Abstract
Congenital Diaphragmatic Hernia (CDH) is defined by the presence of an orifice in the diaphragm, more often left and posterolateral that permits the herniation of abdominal contents into the thorax. The lungs are hypoplastic and have abnormal vessels that cause respiratory insufficiency and persistent pulmonary hypertension with high mortality. About one third of cases have cardiovascular malformations and lesser proportions have skeletal, neural, genitourinary, gastrointestinal or other defects. CDH can be a component of Pallister-Killian, Fryns, Ghersoni-Baruch, WAGR, Denys-Drash, Brachman-De Lange, Donnai-Barrow or Wolf-Hirschhorn syndromes. Some chromosomal anomalies involve CDH as well. The incidence is < 5 in 10,000 live-births. The etiology is unknown although clinical, genetic and experimental evidence points to disturbances in the retinoid-signaling pathway during organogenesis. Antenatal diagnosis is often made and this allows prenatal management (open correction of the hernia in the past and reversible fetoscopic tracheal obstruction nowadays) that may be indicated in cases with severe lung hypoplasia and grim prognosis. Treatment after birth requires all the refinements of critical care including extracorporeal membrane oxygenation prior to surgical correction. The best hospital series report 80% survival but it remains around 50% in population-based studies. Chronic respiratory tract disease, neurodevelopmental problems, neurosensorial hearing loss and gastroesophageal reflux are common problems in survivors. Much more research on several aspects of this severe condition is warranted.
Keywords: Congenital, Diaphragm, Hernia, Retinoids, Lung, Hypoplasia, Pulmonary, Hypertension, Surgery, Fetoscopy
Disease name/synonyms
Congenital Diaphragmatic Hernia (CDH), ORPHA2140, OMIM 142340, 610187, 306950 and 222400
Definition
CDH consists of a posterolateral defect of the diaphragm, generally located on the left side, that allows passage of the abdominal viscera into the thorax. The mediastinum is displaced to the contralateral side, the lungs are hypoplastic (Figure 1) and their arterioles are abnormal causing pulmonary hypertension. Respiratory and cardiovascular functions are severely compromised at birth and this, together with the frequently associated malformations, cause considerable mortality and morbidity. CDH was described many years ago [1,2] but survival after repair was not achieved until the 20th century. Pioneers of pediatric surgery [3] reported amazingly low mortalities until the actual severity of the condition surfaced when abortions, stillbirths and pre-hospital deaths were considered, adding a "hidden mortality" to operative and postoperative demises [4]. The pathophysiology of lung insufficiency and persistent pulmonary hypertension that threaten survival are currently better understood, but the results remain disappointing since mortalities near 50% are still reported when all deaths are taken into account in population-based series [5]. CDH management indeed remains one of the major challenges of perinatal medicine and surgery and active research on its mechanisms is warranted.
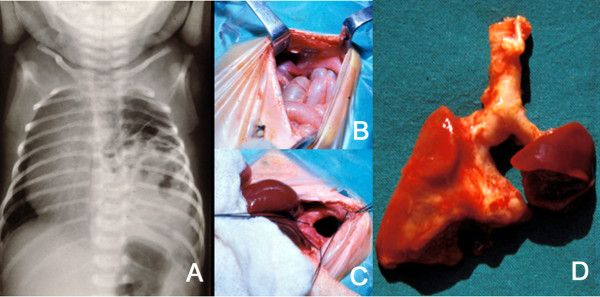
Figure 1.
A: Plain X-ray of the thorax of a newborn with CDH. There are bowel loops into the left hemi-thorax, the mediastinum is displaced to the contralateral side and the space occupied by the lung is reduced. B and C: At laparotomy, a left, posterolateral diaphragmatic hernia was discovered. In B, small bowel loops can be seen entering the thorax through the orifice. In C, this is seen after reducing the contents of the hernia. D: The patient died of severe persistent pulmonary hypertension days later. At autopsy, extreme left lung hypoplasia and less severe right lung hypoplasia were discovered.
Epidemiology
CDH is a rare condition that occurs in < 1-5:10000 births [6]. It seems to be slightly more frequent in males and less frequent in blacks [7,8].
Clinical features
CDH can be detected during fetal life when screening ultrasonography demonstrates herniation of the intestine and/or the liver into the thorax. Polyhydramnios may lead to antenatal diagnosis in some severe cases [9].
Neonatal symptoms of CDH are heralded by respiratory distress with insufficient oxygenation, excavated abdomen with sternal protrusion and displacement of the heart sounds to the contralateral side. In severe cases, APGAR scores at 1 and 5 minutes are low [10,11]. Respiratory bruits are absent or decreased on the affected side. Unless energetic treatment is undertaken, respiratory condition deteriorates rapidly until the patient dies. The symptoms of insufficient gas exchange are associated with those of persistent pulmonary hypertension [12,13] caused by arteriolar constriction and closure of the pulmonary arterial bed that forces maintenance of a pattern of persistent fetal circulation in which the blood from the right ventricle is shunted to the left heart preventing effective gas exchange. In some cases, this pulmonary hypertension intervenes after some hours during which adaptation to a post-natal circulatory pattern with patent pulmonary circulation had taken place. Hypoxia, acidosis, stress or other causes may bring this "honeymoon" period to an end and re-establish the fetal pattern [14].
In some cases without neonatal symptoms, CDH may manifest itself at any age by mild respiratory distress or it can even be an unexpected finding during a medical check-up for other reasons [15]. In these cases, a hernial sac is more often present [16].
Other organs may be involved in CDH [17] because associated malformations are frequent [18]. The heart and great vessels are often abnormal in CDH patients. Cardiovascular defects like peri-membranous ventricular septal defect, cardiac outflow anomalies (tetralogy of Fallot, double outlet right ventricle, transposition of the great vessels and others) and abnormal great vessels (right aortic arch, double aortic arch, truncus arteriosus, abnormal subclavian arteries and others) are found in about one third of CDH patients [19,20]. Heart hypoplasia, particularly of the left side [21], has also been described [22] but its participation in the clinical picture is still unclear.
Musculoskeletal defects like anomalies of the limbs or of the number and shape of the vertebral bodies and/or ribs [18,23-25], neural tube defects [18,26], abdominal wall defects [27], craniofacial defects [28] or urinary tract anomalies [29] are also found. The parafollicular C-cells [30] and enteric innervation are deficient and might account for some dysfunctions [31]. Finally, the presence of the intestine in the thorax during late fetal development causes malrotation and/or malfixation [32] that can further complicate the disease [33].
Etiology
The causes of CDH are largely unknown. Most cases are isolated, but associated malformations are often observed [34], sometimes as components of Pallister-Killian and Fryns [35], Ghersoni-Baruch [36], WAGR and Denys-Drash [29,37], Brachman-De Lange [38], Donnai-Barrow [39] or Wolf-Hirschhorn [40] syndromes. CDH is also observed in some chromosomal anomalies whether related or not to these syndromes like 9p tetrasomy [41], 11q23-qter duplication [42], 15q24-26 [43], 15q26 [44], 1q41-q42.12 [39,45] and 8p23.1 [46] deletions. Null-mice for several genes, like shh transcription factors Gli 2 and Gli3 [47], Slit3 [48,49], COUP-TFII [50], Fog2 [51], Wt1 [52] and FGFLR-1 [53] have CDH.
The orifice in the diaphragm is caused by delayed or disturbed separation of the thoracic and abdominal compartments of the body by closure of embryonic pleuroperitoneal canals effected by growth of the post-hepatic mesenchymal plate and of the pleuroperitoneal folds [54-57].
CDH occurs more often on the left side (4:1) [58]. In some rare instances [59], the defect is a true agenesis of the hemidiaphragm [60], but in most cases it is limited to the posterolateral area. Less often, there is a hernial sac devoid of muscular fibers [16]. When the defect is located on the left side, the thorax may contain small and large bowel, the spleen, the stomach, the left lobe of the liver and, occasionally, the kidney. Right-sided CDHs usually contain part of the right lobe of the liver and sometimes the bowel and/or the kidney.
The lung is obviously hypoplastic on the side of the hernia, but the contralateral one is also affected to a variable extent. Lung weight is decreased and the number of alveoli is reduced due to insufficient branching [61]. Respiratory epithelial maturity is delayed with hyaline membrane disease patterns similar to those found in prematures [62]. Surfactant deficiency has been shown [63-65] although this issue is controversial [66]. The distal bronchiolar arteries have muscularized walls and the wall thickness is increased particularly at the expense of the media and adventitial layers [67,68].
The lung hypoplasia, immaturity and arteriolar thickening that accompany human CDH can be due to prenatal compression of the lung by the herniated viscera. All these lesions can be reproduced by surgically creating a CDH in rabbits [69-71] lambs [72-74] and primates [75]. These models were used for investigating various aspects of CDH and also for developing fetal surgery [76-81]. However, the associated malformations are absent in these mechanical or surgical models and this limits to some extent their validity as research tools. In fact, the assumption that lung hypoplasia is purely mechanical is probably incorrect because it is already present in teratogenic models of CDH before any herniation has taken place. A "dual hit" pathogenesis [82], in which abnormal development followed by compression cause lung hypoplasia is widely accepted. Regardless of the origin of the lung lesions, these consist of insufficient airway branching [61,63,83], reduced gas exchange surface, abnormal sacculo-alveolar maturation [64,84] and abnormal, muscularized distal arterioles [68,85].
Pharmacologic or teratogenic models of CDH have been crucial for unveiling some pathogenic mechanisms. Prenatal administration of oxidant chemicals like the herbicide nitrofen (2,4-dichloro-phenyl-p-nitrophenyl ether), 4-biphenyl carboxylic acid, bisdiamine, and SB-210661, induce CDH in rodent fetuses. These chemicals inhibit in vitro retinol-dehydrogenase-2 (RALDH-2) a key enzyme for the production of retinoic acid (RA) [86]. Among these agents, nitrofen has been the more extensively used [54-56,87-97] because of the striking resemblance of the diaphragmatic, pulmonary and other associated defects to those of the human condition (Figure 2). Proliferation of the pleuroperitoneal folds is arrested in rats with CDH [98] and the timing of this arrest probably conditions the laterality of the defect. Fibroblast growth factor (FGF) receptor-like 1 (FGFRL1) [99] and WT1 [97] are downregulated in these diaphragms.
Figure 2.
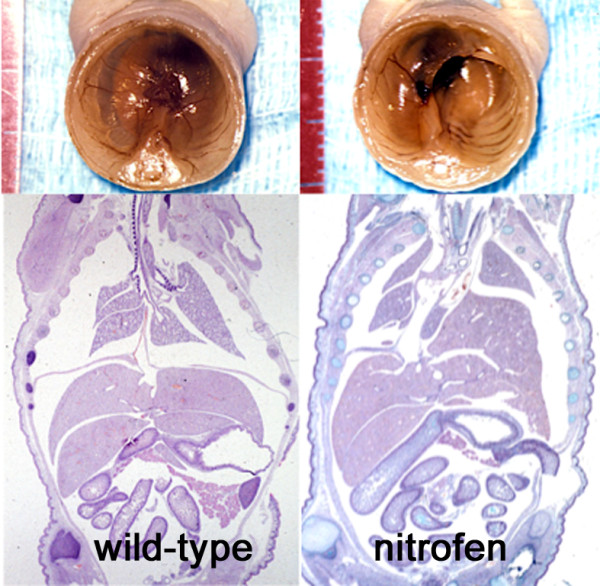
Experimental CDH in rats. Administration of 100 mg of the herbicide nitrofen on gestational day 9.5 to pregnant rats induced the malformation in 40 to 80% of the offspring. On the left, neonatal autopsy of an untreated, wild-type rat shows an intact diaphragm (above) that can also be seen in the frontal section of the trunk (below). On the right, similar preparations of a rat with nitrofen-induced CDH show a large postero-lateral diaphragmatic orifice, herniation of the liver into the thorax, displacement of the mediastinum to the contralateral side and lung hypoplasia.
Although the mechanism by which CDH is induced in these models is not well known, disturbances of the retinoid signalling pathway, a key regulator of embryonic morphogenesis, are likely [90]. Vitamin A deficient-rats give birth to pups with CDH [100]. RA metabolism perturbations caused by nitrofen can be alleviated by addition of either vitamin A or RA [95,101-103]. Hypoplastic lungs from rats with nitrofen-induced CDH show decreased RA synthesis [104] and deficient retinol transportation [105], whereas RA rescues lung hypoplasia [93] probably by upregulating COUP-TFII, FOG2, and GATA4 [106] that are known to be necessary for both pulmonary and diaphragmatic development [39,50]. On the other hand, compound knock-out mice for retinoic acid receptors (RAR) have phenotypes involving CDH and several of the associated malformations seen in this condition [107] whereas blockade of RAR with BMS493 induces CDH [108] and administration of BMS-189453, a RA antagonist, to pregnant mice reproduces the phenotype of CDH [109].
A similar involvement of the retinoid signaling pathway is likely in human CDH because retinol and retinol-binding protein were decreased in the blood of a group of newborns with this malformation [110]. This has recently been confirmed in a larger case-control study [111]. Moreover, some of the genes involved in the pathogenesis of human CDH are tightly related to retinoid signaling [112-115].
Another factor that might contribute to lung hypoplasia in CDH is decreased airway pressure during fetal life. Fetuses with tracheal atresia have large lungs [116,117] whereas fetal tracheostomy induces lung hypoplasia [118]. It has been shown that airway peristalsis is abnormal in lung explants from rat embryos treated with nitrofen and it is likely that decreased airway pressure could contribute to hypoplasia [92,119].
Diagnosis
As mentioned before, CDH is often ultrasonographically diagnosed before birth [120,121]. The intestine and/or the liver may be in the thorax and the lungs are small. US scan allows detailed assessment of the heart. Lung growth is measured as a proportion of head growth. This lung-to-head ratio (LHR) has some prognostic value [122-127] because when it is below 1, survival is compromised [125,128,129]. However, the accuracy of these measurements is questionable [130] and other alternatives like lung/thorax (L/T) transverse area ratio [126,131] or volumetry by MRI have been developed [132-136]. The observed-to-expected LHR (o/e LHR) seems to be a reliable predictor of severity (patch requirement, ECMO) and survival [127,137]. The position of the liver is also of unquestionable value, since liver-up cases more often require ECMO support and have worse survival [138-140]. The intra-thoracic position of the stomach has less value for this purpose [141,142]. When diagnosis is made in utero, amniocentesis is often performed for detecting chromosomal aberrations [143] and may help to estimate lung maturity [64].
After birth, the diagnosis is readily made on the basis of symptoms and physical signs. A plain X-ray of the thorax and abdomen informs of the position of the herniated viscera. Blood gases and pH status reflect the efficiency of gas exchange and other derived indexes refine this assessment [144,145]. Ultrasonography of the heart is necessary for ruling-out associated malformations, for measuring the right-to-left shunt and for estimating the severity of pulmonary hypertension [146-148]. Measurements of the pulmonary artery diameters and the use of some indexes derived from them may facilitate this task [149-151]. Cardiac U.S. is one of the more reliable methods for determining when the patient is "stabilized".
Differential diagnosis
It is rarely necessary to rule out other conditions because CDH is often detected before birth and because diagnosis is easy. Physical exam may suffice but passing a naso-gastric catheter into the stomach before a plain X-ray of the thorax and abdomen may help to locate it or to detect esophageal displacement. In some rare instances, X-rays may suggest a cystic malformation of the lung but again the position of the stomach and the contour of the intra-abdominal gas bubbles facilitate distinction of both conditions.
