Abstract
Cigarette smoke (CS) causes considerable morbidity and mortality by inducing cancer, chronic lung and vascular diseases, and oral disease. Despite the well-recognized risks associated with smoking, the habit remains unacceptably prevalent. Several toxins present in CS have immunomodulatory effects. CS also contains trace amounts of microbial cell components, including bacterial lipopolysaccharide. These and other CS constituents induce chronic inflammation at mucosal surfaces and modify host responses to exogenous antigens. The effects of CS on immunity are far-reaching and complex; both pro-inflammatory and suppressive effects may be induced. The net effect of CS on immunity depends on many variables, including the dose and type of tobacco, the route and chronicity of exposure, and the presence of other factors at the time of immune cell stimulation, such as Toll receptor ligands or other inflammatory mediators. CS impairs innate defenses against pathogens, modulates antigen presentation, and promotes autoimmunity. CS also impairs immunity in the oral cavity and promotes gingival and periodontal disease and oral cancer. The recognition of specific mechanisms by which CS affects host immunity is an important step toward elucidating mechanisms of tobacco-induced disease and may identify novel therapeutic approaches for the management of diseases that afflict smokers.
Abbreviations: AP-1, activator protein-1; CD, cluster of differentiation; COPD, chronic obstructive pulmonary disease; HLA, human leukocyte antigen; IFNγ, interferon gamma; IL, interleukin; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor kappa-B; RAGE, receptors for advanced glycation end-products; ROS, reactive oxidative species; RORγτ, retinoic acid receptor-related orphan receptor transcription factor; STAT, signal transducer and activator of transcription; T-bet, T-box transcription factor; Th, T-helper; TLR, Toll-like receptors; TNFα, Tumor necrosis factor alpha; and TSLP, thymic stromal lymphopoeitin.
Keywords: tobacco, immunity, inflammation, autoimmunity, cigarette smoke, nicotine
Introduction
More than 400,000 people die each year in the United States alone as a result of past or current cigarette smoke (CS) use; adult smokers lose an average 13 to 15 yrs of life-expectancy because they smoke (Morris, 1995; CDC, 2002; Heidrich et al., 2007). Despite widespread knowledge of the risks posed by CS, the worldwide prevalence of tobacco use is estimated to be in excess of one billion persons (Jha et al., 2002; Proctor, 2004; Samet and Wipfli, 2010). CS is a complex mixture of thousands of chemicals generated upon the burning or heating of tobacco leaves. CS contains thousands of chemicals that have cytotoxic, mutagenic, carcinogenic, or antigenic properties (Fig. 1) (Bluhm et al., 1971; Ding et al., 2008). The passive or active inhalation of CS results in the rapid dissolution of toxins in oral/airway epithelial lining fluids and systemic uptake. Combustion is an important step that produces reactive oxidative substances (ROS) that are otherwise not present in the leaf or the ash (Huang et al., 2005). The products of combustion can be divided into gaseous and particulate components (Bluhm et al., 1971; Witschi, 2005). Most of the toxic CS components are present in the particulate phase (Witschi, 2005). CS has far-reaching effects on host immunity that range from alteration of innate immunity in the oral, nasal, and airway mucosa, to alterations in adaptive immunity at the systemic level. Many toxic effects induced by CS, particularly the induction of carcinogenesis, result from direct genetic or epigenetic effects resulting in altered gene functions (for example, cell cycle, DNA repair, and tumor suppressor genes). While recognizing that CS may induce cancer and other diseases by multiple mechanisms, the current review will focus on molecular and cellular mechanisms by which CS promotes immune dysfunction. The current review aims to synthesize a large body of literature regarding CS-induced immune dysfunction. This review takes into account the inherent difficulty in comparing research findings generated in different model systems of CS exposure. Some of the apparently contradictory findings outlined below may be partially explained by differences in the models of CS-extract generation (filtered or unfiltered CS, extraction in physiologic media, buffered media or organic extracts, solubilization of smoke material captured on a filter, acute or prolonged exposure, etc.) and in vivo CS exposure (nose cone exposure, whole-body exposure, and challenge of animals by intra-tracheal CS-extracts).
