis J, Felder RB. Cardiovascular and renal sympathetic activation by blood-borne TNF-alpha in rat: the role of central prostaglandins. Am J Physiol Regul Integr Comp Physiol. 2003;284:R916–927. doi: 10.1152/ajpregu.00406.2002. [DOI] [PubMed] [Google Scholar]
- 65.Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, D'Andrea F, Molinari AM, Giugliano D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–809. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 66.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]