Treatment before birth
Since the introduction of routine prenatal U.S. screening, a large proportion of fetuses with CDH are diagnosed in utero. Termination of gestation is sometimes preferred, particularly when chromosomal aberrations and syndromes are present [5,152-154]. The possibility of fetal instrumentation directed to alleviate the consequences of the herniation is becoming a progressively more acceptable alternative. Thirty years ago Harrison and coll. started pioneer work in San Francisco aimed at developing animal models of CDH on which to test the effects of prenatal manipulation. After demonstrating in fetal lambs that lung compression led to lung hypoplasia [73] and that prenatal decompression reversed this condition [72], this group undertook the surgical creation of CDH [74] and later on, the demonstration of reversal of lung hypoplasia and arteriolar thickening when the diaphragmatic defect was repaired before birth [155]. Clinical application of this rationale led, with close consideration of all the ethical and technical issues involved, to the first attempts at prenatal repair of CDH in human fetuses [120,156,157]. The position of the fetal liver [138] and the demonstration of the lethal effect of umbilical vein compression during fetal reduction of the left lobe of the liver into the abdomen during surgery [74], limited considerably the number of cases suitable for this approach. Furthermore, a randomized study demonstrated that fetal repair did not produce better outcomes than optimal postnatal treatment [158]. The trial was discontinued and a new approach, based on the observation of lung hyperplasia in congenital tracheal obstruction, was developed. The evidence that airway pressure had something to do with lung development led to assume that tracheal occlusion during fetal life could counteract the effects of lung compression in fetuses with CDH. This was tested in the fetal lamb [77,78,159-161] and then in the human fetus [162,163]. Another randomized trial was discontinued when a significant shortening of gestation was observed in fetuses treated in this manner [164]. However, the effects of tracheal occlusion were indeed very positive in terms of alveolar size and histology [159]. Maturation and surfactant function were apparently not so much improved, unless the plug was reversed before delivery [165].
The concurrent development of minimally invasive surgery had the immediate effect of making fetendoscopic balloon tracheal occlusion possible [75,163]. Considerable experience rapidly accumulated and randomized trials are currently underway concentrating these efforts in fetuses with LHR below 1 considered otherwise unviable [166]. Survivals approaching 50% in this group are encouraging [139] and much information has already accumulated on the structure, maturity and tracheal pathology of survivors.
Experimental and clinical evidence suggest that biochemical lung maturation is delayed in fetuses with CDH [65,167-169] and this led to the proposal that this process be accelerated by administration of maternal corticosteroids as it is done for premature deliveries. The evidence of the benefits of this medication is not totally convincing [170,171] and therefore, a multicenter trial could be necessary [172].
Treatment after birth
Whenever prenatal diagnosis is made, it is advisable to direct the mother to a tertiary perinatal center in which all the necessary obstetric, neonatal and surgical skills are concentrated [173]. Gestation should be prolonged until near term if possible [174] (this can be hard to achieve in cases with polyhydramnios) and, although it is often preferred [175], there is no evidence of the benefits of delivery by Cesarean section [174,176,177].
When CDH becomes symptomatic after birth and in all cases diagnosed prenatally, a careful protocol of respiratory assistance should be implemented. Immediate postnatal intubation avoiding mask ventilation should be carried out, and small-volume, high frequency and reduced peak pressure mechanical ventilation should be started. Oxygen delivery has to be tailored for maintaining pre-established gasometric goals. Surfactant has been used in an attempt to compensate for biochemical immaturity in CDH babies [178,179], but this has not been beneficial [180,181]. The stomach is emptied through a nasogastric tube and vascular accesses (peripheral and central veins, umbilical artery) are secured for infusion of fluids, drug administration and blood sampling. A bladder catheter is inserted and urine output is monitored. Then, a period of assessment of the chances of adequate gas exchange is started adapting all actions to the clinical findings. If arterial oxygenation is maintained above a preset minimum, the lung will probably be capable of adequate gas exchange. Pre-ductal and post-ductal percutaneous oxygen saturation measurements help in the assessment of both the adequacy of the lungs to sustain life and of the magnitude of right-to-left shunting. Inotropic drugs are used as necessary and cardiac function and heart anomalies are carefully evaluated. Persistent pulmonary hypertension may cause right ventricle dysfunction and in these cases, maintenance of the patency of the ductus arteriosus is indicated [182].
After years of frustrating results of application of respiratory assistance goals usually applied to other conditions, it was realized that the hypoplastic and probably immature lung of CDH was severely damaged by excessive oxygen delivery and, particularly by excessive airway pressure that led to pneumothorax, barotrauma and volutrauma [183,184]. Mortality decreased drastically and all these untoward consequences of ventilator assistance became milder when a policy of "gentle ventilation" was implemented [185]. Spontaneous ventilation when possible or high frequency, low pressure ventilation (< 20-25 cm H2O), no relaxation, alkalinisation and adoption of modest gasometric goals (pre-ductal saturation of 80-95%, PaO2 around 60 mmHg and "permissive" hypercapnia of up to 60 mmHg) drastically changed the results [186]. This policy progressively gained adepts and it is nowadays the standard in most developed countries [187-189].
High-frequency, oscillatory ventilation is also widely used in these patients [190-192] in whom it allows adequate oxygenation and CO2 elimination with very low airway pressures [193]. Lung damage is apparently minimized while attaining the modest gasometric objectives set. A multicenter European trial is currently underway [194].
For how long time these treatments are necessary varies widely from one patient to another depending on the degree of lung hypoplasia, on the arteriolar reactivity and therefore on the duration of persistent pulmonary hypertension and on the associated malformations, particularly the cardiovascular ones.
The treatment of pulmonary hypertension is of particular concern because it determines shunting of non-oxygenated blood out of the lungs. Several medications have been used: tolazoline [67,195,196] and prostacyclins [197,198] were tried first but they did not produce good enough results. Prostaglandin 1E is occasionally used [199]. Inhaled nitric oxide, a well known smooth muscle relaxant is, in turn, widely used [200-203] with variable results although there is no strong evidence of its benefits [204]. Inhibitors of phospho-diesterase like sildenafil, known for their vasodilator action, are currently used in some cases [205] but, again, there is only anecdotal evidence of their benefits. These medications are coupled with inotropic agents, like dobutamine (up to 20 mg/kg/min), dopamine (< 10 mg/kg/min) [206] and also, sometimes with peripheral vasoconstrictors, like adrenaline at low doses, aimed at reducing the shunting by increasing pressure in the systemic circulation.
ECMO
Extra-corporeal membrane oxygenation (ECMO) may be a useful adjunct in the treatment of CDH. Cannulation of both the right carotid artery and jugular vein and connection to a circuit with a membrane gas exchange chamber allows oxygenation and CO2 disposal without participation of the lung which is preserved from any pressure insult [207]. Alternatively, veno-venous ECMO avoids cannulation of the carotid artery while permitting adequate gas exchange [208-210].
ECMO can be seen as a safety net maintained until proper gas exchange is demonstrated at weaning test periods. The fact that institutions, particularly in the US, which used ECMO liberally [10] had results comparable to Canadian institutions [211] that rarely, if ever, used it, casted some doubt on the actual need for this complicated and risky procedure [12,212]. The criteria for ECMO indication are variable, but they can be summarized as follows: Inability to reach pre-ductal SatO2 > 85% or post-ductal SatO2 > 70%, oxygenation index (mean airway pressure × FiO2 × 100/PaO2) ≥ 40 or need for high peak inspiratory pressures or a-AdO2 greater than 600 for 6 to 8 hours [213]. The main contraindication is, of course, the inability to obtain SatO2 > 80% at any moment of the initial treatment under FiO2 of 1. In fact, ECMO registries showed that the worst results were obtained precisely in the CDH group of patients [214,215]. This, together with the limitations of the technique (required weight above 2000 g, need for heparinisation), somewhat tempered the initial enthusiasm and the proportion of patients so treated decreased everywhere to more reasonable figures. There is only weak evidence of the benefits of ECMO in this particular group of patients [216] because only non-randomized trials have been carried out on them [171,212] and the only RCT reported in the UK was not specifically directed to babies with CDH [217]. This technique is probably a good adjunct in a limited number of patients in which predicted severe lung hypoplasia would make adequate gas exchange impossible or in those in whom reversal to the fetal pattern of circulation becomes unmanageable.
Surgical repair
Surgical repair of CDH used to be in the past a life-saving emergency. It is presently accepted that it should be undertaken only after cardio-respiratory functions are stable. A policy of "delayed" surgery coupled with gentle ventilation and occasionally ECMO support yields the best results recorded. For how long surgery has to be delayed is unclear, but a few days and even weeks may be beneficial. The goal of all preoperative treatments is to obtain "stabilization" of the patient and this means acceptable oxygenation (PaO2 > 40 mmHg) and CO2 disposal (PaCO2 < 60 mmHg) with stable pulmonary pressures (< 50% of systemic pressure), tolerable shunting, good myocardial function and adequate renal clearance with reduced or withdrawn inotropic drugs. Obviously, there are no magic indicators of stabilization (this may also be impossible) and a decision to operate is only made after a consensus among all the actors of these treatments has been reached. Once again, when critically reviewed, even the alleged benefits of delayed repair are not consistently documented [218].
In many institutions, the operation is carried out in the neonatal ICU in order to minimize changes that might increase stress [219-221]. Under general anesthesia, a subcostal or transverse abdominal incision is made, the herniated viscera are carefully reduced into the abdomen and the diaphragmatic orifice is closed with interrupted sutures without leaving an intercostal tube. A tube was routinely used in the past [58,222] until it was realized that underwater seals cause increased respiratory work and overdistension of the hypoplastic lung that may further reduce ventilation. A tubeless policy was then advised [185,223]. When the defect is too large, a prosthetic patch is used to achieve closure [224,225]. It is sutured to the rims of the orifice with interrupted sutures and, to avoid excessive tension and enlargement of the hemi-thorax, cone-shaping of the patch can be beneficial [226]. The use of a patch seems to increase the risk of re-herniation [227-230] although it should be acknowledged that patients requiring a patch have larger defects which entail higher morbidity [59]. Abdominal wall or latissimus dorsi muscle flaps have also been used for CDH repair [231-234].
Malrotation or non-rotation is usually present but this is rarely a problem in the newborn and the main concern of the surgeon after repair of the hernia is abdominal wall closure without excessive pressure [235,236]. In some cases, another abdominal wall prosthetic patch is necessary to avoid abdominal compartment syndrome [237-239].
Since the advent of minimally invasive surgery (MIS), thoracoscopic [240-246] or laparoscopic [247] approaches have been proposed for CDH repair. Both are indeed possible (with or without patch insertion), although it should never be disregarded that this repair comes only after stable conditions have been secured. Probably, MIS is a good approach in cases diagnosed during infancy or in outborns with less severe symptoms. Nevertheless, some concerns about this particular approach in the newborn period have been expressed on the basis of excessive perioperative hypercapnia and prolonged postoperative low brain oxygenation [248]. The risk of recurrence after a minimally invasive approach has been found to be similar to that of open surgery by some reports [246] but it was indeed increased in other series [248,249].
Prognosis
The main outcome endpoint is, of course, survival and the wide range of figures reported is puzzling. When only hospital, postoperative results were reported, survival approached 70% [250] or more [189,251,252]. However, those institutions with attached maternity facilities that treated mostly inborns, reported higher mortalities. The introduction of ECMO, in parallel with advances in other aspects of treatment, improved the results and the top institutions reported survivals approaching 90% although, sometimes, their statistics excluded chromosomal aberrations, multiple malformations and even some patients that did not reach surgery. The truth is that still nowadays, if terminations of pregnancy, spontaneous abortions, stillborns, pre-hospital and/or preoperative deaths and surgical mortality are taken into account, real mortality is still between 50% and 60%. But if only hospital statistics are considered, there has been indeed a clearcut progress in the results. Many institutions and the multi-institutional registries report 70% to 80% survival with some institutions peaking at 90%. Right-sided hernias seem to have a worse prognosis than left-sided ones and may require more ECMO support [253-255].
Other in-hospital complications can occur after CDH repair. Pleural effusions and chylothorax may require nutritional treatment, medication and drainage [256-260].
Chronic respiratory tract disease is frequent in these patients. Lung hypoplasia can be caught up only in part during the first months of life and oxygen toxicity, barotrauma and volutrauma may occur even after gentle ventilation. True broncho-pulmonary dysplasia is relatively frequent in survivors [250,261], and a number of them require oxygen at home for long periods of time [228,251]. Restrictive [262-264] and obstructive [263-265] lung diseases have been reported in CDH survivors many years after operation and diaphragmatic rigidity and thoracic deformities can also play a minor role in chronic lung disease [266-268].
Gastroesophageal reflux is frequent in CDH survivors. The diaphragmatic sling may be malformed or absent and the repair changes the anatomy of the region. In addition, mal-rotation may delay gastric emptying, and the abnormal balance of pressures in the thorax and abdomen in the course of the respiratory cycle facilitates retrograde passage of gastric contents to the esophagus [269]. Finally, there is evidence of abnormal enteric innervation in CDH and it is likely for esophago-gastric peristalsis to be abnormal [96,270]. Reflux can complicate the pre-existing respiratory disease and, for all these reasons, a considerable proportion of these patients [271,272] respond poorly to medical treatment and ultimately require anti-reflux surgery [227,273-276]. Prophylactic fun-doplication during surgical repair has been proposed in patients with CDH [277]
Neurodevelopmental deficits are always possible in these patients in whom brain oxygenation was marginal for long periods of time and particularly when ECMO required major vascular occlusion [228,278-283]. Neurosensorial deafness occurs in a small proportion of children surviving CDH [283-290]. This is progressive [287,291] and it is generally considered to be accounted for by prolonged antibiotic treatments [288]. However, other newborns with similar treatments suffer this deafness more rarely and it should be acknowledged that perhaps CDH patients have a particular sensitivity or a developmental defect of the inner ear.
Other sequelae are possible in this particular group of patients. This, and the frequent associated malformations, require long-term follow-up and permanent support.
Genetic counseling
Most cases are sporadic but there are some reports of familial clusters that suggest multifactoral [292-296] rather than autosomal recessive [297,298] patterns of inheritance. Genetic aspects of CDH were recently addressed [299-302]. Two thirds of these cases were males and the risk of recurrence in sibs is 2% [294].
Unresolved questions
CDH remains one of the most difficult problems of perinatology and neonatal surgery. Its mechanisms are beginning to be unveiled, but the severity of the lung and other lesions require the use of the entire armamentarium of sophisticated neonatal care. This, together with the rarity of the condition, make the setting of solid evidence-based protocols of management very difficult. The perspectives of fetal manipulation or even of prophylactic drug treatment remain still at an embryonal stage and, most probably, this condition will remain a hot topic of research in the coming years.
Conclusions
CDH is a complex condition probably caused by disturbed molecular signaling during organogenesis. The diaphragmatic orifice is invariably accompanied by pulmonary hypoplasia with vascular hyper-reactivity that causes deficient gas exchange and persistent pulmonary hypertension. In addition, other malformations that further complicate the clinical course may be present. All efforts are directed at enhancing antenatal lung growth in prenatally diagnosed cases and at protecting the lung during the intensive care pre and post-operative phases in all cases. Fetoscopic reversible tracheal obstruction seems promising before birth and gentle ventilation with occasional ECMO use yields the best results post-natally. Nevertheless, many aspects of the disease are still unknown and, given that the incidence is relatively high, that the expenses involved in the current treatments are overwhelming and that the sequelae are frequent, more research efforts into causation, prevention and treatment are warranted.
Competing interests
The author declares that they have no competing interests.