Figure 1.
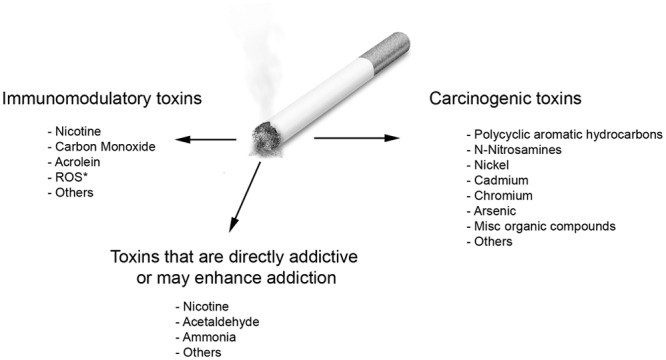
Cigarette smoke is a mixture of thousands of chemicals generated from the burning or heating of tobacco. The extraordinarily large collection of compounds present in cigarette smoke may be classified into broad groups, based on known target effects. Many carcinogenic toxins are now known to exist in cigarette smoke. Nicotine is the predominant addictive cigarette smoke constituent, although other chemicals contribute directly or indirectly to the addictive nature of CS (some by modifying nicotine effects). Nicotine, carbon monoxide, ROS, and acrolein are among the more important cigarette smoke toxins with immunomodulatory potential, although many more may exist. *ROS refers to reactive oxidant substances.
Cigarette Smoke Exerts Inflammatory and Suppressive Effects on Immune Cells
Gaseous and particulate CS constituents first interface with the immune system at the mucosal surfaces lining the oral cavity, sinuses, and airways. Thousands of ROS are produced in the burning cigarette and are not removed by cigarette butt filters (Huang et al., 2005). ROS contained in the gaseous phase are often short-lived and affect primarily the upper airways. Those in the particulate phase, particularly the semiquinone radicals, have the ability to secondarily generate more free radicals (Huang et al., 2005). ROS damage epithelial cells lining the airways by inducing peroxidation of lipids and other cell membrane constituents, activate oxidative-sensitive cellular pathways, and induce DNA damage (Valavanidis et al., 2009). CS constituents (particularly ROS) activate epithelial cell intracellular signaling cascades that lead to inflammatory gene activation [e.g., interleukin-8 or IL-8 and tumor necrosis factor-alpha (TNFα)] (Churg et al., 2002; Chung, 2005). The secretion of these inflammatory mediators promotes chronic immune cell recruitment and inflammation.
Not all effects of CS on host immunity are stimulatory. T-cells may be induced to proliferate and secrete cytokines that mediate important biological functions. The ensuing adaptive T-cell inflammatory response may be categorized as T-helper (Th)1, Th2, and Th17-type inflammation (Zhou et al., 2009). The designation of Th cells as Th1, 2, or 17 reflects the preferential activation of specific transcriptional T-cell factors and secretion of cytokines; for example, a preponderance of Th1 polarized T-cells produce interferon-gamma (IFNγ) rather than interleukin-4 (IL-4), which is produced primarily by Th2 polarized cells (Zhou et al., 2009). CS suppresses certain Th1 responses, while facilitating the generation of Th2 inflammation (Cozen et al., 2004; Vassallo et al., 2005; Nakamura et al., 2008; de Heens et al., 2009; Robays et al., 2009). For example, acute exposure of dendritic cells to cigarette smoke extract suppresses their activation by bacterial lipopolysaccharide (LPS), resulting in reduced secretion of interleukin-12 (Th1 polarizing) and interleukin-23 (Th17 polarizing) cytokines (Vassallo et al., 2005; Kroening et al., 2008). Similarly, lung and systemic dendritic cells extracted from mice exposed to CS secrete less IL-12 and IL-23 when activated ex vivo with LPS (Kroening et al., 2008). In an animal model of respiratory syncytial virus intranasal infection, mice exposed to CS showed an increase in lung eosinophils (a marker of Th2 inflammation), and reduced levels of Th1 cytokines, compared with air-exposed mice (Phaybouth et al., 2006). In an animal model of ovalbumin-mediated asthma, Robays et al. showed that CS enhanced Th2 polarized eosinophilic airway inflammation (Robays et al., 2009). In parallel to these findings, de Heens et al. observed that in vitro stimulation of peripheral blood T-cells of individual smokers produced greater amounts of interleukin-13 (Th2 cytokine) than