References
- Irish MS, Holm BA, Glick PL. Congenital diaphragmatic hernia. A historical review. Clin Perinatol. 1996;23:625–653. [PubMed] [Google Scholar]
- Golombek SG. The history of congenital diaphragmatic hernia from 1850s to the present. J Perinatol. 2002;22:242–246. doi: 10.1038/sj.jp.7210701. [DOI] [PubMed] [Google Scholar]
- McNamara JJ, Eraklis AJ, Gross RE. Congenital posterolateral diaphragmatic hernia in the newborn. J Thorac Cardiovasc Surg. 1968;55:55–59. [PubMed] [Google Scholar]
- Harrison MR, Bjordal RI, Langmark F, Knutrud O. Congenital diaphragmatic hernia: the hidden mortality. J Pediatr Surg. 1978;13:227–230. doi: 10.1016/s0022-3468(78)80391-1. [DOI] [PubMed] [Google Scholar]
- Stege G, Fenton A, Jaffray B. Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics. 2003;112:532–535. doi: 10.1542/peds.112.3.532. [DOI] [PubMed] [Google Scholar]
- Gallot D, Boda C, Ughetto S, Perthus I, Robert-Gnansia E, Francannet C, Laurichesse-Delmas H, Jani J, Coste K, Deprest J. et al. Prenatal detection and outcome of congenital diaphragmatic hernia: a French registry-based study. Ultrasound Obstet Gynecol. 2007;29:276–283. doi: 10.1002/uog.3863. [DOI] [PubMed] [Google Scholar]
- Torfs CP, Curry CJ, Bateson TF, Honore LH. A population-based study of congenital diaphragmatic hernia. Teratology. 1992;46:555–565. doi: 10.1002/tera.1420460605. [DOI] [PubMed] [Google Scholar]
- Yang W, Carmichael SL, Harris JA, Shaw GM. Epidemiologic characteristics of congenital diaphragmatic hernia among 2.5 million California births, 1989-1997. Birth Defects Res A Clin Mol Teratol. 2006;76:170–174. doi: 10.1002/bdra.20230. [DOI] [PubMed] [Google Scholar]
- Sinha CK, Islam S, Patel S, Nicolaides K, Greenough A, Davenport M. Congenital diaphragmatic hernia: prognostic indices in the fetal endoluminal tracheal occlusion era. J Pediatr Surg. 2009;44:312–316. doi: 10.1016/j.jpedsurg.2008.10.078. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Lund DP, Lillehei CW, Vacanti JP. Congenital diaphragmatic hernia--a tale of two cities: the Boston experience. J Pediatr Surg. 1997;32:401–405. doi: 10.1016/s0022-3468(97)90590-x. [DOI] [PubMed] [Google Scholar]
- Bedoyan JK, Blackwell SC, Treadwell MC, Johnson A, Klein MD. Congenital diaphragmatic hernia: associated anomalies and antenatal diagnosis. Outcome-related variables at two Detroit hospitals. Pediatr Surg Int. 2004;20:170–176. doi: 10.1007/s00383-004-1138-2. [DOI] [PubMed] [Google Scholar]
- de Buys Roessingh AS, Dinh-Xuan AT. Congenital diaphragmatic hernia: current status and review of the literature. Eur J Pediatr. 2009;168:393–406. doi: 10.1007/s00431-008-0904-x. [DOI] [PubMed] [Google Scholar]
- Keijzer R, Puri P. Congenital diaphragmatic hernia. Semin Pediatr Surg. 2010;19:180–185. doi: 10.1053/j.sempedsurg.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Johnston PW, Liberman R, Gangitano E, Vogt J. Ventilation parameters and arterial blood gases as a prediction of hypoplasia in congenital diaphragmatic hernia. J Pediatr Surg. 1990;25:496–499. doi: 10.1016/0022-3468(90)90558-q. [DOI] [PubMed] [Google Scholar]
- Elhalaby EA, Abo Sikeena MH. Delayed presentation of congenital diaphragmatic hernia. Pediatr Surg Int. 2002;18:480–485. doi: 10.1007/s00383-002-0743-1. [DOI] [PubMed] [Google Scholar]
- Numanoglu A, Steiner Z, Millar A, Cywes S. Delayed presentation of congenital diaphragmatic hernia. S Afr J Surg. 1997;35:74–76. [PubMed] [Google Scholar]
- Stoll C, Alembik Y, Dott B, Roth MP. Associated malformations in cases with congenital diaphragmatic hernia. Genet Couns. 2008;19:331–339. [PubMed] [Google Scholar]
- Sweed Y, Puri P. Congenital diaphragmatic hernia: influence of associated malformations on survival. Arch Dis Child. 1993;69:68–70. doi: 10.1136/adc.69.1_spec_no.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliazza L, Otten C, Xia H, Rodriguez JI, Diez-Pardo JA, Tovar JA. Cardiovascular malformations in congenital diaphragmatic hernia: human and experimental studies. J Pediatr Surg. 1999;34:1352–1358. doi: 10.1016/s0022-3468(99)90010-6. [DOI] [PubMed] [Google Scholar]
- Lin AE, Pober BR, Adatia I. Congenital diaphragmatic hernia and associated cardiovascular malformations: type, frequency, and impact on management. Am J Med Genet C Semin Med Genet. 2007;145C:201–216. doi: 10.1002/ajmg.c.30131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert JR, Haas JE, Beckwith JB. Left ventricular hypoplasia in congenital diaphragmatic hernia. J Pediatr Surg. 1984;19:567–571. doi: 10.1016/s0022-3468(84)80105-0. [DOI] [PubMed] [Google Scholar]
- Baumgart S, Paul JJ, Huhta JC. Left heart hypoplasia in neonates having congenital diaphragmatic hernia and treated with ECMO. J Pediatr Surg. 1998;33:1848. doi: 10.1016/s0022-3468(98)90307-4. [DOI] [PubMed] [Google Scholar]
- Bollmann R, Kalache K, Mau H, Chaoui R, Tennstedt C. Associated malformations and chromosomal defects in congenital diaphragmatic hernia. Fetal Diagn Ther. 1995;10:52–59. doi: 10.1159/000264193. [DOI] [PubMed] [Google Scholar]
- Migliazza L, Xia H, Diez-Pardo JA, Tovar JA. Skeletal malformations associated with congenital diaphragmatic hernia: experimental and human studies. J Pediatr Surg. 1999;34:1624–1629. doi: 10.1016/s0022-3468(99)90630-9. [DOI] [PubMed] [Google Scholar]
- van Dooren MF, Goemaere NN, de Klein A, Tibboel D, de Krijger RR. Postmortem findings and clinicopathological correlation in congenital diaphragmatic hernia. Pediatr Dev Pathol. 2004;7:459–467. doi: 10.1007/s10024-004-1118-2. [DOI] [PubMed] [Google Scholar]
- Sabharwal AJ, Davis CF, Howatson AG. Post-mortem findings in fetal and neonatal congenital diaphragmatic hernia. Eur J Pediatr Surg. 2000;10:96–99. doi: 10.1055/s-2008-1072334. [DOI] [PubMed] [Google Scholar]
- Brownlee EM, Howatson AG, Davis CF, Sabharwal AJ. The hidden mortality of congenital diaphragmatic hernia: a 20-year review. J Pediatr Surg. 2009;44:317–320. doi: 10.1016/j.jpedsurg.2008.10.076. [DOI] [PubMed] [Google Scholar]
- Slavotinek AM. Fryns syndrome: a review of the phenotype and diagnostic guidelines. Am J Med Genet A. 2004;124A:427–433. doi: 10.1002/ajmg.a.20381. [DOI] [PubMed] [Google Scholar]
- Scott DA, Cooper ML, Stankiewicz P, Patel A, Potocki L, Cheung SW. Congenital diaphragmatic hernia in WAGR syndrome. Am J Med Genet A. 2005;134:430–433. doi: 10.1002/ajmg.a.30654. [DOI] [PubMed] [Google Scholar]
- Luis AL, Pederiva F, Encinas JL, Ruiz E, Rodriguez JI, Martinez L, Tovar JA. Parafollicular C-Cells of the Thyroid are Decreased in Patients with Congenital Diaphragmatic Hernia. Eur J Pediatr Surg. 2011;21:246–249. doi: 10.1055/s-0031-1273778. [DOI] [PubMed] [Google Scholar]
- Pederiva F, Rodriguez JI, Ruiz-Bravo E, Martinez L, Tovar JA. Abnormal intrinsic esophageal innervation in congenital diaphragmatic hernia: a likely cause of motor dysfunction. J Pediatr Surg. 2009;44:496–499. doi: 10.1016/j.jpedsurg.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Baoquan Q, Diez-Pardo JA, Tovar JA. Intestinal rotation in experimental congenital diaphragmatic hernia. J Pediatr Surg. 1995;30:1457–1462. doi: 10.1016/0022-3468(95)90407-7. [DOI] [PubMed] [Google Scholar]
- Ford EG, Senac MO Jr, Srikanth MS, Weitzman JJ. Malrotation of the intestine in children. Ann Surg. 1992;215:172–178. doi: 10.1097/00000658-199202000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss I, Kehl S, Link K, Neff W, Schaible T, Sutterlin M, Siemer J. Associated Malformations in Congenital Diaphragmatic Hernia. Am J Perinatol. 2010. [DOI] [PubMed]
- McPherson EW, Ketterer DM, Salsburey DJ. Pallister-Killian and Fryns syndromes: nosology. Am J Med Genet. 1993;47:241–245. doi: 10.1002/ajmg.1320470219. [DOI] [PubMed] [Google Scholar]
- Franceschini P, Guala A, Licata D, Botta G, Flora F, Angeli G, Di Cara G, Franceschini D. Gershoni-Baruch syndrome: report of a new family confirming autosomal recessive inheritance. Am J Med Genet A. 2003;122A:174–179. doi: 10.1002/ajmg.a.20275. [DOI] [PubMed] [Google Scholar]
- Cho HY, Lee BS, Kang CH, Kim WH, Ha IS, Cheong HI, Choi Y. Hydrothorax in a patient with Denys-Drash syndrome associated with a diaphragmatic defect. Pediatr Nephrol. 2006;21:1909–1912. doi: 10.1007/s00467-006-0273-5. [DOI] [PubMed] [Google Scholar]
- Martinez-Frias ML, Bermejo E, Felix V, Jimenez N, Gomez-Ullate J, Lopez JA, Aparicio P, Ayala A, Gairi JM, Galan E. et al. [Brachmann-de-Lange syndrome in our population: clinical and epidemiological characteristics] An Esp Pediatr. 1998;48:293–298. [PubMed] [Google Scholar]
- Kantarci S, Donahoe PK. Congenital diaphragmatic hernia (CDH) etiology as revealed by pathway genetics. Am J Med Genet C Semin Med Genet. 2007;145C:217–226. doi: 10.1002/ajmg.c.30132. [DOI] [PubMed] [Google Scholar]
- van Dooren MF, Brooks AS, Hoogeboom AJ, van den Hoonaard TL, de Klein JE, Wouters CH, Tibboel D. Early diagnosis of Wolf-Hirschhorn syndrome triggered by a life-threatening event: congenital diaphragmatic hernia. Am J Med Genet A. 2004;127A:194–196. doi: 10.1002/ajmg.a.20613. [DOI] [PubMed] [Google Scholar]
- Blancato JK, Hunt M, George J, Katz J, Meck JM. Prenatal diagnosis of tetrasomy 12p by in situ hybridization: varying levels of mosaicism in different fetal tissues. Prenat Diagn. 1992;12:979–983. doi: 10.1002/pd.1970121202. [DOI] [PubMed] [Google Scholar]
- Klaassens M, Scott DA, van Dooren M, Hochstenbach R, Eussen HJ, Cai WW, Galjaard RJ, Wouters C, Poot M, Laudy J. et al. Congenital diaphragmatic hernia associated with duplication of 11q23-qter. Am J Med Genet A. 2006;140:1580–1586. doi: 10.1002/ajmg.a.31321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlembach D, Zenker M, Trautmann U, Ulmer R, Beinder E. Deletion 15q24-26 in prenatally detected diaphragmatic hernia: increasing evidence of a candidate region for diaphragmatic development. Prenat Diagn. 2001;21:289–292. doi: 10.1002/pd.50. [DOI] [PubMed] [Google Scholar]
- Biggio JR Jr, Descartes MD, Carroll AJ, Holt RL. Congenital diaphragmatic hernia: is 15q26.1-26.2 a candidate locus? Am J Med Genet A. 2004;126A:183–185. doi: 10.1002/ajmg.a.20464. [DOI] [PubMed] [Google Scholar]
- Kantarci S, Ackerman KG, Russell MK, Longoni M, Sougnez C, Noonan KM, Hatchwell E, Zhang X, Pieretti Vanmarcke R, Anyane-Yeboa K. et al. Characterization of the chromosome 1q41q42.12 region, and the candidate gene DISP1, in patients with CDH. Am J Med Genet A. 2010;152A:2493–2504. doi: 10.1002/ajmg.a.33618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wat MJ, Shchelochkov OA, Holder AM, Breman AM, Dagli A, Bacino C, Scaglia F, Zori RT, Cheung SW, Scott DA, Kang SH. Chromosome 8p23.1 deletions as a cause of complex congenital heart defects and diaphragmatic hernia. Am J Med Genet A. 2009;149A:1661–1677. doi: 10.1002/ajmg.a.32896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PC, Mo R, Hui Cc C. Murine models of VACTERL syndrome: Role of sonic hedgehog signaling pathway. J Pediatr Surg. 2001;36:381–384. doi: 10.1053/jpsu.2001.20722. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang L, Wang D, Shen H, Jiang M, Mei P, Hayden PS, Sedor JR, Hu H. Congenital diaphragmatic hernia, kidney agenesis and cardiac defects associated with Slit3-deficiency in mice. Mech Dev. 2003;120:1059–1070. doi: 10.1016/s0925-4773(03)00161-8. [DOI] [PubMed] [Google Scholar]
- Yuan W, Rao Y, Babiuk RP, Greer JJ, Wu JY, Ornitz DM. A genetic model for a central (septum transversum) congenital diaphragmatic hernia in mice lacking Slit3. Proc Natl Acad Sci USA. 2003;100:5217–5222. doi: 10.1073/pnas.0730709100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You LR, Takamoto N, Yu CT, Tanaka T, Kodama T, Demayo FJ, Tsai SY, Tsai MJ. Mouse lacking COUP-TFII as an animal model of Bochdalek-type congenital diaphragmatic hernia. Proc Natl Acad Sci USA. 2005;102:16351–16356. doi: 10.1073/pnas.0507832102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman KG, Herron BJ, Vargas SO, Huang H, Tevosian SG, Kochilas L, Rao C, Pober BR, Babiuk RP, Epstein JA. et al. Fog2 is required for normal diaphragm and lung development in mice and humans. PLoS Genet. 2005;1:58–65. doi: 10.1371/journal.pgen.0010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordenskjold A, Tapper-Persson M, Anvret M. No evidence of WT1 gene mutations in children with congenital diaphragmatic hernia. J Pediatr Surg. 1996;31:925–927. doi: 10.1016/s0022-3468(96)90412-1. [DOI] [PubMed] [Google Scholar]
- LopezJimenez N, Gerber S, Popovici V, Mirza S, Copren K, Ta L, Shaw GM, Trueb B, Slavotinek AM. Examination of FGFRL1 as a candidate gene for diaphragmatic defects at chromosome 4p16.3 shows that Fgfrl1 null mice have reduced expression of Tpm3, sarcomere genes and Lrtm1 in the diaphragm. Hum Genet. 2009;127:325–336. doi: 10.1007/s00439-009-0777-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluth D, Keijzer R, Hertl M, Tibboel D. Embryology of congenital diaphragmatic hernia. Semin Pediatr Surg. 1996;5:224–233. [PubMed] [Google Scholar]
- Allan DW, Greer JJ. Pathogenesis of nitrofen-induced congenital diaphragmatic hernia in fetal rats. J Appl Physiol. 1997;83:338–347. doi: 10.1152/jappl.1997.83.2.338. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Allan DW, Babiuk RP, Lemke RP. Recent advances in understanding the pathogenesis of nitrofen-induced congenital diaphragmatic hernia. Pediatr Pulmonol. 2000;29:394–399. doi: 10.1002/(sici)1099-0496(200005)29:5<394::aid-ppul9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Babiuk RP, Zhang W, Clugston R, Allan DW, Greer JJ. Embryological origins and development of the rat diaphragm. J Comp Neurol. 2003;455:477–487. doi: 10.1002/cne.10503. [DOI] [PubMed] [Google Scholar]
- Clark RH, Hardin WD Jr, Hirschl RB, Jaksic T, Lally KP, Langham MR Jr, Wilson JM. Current surgical management of congenital diaphragmatic hernia: a report from the Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg. 1998;33:1004–1009. doi: 10.1016/s0022-3468(98)90522-x. [DOI] [PubMed] [Google Scholar]
- Lally KP, Lally PA, Lasky RE, Tibboel D, Jaksic T, Wilson JM, Frenckner B, Van Meurs KP, Bohn DJ, Davis CF, Hirschl RB. Defect size determines survival in infants with congenital diaphragmatic hernia. Pediatrics. 2007;120:e651–657. doi: 10.1542/peds.2006-3040. [DOI] [PubMed] [Google Scholar]
- Tsang TM, Tam PK, Dudley NE, Stevens J. Diaphragmatic agenesis as a distinct clinical entity. J Pediatr Surg. 1994;29:1439–1441. doi: 10.1016/0022-3468(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Schnitzer JJ. Control and regulation of pulmonary hypoplasia associated with congenital diaphragmatic hernia. Semin Pediatr Surg. 2004;13:37–43. doi: 10.1053/j.sempedsurg.2003.09.006. [DOI] [PubMed] [Google Scholar]
- George DK, Cooney TP, Chiu BK, Thurlbeck WM. Hypoplasia and immaturity of the terminal lung unit (acinus) in congenital diaphragmatic hernia. Am Rev Respir Dis. 1987;136:947–950. doi: 10.1164/ajrccm/136.4.947. [DOI] [PubMed] [Google Scholar]
- Wigglesworth JS, Desai R, Guerrini P. Fetal lung hypoplasia: biochemical and structural variations and their possible significance. Arch Dis Child. 1981;56:606–615. doi: 10.1136/adc.56.8.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya FR, Thomas VL, Romaguera J, Mysore MR, Maberry M, Bernard A, Freund M. Fetal lung maturation in congenital diaphragmatic hernia. Am J Obstet Gynecol. 1995;173:1401–1405. doi: 10.1016/0002-9378(95)90624-x. [DOI] [PubMed] [Google Scholar]
- Valls-i-Soler A, Alfonso LF, Arnaiz A, Alvarez FJ, Tovar JA. Pulmonary surfactant dysfunction in congenital diaphragmatic hernia: experimental and clinical findings. Biol Neonate. 1996;69:318–326. doi: 10.1159/000244326. [DOI] [PubMed] [Google Scholar]
- Boucherat O, Benachi A, Chailley-Heu B, Franco-Montoya ML, Elie C, Martinovic J, Bourbon JR. Surfactant maturation is not delayed in human fetuses with diaphragmatic hernia. PLoS Med. 2007;4:e237. doi: 10.1371/journal.pmed.0040237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shochat SJ, Naeye RL, Ford WD, Whitman V, Maisels MJ. Congenital diaphragmatic hernia. New concept in management. Ann Surg. 1979;190:332–341. doi: 10.1097/00000658-197909000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamataka T, Puri P. Pulmonary artery structural changes in pulmonary hypertension complicating congenital diaphragmatic hernia. J Pediatr Surg. 1997;32:387–390. doi: 10.1016/s0022-3468(97)90587-x. [DOI] [PubMed] [Google Scholar]
- Ohi R, Suzuki H, Kato T, Kasai M. Development of the lung in fetal rabbits with experimental diaphragmatic hernia. J Pediatr Surg. 1976;11:955–959. doi: 10.1016/s0022-3468(76)80073-5. [DOI] [PubMed] [Google Scholar]
- Fauza DO, Tannuri U, Ayoub AA, Capelozzi VL, Saldiva PH, Maksoud JG. Surgically produced congenital diaphragmatic hernia in fetal rabbits. J Pediatr Surg. 1994;29:882–886. doi: 10.1016/0022-3468(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Wu J, Yamamoto H, Gratacos E, Ge X, Verbeken E, Sueishi K, Hashimoto S, Vanamo K, Lerut T, Deprest J. Lung development following diaphragmatic hernia in the fetal rabbit. Hum Reprod. 2000;15:2483–2488. doi: 10.1093/humrep/15.12.2483. [DOI] [PubMed] [Google Scholar]
- Harrison MR, Bressack MA, Churg AM, de Lorimier AA. Correction of congenital diaphragmatic hernia in utero. II. Simulated correction permits fetal lung growth with survival at birth. Surgery. 1980;88:260–268. [PubMed] [Google Scholar]
- Harrison MR, Jester JA, Ross NA. Correction of congenital diaphragmatic hernia in utero. I. The model: intrathoracic balloon produces fatal pulmonary hypoplasia. Surgery. 1980;88:174–182. [PubMed] [Google Scholar]
- Harrison MR, Ross NA, de Lorimier AA. Correction of congenital diaphragmatic hernia in utero. III. Development of a successful surgical technique using abdominoplasty to avoid compromise of umbilical blood flow. J Pediatr Surg. 1981;16:934–942. doi: 10.1016/s0022-3468(81)80849-4. [DOI] [PubMed] [Google Scholar]
- Deprest JA, Lerut TE, Vandenberghe K. Operative fetoscopy: new perspective in fetal therapy? Prenat Diagn. 1997;17:1247–1260. [PubMed] [Google Scholar]
- Soper RT, Pringle KC, Scofield JC. Creation and repair of diaphragmatic hernia in the fetal lamb: techniques and survival. J Pediatr Surg. 1984;19:33–40. doi: 10.1016/s0022-3468(84)80011-1. [DOI] [PubMed] [Google Scholar]
- DiFiore JW, Fauza DO, Slavin R, Peters CA, Fackler JC, Wilson JM. Experimental fetal tracheal ligation reverses the structural and physiological effects of pulmonary hypoplasia in congenital diaphragmatic hernia. J Pediatr Surg. 1994;29:248–256. doi: 10.1016/0022-3468(94)90328-x. [DOI] [PubMed] [Google Scholar]
- Hedrick MH, Estes JM, Sullivan KM, Bealer JF, Kitterman JA, Flake AW, Adzick NS, Harrison MR. Plug the lung until it grows (PLUG): a new method to treat congenital diaphragmatic hernia in utero. J Pediatr Surg. 1994;29:612–617. doi: 10.1016/0022-3468(94)90724-2. [DOI] [PubMed] [Google Scholar]
- Benachi A, Dommergues M, Delezoide AL, Bourbon J, Dumez Y, Brunnelle F. Tracheal obstruction in experimental diaphragmatic hernia: an endoscopic approach in the fetal lamb. Prenat Diagn. 1997;17:629–634. [PubMed] [Google Scholar]
- Luks FI, Wild YK, Piasecki GJ, De Paepe ME. Short-term tracheal occlusion corrects pulmonary vascular anomalies in the fetal lamb with diaphragmatic hernia. Surgery. 2000;128:266–272. doi: 10.1067/msy.2000.107373. [DOI] [PubMed] [Google Scholar]
- Bratu I, Flageole H, Laberge JM, Kovacs L, Faucher D, Piedboeuf B. Lung function in lambs with diaphragmatic hernia after reversible fetal tracheal occlusion. J Pediatr Surg. 2004;39:1524–1531. doi: 10.1016/j.jpedsurg.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Keijzer R, Liu J, Deimling J, Tibboel D, Post M. Dual-hit hypothesis explains pulmonary hypoplasia in the nitrofen model of congenital diaphragmatic hernia. Am J Pathol. 2000;156:1299–1306. doi: 10.1016/S0002-9440(10)65000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose K, Kamata S, Sawai T, Tazuke Y, Usui N, Kawahara H, Okada A. Airway anomalies in patients with congenital diaphragmatic hernia. J Pediatr Surg. 2000;35:1562–1565. doi: 10.1053/jpsu.2000.18310. [DOI] [PubMed] [Google Scholar]
- Alfonso LF, Arnaiz A, Alvarez FJ, Qi B, Diez-Pardo JA, Vallis-i-Soler A, Tovar JA. Lung hypoplasia and surfactant system immaturity induced in the fetal rat by prenatal exposure to nitrofen. Biol Neonate. 1996;69:94–100. doi: 10.1159/000244283. [DOI] [PubMed] [Google Scholar]
- Geggel RL, Murphy JD, Langleben D, Crone RK, Vacanti JP, Reid LM. Congenital diaphragmatic hernia: arterial structural changes and persistent pulmonary hypertension after surgical repair. J Pediatr. 1985;107:457–464. doi: 10.1016/s0022-3476(85)80534-5. [DOI] [PubMed] [Google Scholar]
- Mey J, Babiuk RP, Clugston R, Zhang W, Greer JJ. Retinal dehydrogenase-2 is inhibited by compounds that induce congenital diaphragmatic hernias in rodents. Am J Pathol. 2003;162:673–679. doi: 10.1016/s0002-9440(10)63861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluth D, Kangah R, Reich P, Tenbrinck R, Tibboel D, Lambrecht W. Nitrofen-induced diaphragmatic hernias in rats: an animal model. J Pediatr Surg. 1990;25:850–854. doi: 10.1016/0022-3468(90)90190-k. [DOI] [PubMed] [Google Scholar]
- Tenbrinck R, Tibboel D, Gaillard JL, Kluth D, Bos AP, Lachmann B, Molenaar JC. Experimentally induced congenital diaphragmatic hernia in rats. J Pediatr Surg. 1990;25:426–429. doi: 10.1016/0022-3468(90)90386-n. [DOI] [PubMed] [Google Scholar]
- Alfonso LF, Vilanova J, Aldazabal P, Lopez de Torre B, Tovar JA. Lung growth and maturation in the rat model of experimentally induced congenital diaphragmatic hernia. Eur J Pediatr Surg. 1993;3:6–11. doi: 10.1055/s-2008-1063498. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Babiuk RP, Thebaud B. Etiology of congenital diaphragmatic hernia: the retinoid hypothesis. Pediatr Res. 2003;53:726–730. doi: 10.1203/01.PDR.0000062660.12769.E6. [DOI] [PubMed] [Google Scholar]
- Van Tuyl M, Blommaart PE, Keijzer R, Wert SE, Ruijter JM, Lamers WH, Tibboel D. Pulmonary surfactant protein A, B, and C mRNA and protein expression in the nitrofen-induced congenital diaphragmatic hernia rat model. Pediatr Res. 2003;54:641–652. doi: 10.1203/01.PDR.0000086906.19683.42. [DOI] [PubMed] [Google Scholar]
- Jesudason EC, Smith NP, Connell MG, Spiller DG, White MR, Fernig DG, Losty PD. Peristalsis of airway smooth muscle is developmentally regulated and uncoupled from hypoplastic lung growth. Am J Physiol Lung Cell Mol Physiol. 2006;291:L559–565. doi: 10.1152/ajplung.00498.2005. [DOI] [PubMed] [Google Scholar]
- Montedonico S, Nakazawa N, Puri P. Retinoic acid rescues lung hypoplasia in nitrofen-induced hypoplastic foetal rat lung explants. Pediatr Surg Int. 2006;22:2–8. doi: 10.1007/s00383-005-1571-x. [DOI] [PubMed] [Google Scholar]
- Takayasu H, Nakazawa N, Montedonico S, Puri P. Reduced expression of aquaporin 5 water channel in nitrofen-induced hypoplastic lung with congenital diaphragmatic hernia rat model. J Pediatr Surg. 2007;42:415–419. doi: 10.1016/j.jpedsurg.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Montedonico S, Sugimoto K, Felle P, Bannigan J, Puri P. Prenatal treatment with retinoic acid promotes pulmonary alveologenesis in the nitrofen model of congenital diaphragmatic hernia. J Pediatr Surg. 2008;43:500–507. doi: 10.1016/j.jpedsurg.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Martinez L, Pederiva F, Martinez-Calonge W, Aras-Lopez R, Tovar JA. The myenteric plexus of the esophagus is abnormal in an experimental congenital diaphragmatic hernia model. Eur J Pediatr Surg. 2009;19:163–167. doi: 10.1055/s-0029-1202854. [DOI] [PubMed] [Google Scholar]
- Dingemann J, Doi T, Ruttenstock E, Puri P. Expression of the Wilm's tumor gene WT1 during diaphragmatic development in the nitrofen model for congenital diaphragmatic hernia. Pediatr Surg Int. 2010. [DOI] [PubMed]
- Clugston RD, Zhang W, Greer JJ. Early development of the primordial mammalian diaphragm and cellular mechanisms of nitrofen-induced congenital diaphragmatic hernia. Birth Defects Res A Clin Mol Teratol. 2010;88:15–24. doi: 10.1002/bdra.20613. [DOI] [PubMed] [Google Scholar]
- Dingemann J, Doi T, Ruttenstock EM, Puri P. Downregulation of FGFRL1 Contributes to the Development of the Diaphragmatic Defect in the Nitrofen Model of Congenital Diaphragmatic Hernia. Eur J Pediatr Surg. [DOI] [PubMed]
- Andersen DH. Effect of diet during pregnancy upon the incidence of congenital hereditary diaphragmatic hernia in the rat; failure to produce cystic fibrosis of the pancreas by maternal vitamin A deficiency. Am J Pathol. 1949;25:163–185. [PMC free article] [PubMed] [Google Scholar]
- Thebaud B, Tibboel D, Rambaud C, Mercier JC, Bourbon JR, Dinh-Xuan AT, Archer SL. Vitamin A decreases the incidence and severity of nitrofen-induced congenital diaphragmatic hernia in rats. Am J Physiol. 1999;277:L423–429. doi: 10.1152/ajplung.1999.277.2.L423. [DOI] [PubMed] [Google Scholar]
- Thebaud B, Barlier-Mur AM, Chailley-Heu B, Henrion-Caude A, Tibboel D, Dinh-Xuan AT, Bourbon JR. Restoring effects of vitamin A on surfactant synthesis in nitrofen-induced congenital diaphragmatic hernia in rats. Am J Respir Crit Care Med. 2001;164:1083–1089. doi: 10.1164/ajrccm.164.6.2010115. [DOI] [PubMed] [Google Scholar]
- Babiuk RP, Thebaud B, Greer JJ. Reductions in the incidence of nitrofen-induced diaphragmatic hernia by vitamin A and retinoic acid. Am J Physiol Lung Cell Mol Physiol. 2004;286:L970–973. doi: 10.1152/ajplung.00403.2003. [DOI] [PubMed] [Google Scholar]
- Nakazawa N, Takayasu H, Montedonico S, Puri P. Altered regulation of retinoic acid synthesis in nitrofen-induced hypoplastic lung. Pediatr Surg Int. 2007;23:391–396. doi: 10.1007/s00383-006-1848-8. [DOI] [PubMed] [Google Scholar]