that of control non-smokers (de Heens et al., 2009). Whether CS promotes Th17 inflammation is not definitively established, although there is evidence suggesting that, in certain individuals, chronic CS exposure may promote adaptive Th17 immunity to self-antigen (Shan et al., 2009). Analysis of the epidemiologic data showing increased prevalence of inflammatory diseases associated with Th17 inflammation in smokers also suggests that chronic CS exposure may promote Th17 polarized immunity (Heliovaara et al., 1993; Zivadinov et al., 2009). The mechanisms by which CS promotes preferential Th2 priming (and potentially Th17 inflammation) are not fully understood, but may involve suppression of Th1 polarizing cytokine production (Vassallo et al., 2005), altered antigen-presenting cell activation (Kroening et al., 2008; Robays et al., 2009), induction of Th2 polarizing factors from epithelial or other cells (Nakamura et al., 2008; Smelter et al., 2010), host genetic factors, and possibly direct effects on T-cells. While many studies describe CS-extract or CS as an adjuvant of adaptive Th2 immunity (Byron et al., 1994; Vassallo et al., 2005; Nakamura et al., 2008; Van Hove et al., 2008; de Heens et al., 2009; Robays et al., 2009; Smelter et al., 2010), other studies implicate a role for CS as an inducer of Th1 immunity (Kang et al., 2008; Huang et al., 2010). These seemingly contradictory observations may be the result of differences in techniques used to prepare CS-extracts, different rodent models of CS exposure, and other co-factors.
CS is one of the most important modifiers of host responses to pathogens (Zambon et al., 1996; Nuorti et al., 2000). Outcomes and severity of pneumonia due to Streptococcus pneumoniae and influenza infection are substantially worse in smokers (Kark et al., 1982; Nuorti et al., 2000). Smokers are also more likely to develop both latent and active tuberculosis (Kolappan and Gopi, 2002). There are many potential mechanisms by which CS hinders immunity to infection, including the modulation of intracellular epithelial and immune cell signaling (Gualano et al., 2008; Modestou et al., 2010) and suppression of innate and adaptive immune cell activation (Modestou et al., 2010).
Cigarette Smoke Alters Mucosal Immunity
The airway epithelium is a regulator of immune responses to a variety of insults, including CS. CS directly activates epithelial cells and induces chemokine and inflammatory mediator release (Fig. 2) (Mio et al., 1997; Kode et al., 2006; Pace et al., 2008). Epithelial cells express Toll-like receptors (TLRs) that recognize pathogen-associated molecules (Pace et al., 2008). For example, airway epithelial cells express TLR3, which binds double-stranded viral RNA (Yamin et al., 2008). Epithelial cells stimulated with a combination of CS-extract and viral double-stranded RNA produce significantly higher levels of chemokines than when incubated with either stimulus independently (Yamin et al., 2008). Despite this heightened cellular activation, epithelial-mediated innate immune responses to infectious pathogens are compromised by CS. CS-extracts suppress human beta-defensin-2 production (an endogenous secreted antimicrobial peptide) by gingival cells (van der Toorn et al., 2007). In another study, current or former smoking was associated with significantly reduced beta-defensin-2 levels in pharyngeal fluid and sputum from patients with acute pneumonia (Herr et al., 2009). CS also reduces mucosal ciliary motility, increases goblet cell numbers, and stimulates mucus hypersecretion (Chung, 2005). These effects result in persistent mucosal epithelial activation and diminished anti-microbial functions relevant to the clearance of infection, and may partially explain the higher likelihood of smokers to develop colonization and subsequent infection.