- Nakazawa N, Montedonico S, Takayasu H, Paradisi F, Puri P. Disturbance of retinol transportation causes nitrofen-induced hypoplastic lung. J Pediatr Surg. 2007;42:345–349. doi: 10.1016/j.jpedsurg.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Doi T, Sugimoto K, Puri P. Prenatal retinoic acid up-regulates pulmonary gene expression of COUP-TFII, FOG2, and GATA4 in pulmonary hypoplasia. J Pediatr Surg. 2009;44:1933–1937. doi: 10.1016/j.jpedsurg.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- Clugston RD, Zhang W, Alvarez S, de Lera AR, Greer JJ. Understanding abnormal retinoid signaling as a causative mechanism in congenital diaphragmatic hernia. Am J Respir Cell Mol Biol. 2009;42:276–285. doi: 10.1165/rcmb.2009-0076OC. [DOI] [PubMed] [Google Scholar]
- Cipollone D, Cozzi DA, Businaro R, Marino B. Congenital diaphragmatic hernia after exposure to a triple retinoic acid antagonist during pregnancy. J Cardiovasc Med (Hagerstown) 2010. [DOI] [PubMed]
- Major D, Cadenas M, Fournier L, Leclerc S, Lefebvre M, Cloutier R. Retinol status of newborn infants with congenital diaphragmatic hernia. Pediatr Surg Int. 1998;13:547–549. doi: 10.1007/s003830050399. [DOI] [PubMed] [Google Scholar]
- Beurskens LW, Tibboel D, Lindemans J, Duvekot JJ, Cohen-Overbeek TE, Veenma DC, de Klein A, Greer JJ, Steegers-Theunissen RP. Retinol status of newborn infants is associated with congenital diaphragmatic hernia. Pediatrics. 2010;126:712–720. doi: 10.1542/peds.2010-0521. [DOI] [PubMed] [Google Scholar]
- Goumy C, Gouas L, Marceau G, Coste K, Veronese L, Gallot D, Sapin V, Vago P, Tchirkov A. Retinoid pathway and congenital diaphragmatic hernia: hypothesis from the analysis of chromosomal abnormalities. Fetal Diagn Ther. 2010;28:129–139. doi: 10.1159/000313331. [DOI] [PubMed] [Google Scholar]
- Gallot D, Marceau G, Coste K, Hadden H, Robert-Gnansia E, Laurichesse H, Dechelotte PJ, Labbe A, Dastugue B, Lemery D, Sapin V. Congenital diaphragmatic hernia: a retinoid-signaling pathway disruption during lung development? Birth Defects Res A Clin Mol Teratol. 2005;73:523–531. doi: 10.1002/bdra.20151. [DOI] [PubMed] [Google Scholar]
- Holder AM, Klaassens M, Tibboel D, de Klein A, Lee B, Scott DA. Genetic factors in congenital diaphragmatic hernia. Am J Hum Genet. 2007;80:825–845. doi: 10.1086/513442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montedonico S, Nakazawa N, Puri P. Congenital diaphragmatic hernia and retinoids: searching for an etiology. Pediatr Surg Int. 2008;24:755–761. doi: 10.1007/s00383-008-2140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano Y, Flake AW, Quinn TM, Kanai M, Davies P, Sablich TJ, Schneider C, Adzick NS, von Allmen D. Lung growth induced by tracheal occlusion in the sheep is augmented by airway pressurization. J Pediatr Surg. 2000;35:216–221. doi: 10.1016/s0022-3468(00)90012-5. [DOI] [PubMed] [Google Scholar]
- Kitano Y, Kanai M, Davies P, von Allmen D, Yang EY, Radu A, Adzick NS, Flake AW. BAPS prize-1999: Lung growth induced by prenatal tracheal occlusion and its modifying factors: a study in the rat model of congenital diaphragmatic hernia. J Pediatr Surg. 2001;36:251–259. doi: 10.1053/jpsu.2001.20683. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Hislop AA, Kitterman JA, Johnson P. Effect of tracheostomy on lung development in fetal lambs. J Appl Physiol. 1983;55:1103–1108. doi: 10.1152/jappl.1983.55.4.1103. [DOI] [PubMed] [Google Scholar]
- Jesudason EC. Exploiting mechanical stimuli to rescue growth of the hypoplastic lung. Pediatr Surg Int. 2007;23:827–836. doi: 10.1007/s00383-007-1956-0. [DOI] [PubMed] [Google Scholar]
- Nakayama DK, Harrison MR, Chinn DH, Callen PW, Filly RA, Golbus MS, De Lorimier AA. Prenatal diagnosis and natural history of the fetus with a congenital diaphragmatic hernia: initial clinical experience. J Pediatr Surg. 1985;20:118–124. doi: 10.1016/s0022-3468(85)80282-7. [DOI] [PubMed] [Google Scholar]
- Adzick NS, Vacanti JP, Lillehei CW, O'Rourke PP, Crone RK, Wilson JM. Fetal diaphragmatic hernia: ultrasound diagnosis and clinical outcome in 38 cases. J Pediatr Surg. 1989;24:654–657. doi: 10.1016/s0022-3468(89)80713-4. [DOI] [PubMed] [Google Scholar]
- Knox E, Lissauer D, Khan K, Kilby M. Prenatal detection of pulmonary hypoplasia in fetuses with congenital diaphragmatic hernia: a systematic review and meta-analysis of diagnostic studies. J Matern Fetal Neonatal Med. 2010;23:579–588. doi: 10.3109/14767050903551400. [DOI] [PubMed] [Google Scholar]
- Sandaite I, Claus F, De Keyzer F, Done E, Van Mieghem T, Gucciardo L, Dekoninck P, Jani J, Cannie M, Deprest JA. Examining the Relationship between the Lung-to-Head Ratio Measured on Ultrasound and Lung Volumetry by Magnetic Resonance in Fetuses with Isolated Congenital Diaphragmatic Hernia. Fetal Diagn Ther. 2010. [DOI] [PubMed]
- Harrison MR, Sydorak RM, Farrell JA, Kitterman JA, Filly RA, Albanese CT. Fetoscopic temporary tracheal occlusion for congenital diaphragmatic hernia: prelude to a randomized, controlled trial. J Pediatr Surg. 2003;38:1012–1020. doi: 10.1016/s0022-3468(03)00182-9. [DOI] [PubMed] [Google Scholar]
- Jani J, Peralta CF, Van Schoubroeck D, Deprest J, Nicolaides KH. Relationship between lung-to-head ratio and lung volume in normal fetuses and fetuses with diaphragmatic hernia. Ultrasound Obstet Gynecol. 2006;27:545–550. doi: 10.1002/uog.2735. [DOI] [PubMed] [Google Scholar]
- Usui N, Okuyama H, Sawai T, Kamiyama M, Kamata S, Fukuzawa M. Relationship between L/T ratio and LHR in the prenatal assessment of pulmonary hypoplasia in congenital diaphragmatic hernia. Pediatr Surg Int. 2007;23:971–976. doi: 10.1007/s00383-007-1980-0. [DOI] [PubMed] [Google Scholar]
- Jani JC, Benachi A, Nicolaides KH, Allegaert K, Gratacos E, Mazkereth R, Matis J, Tibboel D, Van Heijst A, Storme L. et al. Prenatal prediction of neonatal morbidity in survivors with congenital diaphragmatic hernia: a multicenter study. Ultrasound Obstet Gynecol. 2009;33:64–69. doi: 10.1002/uog.6141. [DOI] [PubMed] [Google Scholar]
- Metkus AP, Filly RA, Stringer MD, Harrison MR, Adzick NS. Sonographic predictors of survival in fetal diaphragmatic hernia. J Pediatr Surg. 1996;31:148–151. doi: 10.1016/s0022-3468(96)90338-3. [DOI] [PubMed] [Google Scholar]
- Lipshutz GS, Albanese CT, Feldstein VA, Jennings RW, Housley HT, Beech R, Farrell JA, Harrison MR. Prospective analysis of lung-to-head ratio predicts survival for patients with prenatally diagnosed congenital diaphragmatic hernia. J Pediatr Surg. 1997;32:1634–1636. doi: 10.1016/s0022-3468(97)90471-1. [DOI] [PubMed] [Google Scholar]
- Heling KS, Wauer RR, Hammer H, Bollmann R, Chaoui R. Reliability of the lung-to-head ratio in predicting outcome and neonatal ventilation parameters in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 2005;25:112–118. doi: 10.1002/uog.1837. [DOI] [PubMed] [Google Scholar]
- Tsukimori K, Masumoto K, Morokuma S, Yoshimura T, Taguchi T, Hara T, Sakaguchi Y, Takahashi S, Wake N, Suita S. The lung-to-thorax transverse area ratio at term and near term correlates with survival in isolated congenital diaphragmatic hernia. J Ultrasound Med. 2008;27:707–713. doi: 10.7863/jum.2008.27.5.707. [DOI] [PubMed] [Google Scholar]
- Mahieu-Caputo D, Sonigo P, Dommergues M, Fournet JC, Thalabard JC, Abarca C, Benachi A, Brunelle F, Dumez Y. Fetal lung volume measurement by magnetic resonance imaging in congenital diaphragmatic hernia. BJOG. 2001;108:863–868. doi: 10.1111/j.1471-0528.2001.00184.x. [DOI] [PubMed] [Google Scholar]
- Gorincour G, Bouvenot J, Mourot MG, Sonigo P, Chaumoitre K, Garel C, Guibaud L, Rypens F, Avni F, Cassart M. et al. Prenatal prognosis of congenital diaphragmatic hernia using magnetic resonance imaging measurement of fetal lung volume. Ultrasound Obstet Gynecol. 2005;26:738–744. doi: 10.1002/uog.2618. [DOI] [PubMed] [Google Scholar]
- Jani J, Breysem L, Maes F, Boulvain M, Roubliova X, Lewi L, Vaast P, Biard JM, Cannie M, Deprest J. Accuracy of magnetic resonance imaging for measuring fetal sheep lungs and other organs. Ultrasound Obstet Gynecol. 2005;25:270–276. doi: 10.1002/uog.1866. [DOI] [PubMed] [Google Scholar]
- Cannie M, Jani JC, De Keyzer F, Devlieger R, Van Schoubroeck D, Witters I, Marchal G, Dymarkowski S, Deprest JA. Fetal body volume: use at MR imaging to quantify relative lung volume in fetuses suspected of having pulmonary hypoplasia. Radiology. 2006;241:847–853. doi: 10.1148/radiol.2413051228. [DOI] [PubMed] [Google Scholar]
- Kilian AK, Schaible T, Hofmann V, Brade J, Neff KW, Busing KA. Congenital diaphragmatic hernia: predictive value of MRI relative lung-to-head ratio compared with MRI fetal lung volume and sonographic lung-to-head ratio. Am J Roentgenol. 2009;192:153–158. doi: 10.2214/AJR.08.1082. [DOI] [PubMed] [Google Scholar]
- Done E, Gucciardo L, Van Mieghem T, Jani J, Cannie M, Van Schoubroeck D, Devlieger R, Catte LD, Klaritsch P, Mayer S. et al. Prenatal diagnosis, prediction of outcome and in utero therapy of isolated congenital diaphragmatic hernia. Prenat Diagn. 2008;28:581–591. doi: 10.1002/pd.2033. [DOI] [PubMed] [Google Scholar]
- Albanese CT, Lopoo J, Goldstein RB, Filly RA, Feldstein VA, Calen PW, Jennings RW, Farrell JA, Harrison MR. Fetal liver position and perinatal outcome for congenital diaphragmatic hernia. Prenat Diagn. 1998;18:1138–1142. doi: 10.1002/(sici)1097-0223(199811)18:11<1138::aid-pd416>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Deprest J, Jani J, Gratacos E, Vandecruys H, Naulaers G, Delgado J, Greenough A, Nicolaides K. Fetal intervention for congenital diaphragmatic hernia: the European experience. Semin Perinatol. 2005;29:94–103. doi: 10.1053/j.semperi.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Hedrick HL, Danzer E, Merchant A, Bebbington MW, Zhao H, Flake AW, Johnson MP, Liechty KW, Howell LJ, Wilson RD, Adzick NS. Liver position and lung-to-head ratio for prediction of extracorporeal membrane oxygenation and survival in isolated left congenital diaphragmatic hernia. Am J Obstet Gynecol. 2007;197:422. doi: 10.1016/j.ajog.2007.07.001. e421-424. [DOI] [PubMed] [Google Scholar]
- Goodfellow T, Hyde I, Burge DM, Freeman NV. Congenital diaphragmatic hernia: the prognostic significance of the site of the stomach. Br J Radiol. 1987;60:993–995. doi: 10.1259/0007-1285-60-718-993. [DOI] [PubMed] [Google Scholar]
- Laudy JA, Van Gucht M, Van Dooren MF, Wladimiroff JW, Tibboel D. Congenital diaphragmatic hernia: an evaluation of the prognostic value of the lung-to-head ratio and other prenatal parameters. Prenat Diagn. 2003;23:634–639. doi: 10.1002/pd.654. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Hayashi S, Miura Y, Tsukamoto K, Kosaki R, Itoh Y, Sago H. Trisomy 9 mosaicism diagnosed in utero. Obstet Gynecol Int. 2010. [DOI] [PMC free article] [PubMed]
- Bohn D, Tamura M, Perrin D, Barker G, Rabinovitch M. Ventilatory predictors of pulmonary hypoplasia in congenital diaphragmatic hernia, confirmed by morphologic assessment. J Pediatr. 1987;111:423–431. doi: 10.1016/s0022-3476(87)80474-2. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Lund DP, Lillehei CW, Vacanti JP. Congenital diaphragmatic hernia: predictors of severity in the ECMO era. J Pediatr Surg. 1991;26:1028–1033. doi: 10.1016/0022-3468(91)90667-i. [DOI] [PubMed] [Google Scholar]
- Haugen SE, Linker D, Eik-Nes S, Kufaas T, Vik T, Eggen BM, Brubakk AM. Congenital diaphragmatic hernia: determination of the optimal time for operation by echocardiographic monitoring of the pulmonary arterial pressure. J Pediatr Surg. 1991;26:560–562. doi: 10.1016/0022-3468(91)90707-z. [DOI] [PubMed] [Google Scholar]
- Tanabe M, Yoshida H, Iwai J, Takahashi H, Ohnuma N, Terai M. Doppler flow patterns through the ductus arteriosus in patients with congenital diaphragmatic hernia. Eur J Pediatr Surg. 2000;10:92–95. doi: 10.1055/s-2008-1072333. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Kohno S, Hasegawa S, Urushihara N, Yoshida A, Kawano S, Saito A, Tanaka Y. Congenital diaphragmatic hernia: efficacy of ultrasound examination in its management. Pediatr Surg Int. 2003;19:176–179. doi: 10.1007/s00383-002-0913-1. [DOI] [PubMed] [Google Scholar]
- Suda K, Bigras JL, Bohn D, Hornberger LK, McCrindle BW. Echocardiographic predictors of outcome in newborns with congenital diaphragmatic hernia. Pediatrics. 2000;105:1106–1109. doi: 10.1542/peds.105.5.1106. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Oishi Y, Ito N, Nanba Y, Tsukamoto K, Nakamura T, Ito Y, Hayashi S, Sago H, Kuroda T, Honna T. Evaluating mortality and disease severity in congenital diaphragmatic hernia using the McGoon and pulmonary artery indices. J Pediatr Surg. 2009;44:2101–2106. doi: 10.1016/j.jpedsurg.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Ikawa H, Fukumoto H, Masuyama H, Konuma K, Kohno M, Nakamura T, Takahashi H. Patent ductus arteriosus flow patterns in the treatment of congenital diaphragmatic hernia. Pediatr Int. 2009;51:555–558. doi: 10.1111/j.1442-200X.2009.02808.x. [DOI] [PubMed] [Google Scholar]
- Garne E, Haeusler M, Barisic I, Gjergja R, Stoll C, Clementi M. Congenital diaphragmatic hernia: evaluation of prenatal diagnosis in 20 European regions. Ultrasound Obstet Gynecol. 2002;19:329–333. doi: 10.1046/j.1469-0705.2002.00635.x. [DOI] [PubMed] [Google Scholar]
- Colvin J, Bower C, Dickinson JE, Sokol J. Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia. Pediatrics. 2005;116:e356–363. doi: 10.1542/peds.2004-2845. [DOI] [PubMed] [Google Scholar]
- Richmond S, Atkins J. A population-based study of the prenatal diagnosis of congenital malformation over 16 years. BJOG. 2005;112:1349–1357. doi: 10.1111/j.1471-0528.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- Adzick NS, Outwater KM, Harrison MR, Davies P, Glick PL, deLorimier AA, Reid LM. Correction of congenital diaphragmatic hernia in utero. IV. An early gestational fetal lamb model for pulmonary vascular morphometric analysis. J Pediatr Surg. 1985;20:673–680. doi: 10.1016/s0022-3468(85)80022-1. [DOI] [PubMed] [Google Scholar]
- Harrison MR, Langer JC, Adzick NS, Golbus MS, Filly RA, Anderson RL, Rosen MA, Callen PW, Goldstein RB, deLorimier AA. Correction of congenital diaphragmatic hernia in utero, V. Initial clinical experience. J Pediatr Surg. 1990;25:47–55. doi: 10.1016/s0022-3468(05)80163-0. [DOI] [PubMed] [Google Scholar]
- Harrison MR, Adzick NS. The fetus as a patient. Surgical considerations. Ann Surg. 1991;213:279–291. doi: 10.1097/00000658-199104000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MR, Adzick NS, Bullard KM, Farrell JA, Howell LJ, Rosen MA, Sola A, Goldberg JD, Filly RA. Correction of congenital diaphragmatic hernia in utero VII: a prospective trial. J Pediatr Surg. 1997;32:1637–1642. doi: 10.1016/s0022-3468(97)90472-3. [DOI] [PubMed] [Google Scholar]
- Harrison MR, Adzick NS, Flake AW, VanderWall KJ, Bealer JF, Howell LJ, Farrell JA, Filly RA, Rosen MA, Sola A, Goldberg JD. Correction of congenital diaphragmatic hernia in utero VIII: Response of the hypoplastic lung to tracheal occlusion. J Pediatr Surg. 1996;31:1339–1348. doi: 10.1016/s0022-3468(96)90824-6. [DOI] [PubMed] [Google Scholar]
- Skarsgard ED, Meuli M, VanderWall KJ, Bealer JF, Adzick NS, Harrison MR. Fetal endoscopic tracheal occlusion ('Fetendo-PLUG') for congenital diaphragmatic hernia. J Pediatr Surg. 1996;31:1335–1338. doi: 10.1016/s0022-3468(96)90823-4. [DOI] [PubMed] [Google Scholar]
- VanderWall KJ, Bruch SW, Meuli M, Kohl T, Szabo Z, Adzick NS, Harrison MR. Fetal endoscopic ('Fetendo') tracheal clip. J Pediatr Surg. 1996;31:1101–1103. doi: 10.1016/s0022-3468(96)90096-2. [DOI] [PubMed] [Google Scholar]
- VanderWall KJ, Skarsgard ED, Filly RA, Eckert J, Harrison MR. Fetendo-clip: a fetal endoscopic tracheal clip procedure in a human fetus. J Pediatr Surg. 1997;32:970–972. doi: 10.1016/s0022-3468(97)90379-1. [DOI] [PubMed] [Google Scholar]
- Harrison MR, Mychaliska GB, Albanese CT, Jennings RW, Farrell JA, Hawgood S, Sandberg P, Levine AH, Lobo E, Filly RA. Correction of congenital diaphragmatic hernia in utero IX: fetuses with poor prognosis (liver herniation and low lung-to-head ratio) can be saved by fetoscopic temporary tracheal occlusion. J Pediatr Surg. 1998;33:1017–1022. doi: 10.1016/s0022-3468(98)90524-3. [DOI] [PubMed] [Google Scholar]
- Harrison MR, Keller RL, Hawgood SB, Kitterman JA, Sandberg PL, Farmer DL, Lee H, Filly RA, Farrell JA, Albanese CT. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med. 2003;349:1916–1924. doi: 10.1056/NEJMoa035005. [DOI] [PubMed] [Google Scholar]
- Flageole H, Evrard VA, Piedboeuf B, Laberge JM, Lerut TE, Deprest JA. The plug-unplug sequence: an important step to achieve type II pneumocyte maturation in the fetal lamb model. J Pediatr Surg. 1998;33:299–303. doi: 10.1016/s0022-3468(98)90451-1. [DOI] [PubMed] [Google Scholar]
- Cannie MM, Jani JC, De Keyzer F, Allegaert K, Dymarkowski S, Deprest J. Evidence and patterns in lung response after fetal tracheal occlusion: clinical controlled study. Radiology. 2009;252:526–533. doi: 10.1148/radiol.2522081955. [DOI] [PubMed] [Google Scholar]
- Suen HC, Catlin EA, Ryan DP, Wain JC, Donahoe PK. Biochemical immaturity of lungs in congenital diaphragmatic hernia. J Pediatr Surg. 1993;28:471–475. doi: 10.1016/0022-3468(93)90250-o. [DOI] [PubMed] [Google Scholar]
- Suen HC, Bloch KD, Donahoe PK. Antenatal glucocorticoid corrects pulmonary immaturity in experimentally induced congenital diaphragmatic hernia in rats. Pediatr Res. 1994;35:523–529. doi: 10.1203/00006450-199405000-00002. [DOI] [PubMed] [Google Scholar]
- Losty PD, Suen HC, Manganaro TF, Donahoe PK, Schnitzer JJ. Prenatal hormonal therapy improves pulmonary compliance in the nitrofen-induced CDH rat model. J Pediatr Surg. 1995;30:420–426. doi: 10.1016/0022-3468(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Lally KP, Bagolan P, Hosie S, Lally PA, Stewart M, Cotten CM, Van Meurs KP, Alexander G. Corticosteroids for fetuses with congenital diaphragmatic hernia: can we show benefit? J Pediatr Surg. 2006;41:668–674. doi: 10.1016/j.jpedsurg.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Logan JW, Rice HE, Goldberg RN, Cotten CM. Congenital diaphragmatic hernia: a systematic review and summary of best-evidence practice strategies. J Perinatol. 2007;27:535–549. doi: 10.1038/sj.jp.7211794. [DOI] [PubMed] [Google Scholar]
- Keijzer R, van Tuyl M, Tibboel D. Hormonal modulation of fetal pulmonary development: relevance for the fetus with diaphragmatic hernia. Eur J Obstet Gynecol Reprod Biol. 2000;92:127–133. doi: 10.1016/s0301-2115(00)00436-x. [DOI] [PubMed] [Google Scholar]
- Shaw KS, Filiatrault D, Yazbeck S, St-Vil D. Improved survival for congenital diaphragmatic hernia, based on prenatal ultrasound diagnosis and referral to a combined obstetric-pediatric surgical center. J Pediatr Surg. 1994;29:1268–1269. doi: 10.1016/0022-3468(94)90821-4. [DOI] [PubMed] [Google Scholar]
- Stevens TP, van Wijngaarden E, Ackerman KG, Lally PA, Lally KP. Timing of delivery and survival rates for infants with prenatal diagnoses of congenital diaphragmatic hernia. Pediatrics. 2009;123:494–502. doi: 10.1542/peds.2008-0528. [DOI] [PubMed] [Google Scholar]
- Betremieux P, Gaillot T, de la Pintiere A, Beuchee A, Pasquier L, Habonimana E, Le Bouar G, Branger B, Milon J, Fremond B. et al. Congenital diaphragmatic hernia: prenatal diagnosis permits immediate intensive care with high survival rate in isolated cases. A population-based study. Prenat Diagn. 2004;24:487–493. doi: 10.1002/pd.909. [DOI] [PubMed] [Google Scholar]
- Frenckner BP, Lally PA, Hintz SR, Lally KP. Prenatal diagnosis of congenital diaphragmatic hernia: how should the babies be delivered? J Pediatr Surg. 2007;42:1533–1538. doi: 10.1016/j.jpedsurg.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Safavi A, Lin Y, Skarsgard ED. Perinatal management of congenital diaphragmatic hernia: when and how should babies be delivered? Results from the Canadian Pediatric Surgery Network. J Pediatr Surg. 2010;45:2334–2339. doi: 10.1016/j.jpedsurg.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Glick PL, Leach CL, Besner GE, Egan EA, Morin FC, Malanowska-Kantoch A, Robinson LK, Brody A, Lele AS, McDonnell M. et al. Pathophysiology of congenital diaphragmatic hernia. III: Exogenous surfactant therapy for the high-risk neonate with CDH. J Pediatr Surg. 1992;27:866–869. doi: 10.1016/0022-3468(92)90386-l. [DOI] [PubMed] [Google Scholar]
- Bae CW, Jang CK, Chung SJ, Choi YM, Oh SM, Lee TS, Shin OY. Exogenous pulmonary surfactant replacement therapy in a neonate with pulmonary hypoplasia accompanying congenital diaphragmatic hernia--a case report. J Korean Med Sci. 1996;11:265–270. doi: 10.3346/jkms.1996.11.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CE, Lally KP, Hintz SR, Lally PA, Tibboel D, Moya FR, VanMeurs KP. Surfactant replacement therapy on ECMO does not improve outcome in neonates with congenital diaphragmatic hernia. J Pediatr Surg. 2004;39:1632–1637. doi: 10.1016/j.jpedsurg.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Van Meurs K. Is surfactant therapy beneficial in the treatment of the term newborn infant with congenital diaphragmatic hernia? J Pediatr. 2004;145:312–316. doi: 10.1016/j.jpeds.2004.04.056. [DOI] [PubMed] [Google Scholar]
- Mohseni-Bod H, Bohn D. Pulmonary hypertension in congenital diaphragmatic hernia. Semin Pediatr Surg. 2007;16:126–133. doi: 10.1053/j.sempedsurg.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Azarow K, Cutz E, Messineo A, Pearl R, Bohn D. Pulmonary barotrauma in congenital diaphragmatic hernia: a clinicopathological correlation. J Pediatr Surg. 1999;34:1813–1817. doi: 10.1016/s0022-3468(99)90319-6. [DOI] [PubMed] [Google Scholar]
- Langham MR Jr, Kays DW, Beierle EA, Chen MK, Mullet TC, Rieger K, Wood CE, Talbert JL. Twenty years of progress in congenital diaphragmatic hernia at the University of Florida. Am Surg. 2003;69:45–52. [PubMed] [Google Scholar]
- Wung JT, Sahni R, Moffitt ST, Lipsitz E, Stolar CJ. Congenital diaphragmatic hernia: survival treated with very delayed surgery, spontaneous respiration, and no chest tube. J Pediatr Surg. 1995;30:406–409. doi: 10.1016/0022-3468(95)90042-x. [DOI] [PubMed] [Google Scholar]
- Boloker J, Bateman DA, Wung JT, Stolar CJ. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg. 2002;37:357–366. doi: 10.1053/jpsu.2002.30834. [DOI] [PubMed] [Google Scholar]
- Frenckner B, Ehren H, Granholm T, Linden V, Palmer K. Improved results in patients who have congenital diaphragmatic hernia using preoperative stabilization, extracorporeal membrane oxygenation, and delayed surgery. J Pediatr Surg. 1997;32:1185–1189. doi: 10.1016/s0022-3468(97)90679-5. [DOI] [PubMed] [Google Scholar]
- Bagolan P, Casaccia G, Crescenzi F, Nahom A, Trucchi A, Giorlandino C. Impact of a current treatment protocol on outcome of high-risk congenital diaphragmatic hernia. J Pediatr Surg. 2004;39:313–318. doi: 10.1016/j.jpedsurg.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Waag KL, Loff S, Zahn K, Ali M, Hien S, Kratz M, Neff W, Schaffelder R, Schaible T. Congenital diaphragmatic hernia: a modern day approach. Semin Pediatr Surg. 2008;17:244–254. doi: 10.1053/j.sempedsurg.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Karl SR, Ballantine TV, Snider MT. High-frequency ventilation at rates of 375 to 1800 cycles per minute in four neonates with congenital diaphragmatic hernia. J Pediatr Surg. 1983;18:822–828. doi: 10.1016/s0022-3468(83)80030-x. [DOI] [PubMed] [Google Scholar]
- Tamura M, Tsuchida Y, Kawano T, Honna T, Ishibashi R, Iwanaka T, Morita Y, Hashimoto H, Tada H, Miyasaka K. Piston-pump-type high frequency oscillatory ventilation for neonates with congenital diaphragmatic hernia: a new protocol. J Pediatr Surg. 1988;23:478–482. doi: 10.1016/s0022-3468(88)80453-6. [DOI] [PubMed] [Google Scholar]
- Fujino Y, Takezawa J, Nishimura M, Imanaka H, Taenaka N, Yoshiya I. High-frequency oscillation for persistent fetal circulation after repair of congenital diaphragmatic hernia. Crit Care Med. 1989;17:376–377. doi: 10.1097/00003246-198904000-00016. [DOI] [PubMed] [Google Scholar]
- Schnitzer JJ, Thompson JE, Hedrick HL. A new ventilator improves CO2 removal in newborn lambs with congenital diaphragmatic hernia. Crit Care Med. 1999;27:109–112. doi: 10.1097/00003246-199901000-00037. [DOI] [PubMed] [Google Scholar]
- Reiss I, Schaible T, van den Hout L, Capolupo I, Allegaert K, van Heijst A, Gorett Silva M, Greenough A, Tibboel D. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium consensus. Neonatology. 2010;98:354–364. doi: 10.1159/000320622. [DOI] [PubMed] [Google Scholar]
- Collins DL, Pomerance JJ, Travis KW, Turner SW, Pappelbaum SJ. A new approach to congenital posterolateral diaphragmatic hernia. J Pediatr Surg. 1977;12:149–156. doi: 10.1016/s0022-3468(77)80001-8. [DOI] [PubMed] [Google Scholar]
- Sumner E, Frank JD. Tolazoline in the treatment of congenital diaphragmatic hernias. Arch Dis Child. 1981;56:350–353. doi: 10.1136/adc.56.5.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos AP, Tibboel D, Koot VC, Hazebroek FW, Molenaar JC. Persistent pulmonary hypertension in high-risk congenital diaphragmatic hernia patients: incidence and vasodilator therapy. J Pediatr Surg. 1993;28:1463–1465. doi: 10.1016/0022-3468(93)90431-j. [DOI] [PubMed] [Google Scholar]
- Weinberger B, Weiss K, Heck DE, Laskin DL, Laskin JD. Pharmacologic therapy of persistent pulmonary hypertension of the newborn. Pharmacol Ther. 2001;89:67–79. doi: 10.1016/s0163-7258(00)00104-2. [DOI] [PubMed] [Google Scholar]
- Buss M, Williams G, Dilley A, Jones O. Prevention of heart failure in the management of congenital diaphragmatic hernia by maintaining ductal patency. A case report. J Pediatr Surg. 2006;41:e9–11. doi: 10.1016/j.jpedsurg.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Finer NN, Etches PC, Kamstra B, Tierney AJ, Peliowski A, Ryan CA. Inhaled nitric oxide in infants referred for extracorporeal membrane oxygenation: dose response. J Pediatr. 1994;124:302–308. doi: 10.1016/s0022-3476(94)70324-8. [DOI] [PubMed] [Google Scholar]