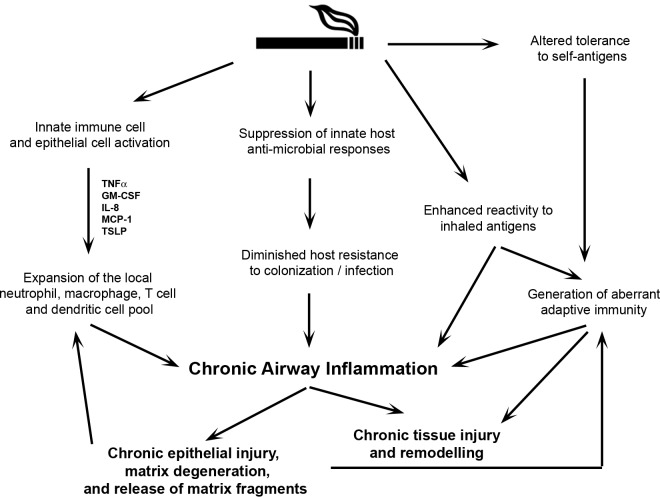
Figure 2.
Cigarette smoke modulates inflammation and promotes chronic inflammation in the conducting airways by a variety of mechanisms. Direct activation of epithelial and immune cells in the oral and conducting airways induces the secretion of pro-inflammatory factors (some of which are listed in Fig. 1) that promote the recruitment and survival of other immune cells, including neutrophils, macrophages, T-cells, and dendritic cells. Simultaneously, CS impairs innate host defense mechanisms, subdues innate responses to pathogens, and alters adaptive immune responses to inhaled antigens. The net result of these effects is a state of chronic injury and inflammation of the airways. Repetitive injury coupled with abnormal tolerogenic responses to self-antigens and co-existent pathogen colonization may promote the development of auto-reactive immunity, which further perpetuates tissue injury and inflammation.
CS alters many cell-signaling pathways involved in cellular activation. CS constituents activate several cell-signaling pathways, including mitogen-activated protein kinases (MAPK), nuclear factor kappa-B (NF-κB), signal transducer and activator of transcription (STAT), and activatory protein-1 (AP-1), all of which are also involved in the regulation of inflammatory, cell cycle, and other genes (Iles et al., 2005; Kroening et al., 2008; Liu et al., 2008; Smelter et al., 2010). Among these, CS-induced activation of the NF-κB and AP-1 transcription factors is critically involved in the regulation of inflammatory chemokine generation, altered corticosteroid resistance, altered responsiveness to acute pathogen challenge, and altered cell death regulation (Laan et al., 2004; Walters et al., 2005; Liu et al., 2008; Modestou et al., 2010). Liu et al. reported that CS-extract induces up-regulation of anti-apoptotic factors and activates NF-κB, the latter response being essential to prevent CS-induced cell death (Liu et al., 2008). Epithelial cells exposed to CS-extract also display significantly increased activity of the intracellular signaling molecule Ras, an effect that is at least partially dependent on the activation of receptors for advanced glycation end-products (RAGE) by CS-constituents (Reynolds et al., 2010). Elevated Ras activity, a characteristic feature of epithelial-derived lung cancers (Bos, 1989), has been shown to be a key cellular checkpoint through which CS-induced RAGE activation converges, culminating in downstream NF-κB activation and inflammatory gene expression (Reynolds et al., 2010). In addition to directly inducing NF-κB activation in epithelial cells, CS constituents also may prevent homeostatic NF-κB activation in stressed epithelial cells during pathogen challenge (Manzel et al., 2011). Manzel et al. showed that, following an acute exposure to combined mainstream and sidestream CS, mice challenged with H. influenzae showed suppressed NF-κB and downstream defense gene expression when compared with wild-type mice, indicating that CS constituents not only induce NF-κB components directly, but can also modulate cellular inflammatory NF-κB-dependent activation in the context of infection (Manzel et al., 2011). ROS in CS also activate AP-1, which is important in the induction of monocyte and macrophage activation and IL-8 production (Walters et al., 2005). The activation of AP-1 is also important in the development of corticosteroid-resistant inflammation (Walters et al., 2005). Although CS itself induces AP-1 activation, in the setting of acute LPS challenge of cells previously treated with CS-extracts, activation of AP-1 in bronchial epithelial cells is blunted (Laan et al., 2004). These findings support the notion that CS induces chronic inflammation in the airways while simultaneously modulating mucosal functions, resulting in diminished acute responsiveness to infectious challenge.