- Karamanoukian HL, Glick PL, Zayek M, Steinhorn RH, Zwass MS, Fineman JR, Morin FC. Inhaled nitric oxide in congenital hypoplasia of the lungs due to diaphragmatic hernia or oligohydramnios. Pediatrics. 1994;94:715–718. [PubMed] [Google Scholar]
- Shah N, Jacob T, Exler R, Morrow S, Ford H, Albanese C, Wiener E, Rowe M, Billiar T, Simmons R. et al. Inhaled nitric oxide in congenital diaphragmatic hernia. J Pediatr Surg. 1994;29:1010–1014. doi: 10.1016/0022-3468(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Kinsella JP, Parker TA, Ivy DD, Abman SH. Noninvasive delivery of inhaled nitric oxide therapy for late pulmonary hypertension in newborn infants with congenital diaphragmatic hernia. J Pediatr. 2003;142:397–401. doi: 10.1067/mpd.2003.140. [DOI] [PubMed] [Google Scholar]
- Lally KP. Congenital diaphragmatic hernia. Curr Opin Pediatr. 2002;14:486–490. doi: 10.1097/00008480-200208000-00022. [DOI] [PubMed] [Google Scholar]
- Hunter L, Richens T, Davis C, Walker G, Simpson JH. Sildenafil use in congenital diaphragmatic hernia. Arch Dis Child Fetal Neonatal Ed. 2009;94:F467. doi: 10.1136/adc.2008.153684. [DOI] [PubMed] [Google Scholar]
- Masumoto K, Teshiba R, Esumi G, Nagata K, Takahata Y, Hikino S, Hara T, Hojo S, Tsukimori K, Wake N. et al. Improvement in the outcome of patients with antenatally diagnosed congenital diaphragmatic hernia using gentle ventilation and circulatory stabilization. Pediatr Surg Int. 2009;25:487–492. doi: 10.1007/s00383-009-2370-6. [DOI] [PubMed] [Google Scholar]
- Bartlett RH, Gazzaniga AB, Toomasian J, Coran AG, Roloff D, Rucker R. Extracorporeal membrane oxygenation (ECMO) in neonatal respiratory failure. 100 cases. Ann Surg. 1986;204:236–245. doi: 10.1097/00000658-198609000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmitt RA, Moss RL, Rhine WD, Benitz WE, Henry MC, Vanmeurs KP. Venoarterial versus venovenous extracorporeal membrane oxygenation in congenital diaphragmatic hernia: the Extracorporeal Life Support Organization Registry, 1990-1999. J Pediatr Surg. 2001;36:1199–1204. doi: 10.1053/jpsu.2001.25762. [DOI] [PubMed] [Google Scholar]
- Guner YS, Khemani RG, Qureshi FG, Wee CP, Austin MT, Dorey F, Rycus PT, Ford HR, Friedlich P, Stein JE. Outcome analysis of neonates with congenital diaphragmatic hernia treated with venovenous vs venoarterial extracorporeal membrane oxygenation. J Pediatr Surg. 2009;44:1691–1701. doi: 10.1016/j.jpedsurg.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Keckler SJ, Laituri CA, Ostlie DJ, St Peter SD. A review of venovenous and venoarterial extracorporeal membrane oxygenation in neonates and children. Eur J Pediatr Surg. 2009;20:1–4. doi: 10.1055/s-0029-1231053. [DOI] [PubMed] [Google Scholar]
- Azarow K, Messineo A, Pearl R, Filler R, Barker G, Bohn D. Congenital diaphragmatic hernia--a tale of two cities: the Toronto experience. J Pediatr Surg. 1997;32:395–400. doi: 10.1016/s0022-3468(97)90589-3. [DOI] [PubMed] [Google Scholar]
- Morini F, Goldman A, Pierro A. Extracorporeal membrane oxygenation in infants with congenital diaphragmatic hernia: a systematic review of the evidence. Eur J Pediatr Surg. 2006;16:385–391. doi: 10.1055/s-2006-924751. [DOI] [PubMed] [Google Scholar]
- Stolar CJ, Snedecor SM, Bartlett RH. Extracorporeal membrane oxygenation and neonatal respiratory failure: experience from the extracorporeal life support organization. J Pediatr Surg. 1991;26:563–571. doi: 10.1016/0022-3468(91)90708-2. [DOI] [PubMed] [Google Scholar]
- Payne NR, Kriesmer P, Mammel M, Meyer CL. Comparison of six ECMO selection criteria and analysis of factors influencing their accuracy. Pediatr Pulmonol. 1991;11:223–232. doi: 10.1002/ppul.1950110308. [DOI] [PubMed] [Google Scholar]
- Karimova A, Brown K, Ridout D, Beierlein W, Cassidy J, Smith J, Pandya H, Firmin R, Liddell M, Davis C, Goldman A. Neonatal extracorporeal membrane oxygenation: practice patterns and predictors of outcome in the UK. Arch Dis Child Fetal Neonatal Ed. 2009;94:F129–132. doi: 10.1136/adc.2008.141051. [DOI] [PubMed] [Google Scholar]
- Mugford M, Elbourne D, Field D. Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants. Cochrane Database Syst Rev. 2008. p. CD001340. [DOI] [PubMed]
- UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. UK Collaborative ECMO Trail Group. Lancet. 1996;348:75–82. [PubMed] [Google Scholar]
- Moyer V, Moya F, Tibboel R, Losty P, Nagaya M, Lally KP. Late versus early surgical correction for congenital diaphragmatic hernia in newborn infants. Cochrane Database Syst Rev. 2002. p. CD001695. [DOI] [PubMed]
- Liem NT, Dien TM, Ung NQ. Thoracoscopic repair in the neonatal intensive care unit for congenital diaphragmatic hernia during high-frequency oscillatory ventilation. J Laparoendosc Adv Surg Tech A. 2009;20:111–114. doi: 10.1089/lap.2008.0412. [DOI] [PubMed] [Google Scholar]
- Gavilanes AW, Heineman E, Herpers MJ, Blanco CE. Use of neonatal intensive care unit as a safe place for neonatal surgery. Arch Dis Child Fetal Neonatal Ed. 1997;76:F51–53. doi: 10.1136/fn.76.1.f51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago P, Meneghini L, Chiandetti L, Tormena F, Metrangolo S, Gamba P. Congenital diaphragmatic hernia: intensive care unit or operating room? Am J Perinatol. 2005;22:189–197. doi: 10.1055/s-2005-866602. [DOI] [PubMed] [Google Scholar]
- Tyson KR, Schwartz MZ, Marr CC. "Balanced" thoracic drainage is the method of choice to control intrathoracic pressure following repair of diaphragmatic hernia. J Pediatr Surg. 1985;20:415–417. doi: 10.1016/s0022-3468(85)80231-1. [DOI] [PubMed] [Google Scholar]
- Migliazza L, Bellan C, Alberti D, Auriemma A, Burgio G, Locatelli G, Colombo A. Retrospective study of 111 cases of congenital diaphragmatic hernia treated with early high-frequency oscillatory ventilation and presurgical stabilization. J Pediatr Surg. 2007;42:1526–1532. doi: 10.1016/j.jpedsurg.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Grethel EJ, Cortes RA, Wagner AJ, Clifton MS, Lee H, Farmer DL, Harrison MR, Keller RL, Nobuhara KK. Prosthetic patches for congenital diaphragmatic hernia repair: Surgisis vs Gore-Tex. J Pediatr Surg. 2006;41:29–33. doi: 10.1016/j.jpedsurg.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Fei L, Saviano C, Moccia F, del Genio G, Trapani V, Nunziale A, Lombardi G, Cecchi M. ePTFE soft tissue patch reconstruction of hemidiaphragmatic agenesis with late clinical presentation. Hernia. 2008;12:103–106. doi: 10.1007/s10029-007-0254-z. [DOI] [PubMed] [Google Scholar]
- Loff S, Wirth H, Jester I, Hosie S, Wollmann C, Schaible T, Ataman O, Waag KL. Implantation of a cone-shaped double-fixed patch increases abdominal space and prevents recurrence of large defects in congenital diaphragmatic hernia. J Pediatr Surg. 2005;40:1701–1705. doi: 10.1016/j.jpedsurg.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Atkinson JB, Poon MW. ECMO and the management of congenital diaphragmatic hernia with large diaphragmatic defects requiring a prosthetic patch. J Pediatr Surg. 1992;27:754–756. doi: 10.1016/s0022-3468(05)80109-5. [DOI] [PubMed] [Google Scholar]
- Van Meurs KP, Robbins ST, Reed VL, Karr SS, Wagner AE, Glass P, Anderson KD, Short BL. Congenital diaphragmatic hernia: long-term outcome in neonates treated with extracorporeal membrane oxygenation. J Pediatr. 1993;122:893–899. doi: 10.1016/s0022-3476(09)90013-0. [DOI] [PubMed] [Google Scholar]
- Moss RL, Chen CM, Harrison MR. Prosthetic patch durability in congenital diaphragmatic hernia: a long-term follow-up study. J Pediatr Surg. 2001;36:152–154. doi: 10.1053/jpsu.2001.20037. [DOI] [PubMed] [Google Scholar]
- Laituri CA, Garey CL, Valusek PA, Fike FB, Kaye AJ, Ostlie DJ, Snyder CL, St Peter SD. Outcome of congenital diaphragmatic hernia repair depending on patch type. Eur J Pediatr Surg. 2010;20:363–365. doi: 10.1055/s-0030-1261939. [DOI] [PubMed] [Google Scholar]
- Nasr A, Struijs MC, Ein SH, Langer JC, Chiu PP. Outcomes after muscle flap vs prosthetic patch repair for large congenital diaphragmatic hernias. J Pediatr Surg. 2010;45:151–154. doi: 10.1016/j.jpedsurg.2009.10.024. [DOI] [PubMed] [Google Scholar]
- Scaife ER, Johnson DG, Meyers RL, Johnson SM, Matlak ME. The split abdominal wall muscle flap--a simple, mesh-free approach to repair large diaphragmatic hernia. J Pediatr Surg. 2003;38:1748–1751. doi: 10.1016/j.jpedsurg.2003.08.045. [DOI] [PubMed] [Google Scholar]
- Sydorak RM, Hoffman W, Lee H, Yingling CD, Longaker M, Chang J, Smith B, Harrison MR, Albanese CT. Reversed latissimus dorsi muscle flap for repair of recurrent congenital diaphragmatic hernia. J Pediatr Surg. 2003;38:296–300. doi: 10.1053/jpsu.2003.50097. [DOI] [PubMed] [Google Scholar]
- Joshi SB, Sen S, Chacko J, Thomas G, Karl S. Abdominal muscle flap repair for large defects of the diaphragm. Pediatr Surg Int. 2005;21:677–680. doi: 10.1007/s00383-005-1438-1. [DOI] [PubMed] [Google Scholar]
- Hosgor M, Karaca I, Karkiner A, Ucan B, Temir G, Erdag G, Fescekoglu O. Associated malformations in delayed presentation of congenital diaphragmatic hernia. J Pediatr Surg. 2004;39:1073–1076. doi: 10.1016/j.jpedsurg.2004.03.050. [DOI] [PubMed] [Google Scholar]
- Imamoglu M, Cay A, Kosucu P, Ozdemir O, Orhan F, Sapan L, Sarihan H. Congenital paraesophageal hiatal hernia: pitfalls in the diagnosis and treatment. J Pediatr Surg. 2005;40:1128–1133. doi: 10.1016/j.jpedsurg.2005.03.060. [DOI] [PubMed] [Google Scholar]
- Schnitzer JJ, Kikiros CS, Short BL, O'Brien A, Anderson KD, Newman KD. Experience with abdominal wall closure for patients with congenital diaphragmatic hernia repaired on ECMO. J Pediatr Surg. 1995;30:19–22. doi: 10.1016/0022-3468(95)90600-2. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Yoshioka Y, Sasaki T, Iwasaki Y, Tazuke Y, Sumimura J, Ban H, Dezawa T. Abdominal wall plasty for a premature infant with congenital diaphragmatic hernia. Pediatr Surg Int. 1998;13:48–51. doi: 10.1007/s003830050242. [DOI] [PubMed] [Google Scholar]
- Kyzer S, Sirota L, Chaimoff C. Abdominal wall closure with a silastic patch after repair of congenital diaphragmatic hernia. Arch Surg. 2004;139:296–298. doi: 10.1001/archsurg.139.3.296. [DOI] [PubMed] [Google Scholar]
- Nguyen TL, Le AD. Thoracoscopic repair for congenital diaphragmatic hernia: lessons from 45 cases. J Pediatr Surg. 2006;41:1713–1715. doi: 10.1016/j.jpedsurg.2006.05.043. [DOI] [PubMed] [Google Scholar]
- Becmeur F, Reinberg O, Dimitriu C, Moog R, Philippe P. Thoracoscopic repair of congenital diaphragmatic hernia in children. Semin Pediatr Surg. 2007;16:238–244. doi: 10.1053/j.sempedsurg.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Shalaby R, Gabr K, Al-Saied G, Ibrahem M, Shams AM, Dorgham A, Ismail M. Thoracoscopic repair of diaphragmatic hernia in neonates and children: a new simplified technique. Pediatr Surg Int. 2008;24:543–547. doi: 10.1007/s00383-008-2128-6. [DOI] [PubMed] [Google Scholar]
- Cho SD, Krishnaswami S, McKee JC, Zallen G, Silen ML, Bliss DW. Analysis of 29 consecutive thoracoscopic repairs of congenital diaphragmatic hernia in neonates compared to historical controls. J Pediatr Surg. 2009;44:80–86. doi: 10.1016/j.jpedsurg.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Gourlay DM, Cassidy LD, Sato TT, Lal DR, Arca MJ. Beyond feasibility: a comparison of newborns undergoing thoracoscopic and open repair of congenital diaphragmatic hernias. J Pediatr Surg. 2009;44:1702–1707. doi: 10.1016/j.jpedsurg.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Kim AC, Bryner BS, Akay B, Geiger JD, Hirschl RB, Mychaliska GB. Thoracoscopic repair of congenital diaphragmatic hernia in neonates: lessons learned. J Laparoendosc Adv Surg Tech A. 2009;19:575–580. doi: 10.1089/lap.2009.0129. [DOI] [PubMed] [Google Scholar]
- Keijzer R, van de Ven C, Vlot J, Sloots C, Madern G, Tibboel D, Bax K. Thoracoscopic repair in congenital diaphragmatic hernia: patching is safe and reduces the recurrence rate. J Pediatr Surg. 2010;45:953–957. doi: 10.1016/j.jpedsurg.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Meehan JJ, Sandler A. Robotic repair of a Bochdalek congenital diaphragmatic hernia in a small neonate: robotic advantages and limitations. J Pediatr Surg. 2007;42:1757–1760. doi: 10.1016/j.jpedsurg.2007.06.013. [DOI] [PubMed] [Google Scholar]
- McHoney M, Giacomello L, Nah SA, De Coppi P, Kiely EM, Curry JI, Drake DP, Eaton S, Pierro A. Thoracoscopic repair of congenital diaphragmatic hernia: intraoperative ventilation and recurrence. J Pediatr Surg. 2010;45:355–359. doi: 10.1016/j.jpedsurg.2009.10.072. [DOI] [PubMed] [Google Scholar]
- Lansdale N, Alam S, Losty PD, Jesudason EC. Neonatal endosurgical congenital diaphragmatic hernia repair: a systematic review and meta-analysis. Ann Surg. pp. 20–26. [DOI] [PubMed]
- van den Hout L, Reiss I, Felix JF, Hop WC, Lally PA, Lally KP, Tibboel D. Risk factors for chronic lung disease and mortality in newborns with congenital diaphragmatic hernia. Neonatology. 2010;98:370–380. doi: 10.1159/000316974. [DOI] [PubMed] [Google Scholar]
- Downard CD, Jaksic T, Garza JJ, Dzakovic A, Nemes L, Jennings RW, Wilson JM. Analysis of an improved survival rate for congenital diaphragmatic hernia. J Pediatr Surg. 2003;38:729–732. doi: 10.1016/jpsu.2003.50194. [DOI] [PubMed] [Google Scholar]
- Doyle NM, Lally KP. The CDH Study Group and advances in the clinical care of the patient with congenital diaphragmatic hernia. Semin Perinatol. 2004;28:174–184. doi: 10.1053/j.semperi.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Hedrick HL, Crombleholme TM, Flake AW, Nance ML, von Allmen D, Howell LJ, Johnson MP, Wilson RD, Adzick NS. Right congenital diaphragmatic hernia: Prenatal assessment and outcome. J Pediatr Surg. 2004;39:319–323. doi: 10.1016/j.jpedsurg.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Fisher JC, Jefferson RA, Arkovitz MS, Stolar CJ. Redefining outcomes in right congenital diaphragmatic hernia. J Pediatr Surg. 2008;43:373–379. doi: 10.1016/j.jpedsurg.2007.10.049. [DOI] [PubMed] [Google Scholar]
- Bryner BS, Kim AC, Khouri JS, Drongowski RA, Bruch SW, Hirschl RB, Mychaliska GB. Right-sided congenital diaphragmatic hernia: high utilization of extracorporeal membrane oxygenation and high survival. J Pediatr Surg. 2009;44:883–887. doi: 10.1016/j.jpedsurg.2009.01.037. [DOI] [PubMed] [Google Scholar]
- Kamiyama M, Usui N, Tani G, Soh H, Kamata S, Nose K, Kubota A, Fukuzawa M. Postoperative chylothorax in congenital diaphragmatic hernia. Eur J Pediatr Surg. 2010;20:391–394. doi: 10.1055/s-0030-1261956. [DOI] [PubMed] [Google Scholar]
- Zavala A, Campos JM, Riutort C, Skorin I, Godoy L, Faunes M, Kattan J. Chylothorax in congenital diaphragmatic hernia. Pediatr Surg Int. 2010;26:919–922. doi: 10.1007/s00383-010-2677-3. [DOI] [PubMed] [Google Scholar]
- Kavvadia V, Greenough A, Davenport M, Karani J, Nicolaides KH. Chylothorax after repair of congenital diaphragmatic hernia--risk factors and morbidity. J Pediatr Surg. 1998;33:500–502. doi: 10.1016/s0022-3468(98)90097-5. [DOI] [PubMed] [Google Scholar]
- Goyal A, Smith NP, Jesudason EC, Kerr S, Losty PD. Octreotide for treatment of chylothorax after repair of congenital diaphragmatic hernia. J Pediatr Surg. 2003;38:E19–20. doi: 10.1016/s0022-3468(03)00294-x. [DOI] [PubMed] [Google Scholar]
- Casaccia G, Crescenzi F, Palamides S, Catalano OA, Bagolan P. Pleural effusion requiring drainage in congenital diaphragmatic hernia: incidence, aetiology and treatment. Pediatr Surg Int. 2006;22:585–588. doi: 10.1007/s00383-006-1706-8. [DOI] [PubMed] [Google Scholar]
- Ijsselstijn H, Tibboel D, Hop WJ, Molenaar JC, de Jongste JC. Long-term pulmonary sequelae in children with congenital diaphragmatic hernia. Am J Respir Crit Care Med. 1997;155:174–180. doi: 10.1164/ajrccm.155.1.9001308. [DOI] [PubMed] [Google Scholar]
- Nakayama DK, Motoyama EK, Mutich RL, Koumbourlis AC. Pulmonary function in newborns after repair of congenital diaphragmatic hernia. Pediatr Pulmonol. 1991;11:49–55. doi: 10.1002/ppul.1950110109. [DOI] [PubMed] [Google Scholar]
- Vanamo K, Rintala R, Sovijarvi A, Jaaskelainen J, Turpeinen M, Lindahl H, Louhimo I. Long-term pulmonary sequelae in survivors of congenital diaphragmatic defects. J Pediatr Surg. 1996;31:1096–1099. doi: 10.1016/s0022-3468(96)90095-0. [DOI] [PubMed] [Google Scholar]
- Peetsold MG, Heij HA, Kneepkens CM, Nagelkerke AF, Huisman J, Gemke RJ. The long-term follow-up of patients with a congenital diaphragmatic hernia: a broad spectrum of morbidity. Pediatr Surg Int. 2009;25:1–17. doi: 10.1007/s00383-008-2257-y. [DOI] [PubMed] [Google Scholar]
- Muratore CS, Kharasch V, Lund DP, Sheils C, Friedman S, Brown C, Utter S, Jaksic T, Wilson JM. Pulmonary morbidity in 100 survivors of congenital diaphragmatic hernia monitored in a multidisciplinary clinic. J Pediatr Surg. 2001;36:133–140. doi: 10.1053/jpsu.2001.20031. [DOI] [PubMed] [Google Scholar]
- Lund DP, Mitchell J, Kharasch V, Quigley S, Kuehn M, Wilson JM. Congenital diaphragmatic hernia: the hidden morbidity. J Pediatr Surg. 1994;29:258–262. doi: 10.1016/0022-3468(94)90329-8. [DOI] [PubMed] [Google Scholar]
- Vanamo K, Peltonen J, Rintala R, Lindahl H, Jaaskelainen J, Louhimo I. Chest wall and spinal deformities in adults with congenital diaphragmatic defects. J Pediatr Surg. 1996;31:851–854. doi: 10.1016/s0022-3468(96)90152-9. [DOI] [PubMed] [Google Scholar]
- Trachsel D, Selvadurai H, Bohn D, Langer JC, Coates AL. Long-term pulmonary morbidity in survivors of congenital diaphragmatic hernia. Pediatr Pulmonol. 2005;39:433–439. doi: 10.1002/ppul.20193. [DOI] [PubMed] [Google Scholar]
- Qi B, Soto C, Diez-Pardo JA, Tovar JA. An experimental study on the pathogenesis of gastroesophageal reflux after repair of diaphragmatic hernia. J Pediatr Surg. 1997;32:1310–1313. doi: 10.1016/s0022-3468(97)90309-2. [DOI] [PubMed] [Google Scholar]
- Martinez L, Gonzalez-Reyes S, Burgos E, Tovar JA. The vagus and recurrent laryngeal nerves in experimental congenital diaphragmatic hernia. Pediatr Surg Int. 2004;20:253–257. doi: 10.1007/s00383-003-1121-3. [DOI] [PubMed] [Google Scholar]
- Stolar CJ, Berdon WE, Dillon PW, Reyes C, Abramson SJ, Amodio JB. Esophageal dilatation and reflux in neonates supported by ECMO after diaphragmatic hernia repair. AJR Am J Roentgenol. 1988;151:135–137. doi: 10.2214/ajr.151.1.135. [DOI] [PubMed] [Google Scholar]
- Stolar CJ, Levy JP, Dillon PW, Reyes C, Belamarich P, Berdon WE. Anatomic and functional abnormalities of the esophagus in infants surviving congenital diaphragmatic hernia. Am J Surg. 1990;159:204–207. doi: 10.1016/s0002-9610(05)80261-2. [DOI] [PubMed] [Google Scholar]
- Lally KP, Paranka MS, Roden J, Georgeson KE, Wilson JM, Lillehei CW, Breaux CW Jr, Poon M, Clark RH, Atkinson JB. Congenital diaphragmatic hernia. Stabilization and repair on ECMO. Ann Surg. 1992;216:569–573. doi: 10.1097/00000658-199211000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanamo K, Rintala RJ, Lindahl H, Louhimo I. Long-term gastrointestinal morbidity in patients with congenital diaphragmatic defects. J Pediatr Surg. 1996;31:551–554. doi: 10.1016/s0022-3468(96)90494-7. [DOI] [PubMed] [Google Scholar]
- Fasching G, Huber A, Uray E, Sorantin E, Lindbichler F, Mayr J. Gastroesophageal reflux and diaphragmatic motility after repair of congenital diaphragmatic hernia. Eur J Pediatr Surg. 2000;10:360–364. doi: 10.1055/s-2008-1072391. [DOI] [PubMed] [Google Scholar]
- Tovar JA, Luis AL, Encinas JL, Burgos L, Pederiva F, Martinez L, Olivares P. Pediatric surgeons and gastroesophageal reflux. J Pediatr Surg. 2007;42:277–283. doi: 10.1016/j.jpedsurg.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Dariel A, Roze JC, Piloquet H, Podevin G. Impact of prophylactic fundoplication on survival without growth disorder in left congenital diaphragmatic hernia requiring a patch repair. J Pediatr. 2010;157:688–690. doi: 10.1016/j.jpeds.2010.06.009. 690 e681. [DOI] [PubMed] [Google Scholar]
- D'Agostino JA, Bernbaum JC, Gerdes M, Schwartz IP, Coburn CE, Hirschl RB, Baumgart S, Polin RA. Outcome for infants with congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation: the first year. J Pediatr Surg. 1995;30:10–15. doi: 10.1016/0022-3468(95)90598-7. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Gangitano E, Odell RM, Doran R, Durand M. Survival, intracranial lesions, and neurodevelopmental outcome in infants with congenital diaphragmatic hernia treated with extracorporeal membrane oxygenation. J Perinatol. 1999;19:436–440. doi: 10.1038/sj.jp.7200242. [DOI] [PubMed] [Google Scholar]
- Rasheed A, Tindall S, Cueny DL, Klein MD, Delaney-Black V. Neurodevelopmental outcome after congenital diaphragmatic hernia: Extracorporeal membrane oxygenation before and after surgery. J Pediatr Surg. 2001;36:539–544. doi: 10.1053/jpsu.2001.22278. [DOI] [PubMed] [Google Scholar]
- Bagolan P, Morini F. Long-term follow up of infants with congenital diaphragmatic hernia. Semin Pediatr Surg. 2007;16:134–144. doi: 10.1053/j.sempedsurg.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Friedman S, Chen C, Chapman JS, Jeruss S, Terrin N, Tighiouart H, Parsons SK, Wilson JM. Neurodevelopmental outcomes of congenital diaphragmatic hernia survivors followed in a multidisciplinary clinic at ages 1 and 3. J Pediatr Surg. 2008;43:1035–1043. doi: 10.1016/j.jpedsurg.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Lally KP, Engle W. Postdischarge follow-up of infants with congenital diaphragmatic hernia. Pediatrics. 2008;121:627–632. doi: 10.1542/peds.2007-3282. [DOI] [PubMed] [Google Scholar]
- The collaborative UK ECMO (Extracorporeal Membrane Oxygenation) trial: follow-up to 1 year of age. Pediatrics. 1998;101:E1. doi: 10.1542/peds.101.4.e1. [DOI] [PubMed] [Google Scholar]
- Lasky RE, Wiorek L, Becker TR. Hearing loss in survivors of neonatal extracorporeal membrane oxygenation (ECMO) therapy and high-frequency oscillatory (HFO) therapy. J Am Acad Audiol. 1998;9:47–58. [PubMed] [Google Scholar]
- Kuga T, Taniguchi S, Inoue T, Zempo N, Esato K. Hearing loss in infants with congenital diaphragmatic hernia treated without extracorporeal membrane oxygenation: report of two cases. J Pediatr Surg. 2000;35:621–623. doi: 10.1053/jpsu.2000.0350621. [DOI] [PubMed] [Google Scholar]
- Robertson CM, Tyebkhan JM, Hagler ME, Cheung PY, Peliowski A, Etches PC. Late-onset, progressive sensorineural hearing loss after severe neonatal respiratory failure. Otol Neurotol. 2002;23:353–356. doi: 10.1097/00129492-200205000-00022. [DOI] [PubMed] [Google Scholar]
- Fligor BJ, Neault MW, Mullen CH, Feldman HA, Jones DT. Factors associated with sensorineural hearing loss among survivors of extracorporeal membrane oxygenation therapy. Pediatrics. 2005;115:1519–1528. doi: 10.1542/peds.2004-0247. [DOI] [PubMed] [Google Scholar]
- Masumoto K, Nagata K, Uesugi T, Yamada T, Taguchi T. Risk factors for sensorineural hearing loss in survivors with severe congenital diaphragmatic hernia. Eur J Pediatr. 2007;166:607–612. doi: 10.1007/s00431-006-0300-3. [DOI] [PubMed] [Google Scholar]
- Morini F, Capolupo I, Masi R, Ronchetti MP, Locatelli M, Corchia C, Bagolan P. Hearing impairment in congenital diaphragmatic hernia: the inaudible and noiseless foot of time. J Pediatr Surg. 2008;43:380–384. doi: 10.1016/j.jpedsurg.2007.10.048. [DOI] [PubMed] [Google Scholar]
- Javidnia H, Vaccani JP. Progressive sensorineural hearing loss in children with congenital diaphragmatic hernias. J Otolaryngol Head Neck Surg. 2009;38:29–31. [PubMed] [Google Scholar]
- Crane JP. Familial congenital diaphragmatic hernia: prenatal diagnostic approach and analysis of twelve families. Clin Genet. 1979;16:244–252. doi: 10.1111/j.1399-0004.1979.tb00996.x. [DOI] [PubMed] [Google Scholar]
- Wolff G. Familial congenital diaphragmatic defect: review and conclusions. Hum Genet. 1980;54:1–5. doi: 10.1007/BF00279041. [DOI] [PubMed] [Google Scholar]
- Norio R, Kaariainen H, Rapola J, Herva R, Kekomaki M. Familial congenital diaphragmatic defects: aspects of etiology, prenatal diagnosis, and treatment. Am J Med Genet. 1984;17:471–483. doi: 10.1002/ajmg.1320170210. [DOI] [PubMed] [Google Scholar]
- Mishalany H, Gordo J. Congenital diaphragmatic hernia in monozygotic twins. J Pediatr Surg. 1986;21:372–374. doi: 10.1016/s0022-3468(86)80208-1. [DOI] [PubMed] [Google Scholar]
- Frey P, Glanzmann R, Nars P, Herzog B. Familial congenital diaphragmatic defect: transmission from father to daughter. J Pediatr Surg. 1991;26:1396–1398. doi: 10.1016/0022-3468(91)91044-y. [DOI] [PubMed] [Google Scholar]
- Hitch DC, Carson JA, Smith EI, Sarale DC, Rennert OM. Familial congenital diaphragmatic hernia is an autosomal recessive variant. J Pediatr Surg. 1989;24:860–864. doi: 10.1016/s0022-3468(89)80582-2. [DOI] [PubMed] [Google Scholar]
- Gibbs DL, Rice HE, Farrell JA, Adzick NS, Harrison MR. Familial diaphragmatic agenesis: an autosomal-recessive syndrome with a poor prognosis. J Pediatr Surg. 1997;32:366–368. doi: 10.1016/s0022-3468(97)90212-8. [DOI] [PubMed] [Google Scholar]
- Pober BR, Lin A, Russell M, Ackerman KG, Chakravorty S, Strauss B, Westgate MN, Wilson J, Donahoe PK, Holmes LB. Infants with Bochdalek diaphragmatic hernia: sibling precurrence and monozygotic twin discordance in a hospital-based malformation surveillance program. Am J Med Genet A. 2005;138A:81–88. doi: 10.1002/ajmg.a.30904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober BR. Overview of epidemiology, genetics, birth defects, and chromosome abnormalities associated with CDH. Am J Med Genet C Semin Med Genet. 2007;145C:158–171. doi: 10.1002/ajmg.c.30126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinska M, Jay PY, Erlich JM, Mannisto S, Urban Z, Heikinheimo M, Wilson DB. Molecular genetics of congenital diaphragmatic defects. Ann Med. 2007;39:261–274. doi: 10.1080/07853890701326883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober BR. Genetic aspects of human congenital diaphragmatic hernia. Clin Genet. 2008;74:1–15. doi: 10.1111/j.1399-0004.2008.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]