CS regulates the development of Th2 polarized allergic states by directly inducing pro-allergic factors. CS induces the production of thymic stromal lymphopoietin (TSLP) by epithelial (Nakamura et al., 2008) and airway smooth-muscle cells (Smelter et al., 2010). TSLP activates dendritic cells that promote Th2 polarization (Liu et al., 2007). A survey of TSLP gene single-nucleotide polymorphisms revealed gene variants that are more susceptible to activation due to increased affinity for binding of the AP-1 transcription factor (Harada et al., 2009). Furthermore, certain TSLP gene polymorphisms result in higher levels of TSLP production by bronchial epithelial cells in response to viral respiratory infections (Harada et al., 2009). TSLP stimulation is a specific way by which CS may promote a permissive setting for allergic inflammation in the airways and may be an important mechanism by which CS promotes airway inflammation.
CS also directly modulates dendritic cell functions. Dendritic cells are potent antigen-presenting cells that lie beneath mucosal epithelial cells and serve to process and present antigen to lymphocytes (Mellman and Steinman, 2001). Because of their capacity to influence both innate and adaptive immunity, dendritic cells are of critical importance in the regulation of mucosal immunity (Mellman and Steinman, 2001). In myeloid dendritic cells activated by LPS or CD40 ligand, CS may either stimulate (augment production of secreted prostaglandin-E2, IL-8, and IL-10) (Vassallo et al., 2005, 2008) or suppress (IL-12 and IL-23) production of inflammatory mediators (Kroening et al., 2008). Myeloid dendritic cells incubated with CS-extract also display diminished T-cell-stimulatory capacity (Vassallo et al., 2005; Mortaz et al., 2009a). These effects are mediated by different CS constituents and involve activation of diverse cellular signaling mediators (Kroening et al., 2008; Vassallo et al., 2008). For instance, some of the CS-induced inhibitory effects on dendritic cell functions are induced by ROS, while others are mediated by nicotine or other chemicals (Kroening et al., 2008; Vassalloet al., 2008). Robbins et al. showed that in vivo exposure of mice to CS diminishes lung dendritic cell activation and their capacity to induce antigen-specific T-cell proliferation in thoracic draining lymph nodes (Robbins et al., 2008). Although dendritic cells exposed to CS display diminished T-cell-stimulatory capacity and suppressed Th1 polarizing function, their migratory capacity to draining lymph nodes is preserved and potentially even enhanced (Robbins et al., 2008; Robays et al., 2009). CS-extract also suppresses anti-viral cytokine generation from plasmacytoid dendritic cells activated by a TLR9 agonist (Mortaz et al., 2009b). CS can thus affect dendritic cell functions both directly and indirectly.
In addition to dendritic cells, antigen-presenting cell functions are shared with macrophages and B-cells. Although increased numbers of macrophages are present in the airways and sinuses of smokers, these cells are functionally impaired (Green and Carolin, 1967; Hodge et al., 2003; Kirkham et al., 2004). Phagocytic function of alveolar macrophages is dampened by CS-induced oxidative stress (Green, 1968). CS impairs the phagocytic uptake of both bacteria and apoptotic cells, which may result in impaired healing of epithelial wounds and accumulation of apoptotic and inflammatory cellular debris (Hodge et al., 2003; Kirkham et al., 2004). The effect of CS on macrophage secretion of TNFα is not fully defined (Kuschner et al., 1996; Yang et al., 2006). For example, one study showed enhanced constitutive and inducible TNFα secretion by alveolar macrophages obtained by lung lavage of rodents acutely exposed to CS (Pessina et al., 1993), while another study reported a significant attenuation of inducible macrophage TNFα levels in a chronic model of CS exposure (Gaschler et al., 2008).
CS induces qualitative and quantitative defects in circulating natural killer cells which are important in host anti-tumor and viral responses (Ginns et al., 1985; Lu et al., 2007). In smokers, natural killer cells produce significantly less IFNγ and TNFα upon activation, when compared with non-smokers (Mian et al., 2008). CS-extract also reduces cytotoxic functions of natural killer cells (Mian et al., 2008). Using a mouse model of metastatic melanoma, Lu et al. observed a substantial increase in lung metastases among mice exposed to CS compared with control mice, and posited that CS-impaired natural killer cell-dependent tumor immune surveillance is an important mechanism underlying the observed increased predisposition to lung metastases (Lu et al., 2007).
Cigarette Smoke and Autoimmunity
It is increasingly appreciated that CS promotes certain autoimmune diseases like rheumatoid arthritis (Heliovaara et al., 1993; Silman et al., 1996; Hutchinson et al., 2001). A recent meta-analysis identified a two-fold increased risk of developing seropositive rheumatoid arthritis for individuals who smoked for more than 20 yrs, and a three-fold increased risk for rheumatoid arthritis in male smokers (Sugiyama et al., 2010). CS has also been associated with positivity for anti-citrullinated peptide antibodies, implying that smoking promotes the generation of auto-antibodies to citrullinated peptides (Klareskog et al., 2006). Furthermore, it has been linked to extra-articular rheumatoid disease, including lung disease (Harel-Meir et al., 2007). A major gene-environment interaction between rheumatoid arthritis susceptibility HLA-DR genes and CS was observed with a 21-fold increased relative risk of developing seropositive arthritis reported in European studies (Klareskog et al., 2006).
Potential mechanisms by which CS promotes rheumatoid arthritis include the release of intracellular proteins from ROS-activated or injured cells, augmentation of auto-reactive B-cell function (Klareskog et al., 2006), altered presentation of antigens by CS-impaired antigen-presenting cells (Vassallo et al., 2005; Robays et al., 2009), altered regulatory T-cell functions (Lee et al., 2007), and T-cell activation by antigens found in CS. Using an animal model of collagen-induced arthritis, Chujo et al. showed that the addition of CS condensate into the antigen/adjuvant emulsion used to induce arthritis in mice resulted in exacerbation of arthritis (Chujo et al., 2010). The same group also showed that nasal exposure of mice to CS condensate augmented the induction and development of joint changes in collagen-induced arthritis (Okamoto et al., 2011). In contrast, another group reported a delay in the development of arthritis in mice exposed to CS (Lindblad et al., 2009). The contrasting findings reported may reflect differences in methodologies used to expose mice to CS and the animal models of arthritis. A recent study also suggested a role for nicotine in the development of autoimmune arthritis. Mice lacking the alpha7 nicotinic receptor (one of the key nicotinic receptors expressed by immune and non-immune cells) developed milder collagen-induced arthritis and less cartilage destruction, compared with wild-type controls (Westman et al., 2010). Nicotine may differentially affect the severity of rodent autoimmune arthritis, since treatment of rodents with nicotine prior to immunization with antigen aggravated arthritis, while nicotine treatment following onset of arthritis resulted in amelioration of disease (Yu et al., 2011).
Autoimmune mechanisms may also be relevant in the pathogenesis of chronic obstructive pulmonary disease (COPD), another prevalent CS-induced disease. Individuals with COPD have a state of persistent airway inflammation (Garcia-Garcia et al., 1996; Saetta et al., 1998; Demedts et al., 2007). While the influx of inflammatory cells in the lung may be mediated by direct CS effects, the airway inflammation characteristic of COPD persists even following smoking cessation. The smoldering nature of this inflammation suggests that it is mediated by factors independent of direct CS toxicity, including adaptive immune responses to epithelial or other cellular antigens exposed following repeated injury of lung tissue (Fig. 2) (Churg et al., 2002; Chung, 2005; Lee et al., 2007; Cosio et al., 2009; Shan et al., 2009). In COPD patients, specific T-cell responses can be elicited from lung-derived elastin peptides (Lee et al., 2007; Shan et al., 2009). In addition, antibodies to elastin are increased in COPD patients as compared with control individuals, implying that COPD is associated with autoantibody generation (Lee et al., 2007). COPD patients also have significantly fewer regulatory T-cells in the lungs, which has been interpreted as evidence for antigen-specific autoimmunity partially mediated by a failure of usual homeostatic regulatory T-cell function (Lee et al., 2007). In another study, reduced levels of regulatory T-cell numbers were shown to be limited to small, but not large, airways (Isajevs et al., 2009). In a seemingly contrasting report, Smyth et al. (2007) showed that both “healthy” smokers and smokers with COPD have increased numbers of regulatory T-cells in the lung lavage fluid when compared with non-smokers; however, these cells expressed low levels of CD27, implying an impairment in functional suppressive capacity. Whether CS itself is directly responsible for these observed immune abnormalities in COPD is not clear.
Cigarette Smoking, Immunity, and Oral Disease
CS and the chewing of tobacco products are the main modifiable risk factors for chronic periodontitis. Smokers have poorer periodontal health and respond suboptimally to periodontal treatment (MacFarlane et al., 1992). Persistent inflammation and chronic infection are central in the pathogenesis of periodontitis (Delima et al., 2002; Behl et al., 2008; de Heens et al., 2009). Smokers are susceptible to colonization by Porphyromonas gingivalis (P. gingivalis), a causative agent of periodontitis (Zambon et al., 1996). CS exposure modifies the responses of gingival and immune cells to bacteria like P. gingivalis (Payne et al., 1996; Bagaitkar et al., 2009; Borch et al., 2009). While some studies show suppressed P. gingivalis-mediated activation of immune cells following incubation with CS-extract or nicotine (Payne et al., 1996; Borch et al., 2009), others have shown skewing of immune cell activation to P. gingivalis following acute CS-extract exposure, resulting in more pronounced Th2 cytokine production (de Heens et al., 2009). Acute CS exposure also induces the expression of certain bacterial genes potentially relevant to virulence (Bagaitkar et al., 2009). CS promotes an environment permissive for colonization and infection with pathogens like Escherichia coli, Staphylococcus aureus, and the fungi Candida albicans and Aspergillus fumigatus (Kamma et al., 1999).
There are several mechanisms by which CS-induced modulation of innate immune responses in the oral cavity facilitates colonization and chronic infection. Human gingival epithelial cells incubated with CS-extract produce substantially fewer anti-microbial peptides when activated with TLR ligands (Mahanonda et al., 2009). CS also activates cells in the oral cavity in ways that facilitate chronic inflammation, even though many antimicrobial functions are suppressed or modulated (Mahanonda et al., 2009). CS may also dysregulate innate immune responses in the oral cavity by modifying local TLR expression, distribution, and activation, thereby promoting an environment permissive for chronic inflammation (Beklen et al., 2008; Pace et al., 2008). Another potential mechanism by which CS promotes periodontal disease is through up-regulation of RAGE receptors on gingival cells (Katz et al., 2005). Human gingival cells exposed to nornicotine (a nicotine metabolite) up-regulate RAGE expression (Katz et al., 2005). RAGE has various ligands that primarily include endogenous molecules generated or released during cellular stress, including advanced glycation end-products (AGEs) (Sims et al., 2010). It is plausible that CS-induced RAGE expression on epithelial cells promotes the pro-inflammatory effects of AGEs present in the environment (and also present in CS itself), thereby augmenting chronic inflammatory responses in the gingival tissue of smokers. A role for CS-induced RAGE expression and enhanced RAGE signaling in periodontitis is also supported by observations that blockade of RAGE in mice infected with P. gingivalis resulted in significant attenuation of inflammation and periodontal bone loss compared with control mice (Lalla et al., 2000). Aberrant adaptive inflammation mediated by CS effects on CD4+ T-cells may potentially be relevant in the development of bone loss observed in severe periodontal disease (Teng et al., 2000), and it is possible that persistently activated Th2-polarized T-cell inflammation may be involved in the development of more progressive periodontal lesions (Bartova et al., 2000; de Heens et al., 2009).
Final Comments
CS causes diverse changes in immunity that lead to heightened constitutive inflammation, skewing of adaptive T-cell-mediated immunity, impaired responses to pathogens, and suppressed anti-tumor immune cell functions. When the exposure to CS is sustained, a chronic inflammatory process ensues that has the potential to promote enhanced microbial colonization and infection, persistence of apoptotic material and abnormal processing of cellular debris, induction of autoimmunity to self-antigen, and architectural remodeling. The consequences of unchecked CS-induced inflammation and immune dysregulation continue to be an area of active research. While a potential solution to the tobacco disease epidemic may be attainable with widespread and effective smoking cessation methodologies, the unfortunate reality is that tobacco use is actually on the rise on a global level (Samet and Wipfli, 2010). Education regarding mechanisms by which smoking and second-hand CS promote disease remains a crucial component of tobacco control policy, and objective scientific data are critically needed to counteract deceptive marketing strategies by producers of tobacco. Understanding specific mechanisms by which tobacco impairs immunity may also provide important new therapeutic targets for the treatment of many diseases that afflict smokers.
Footnotes
This research was supported by the Flight Attendant Medical Research Institute and National Institutes of Health grants HL96829Z and AR60077.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bagaitkar J, Williams LR, Renaud DE, Bemakanakere MR, Martin M, Scott DA, et al. (2009). Tobacco-induced alterations to Porphyromonas gingivalis-host interactions. Environ Microbiol 11:1242-1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartova J, Kratka-Opatrna Z, Prochazkova J, Krejsa O, Duskova J, Mrklas L, et al. (2000). Th1 and Th2 cytokine profile in patients with early onset periodontitis and their healthy siblings. Mediators Inflamm 9:115-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl Y, Siqueira M, Ortiz J, Li J, Desta T, Faibish D, et al. (2008). Activation of the acquired immune response reduces coupled bone formation in response to a periodontal pathogen. J Immunol 181:8711-8718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beklen A, Hukkanen M, Richardson R, Konttinen YT. (2008). Immunohistochemical localization of Toll-like receptors 1-10 in periodontitis. Oral Microbiol Immunol 23:425-431 [DOI] [PubMed] [Google Scholar]
- Bluhm AL, Weinstein J, Sousa JA. (1971). Free radicals in tobacco smoke. Nature 229:500. [DOI] [PubMed] [Google Scholar]
- Borch TS, Lobner M, Bendtzen K, Holmstrup P, Nielsen CH. (2009). Decreased interleukin-2 responses to Fusobacterium nucleatum and Porphyromonas gingivalis in generalized aggressive periodontitis. J Periodontol 80:800-807 [DOI] [PubMed] [Google Scholar]
- Bos JL. (1989). RAS oncogenes in human cancer: a review. Cancer Res 49:4682-4689; erratum in Cancer Res 50:1352, 1990 [PubMed] [Google Scholar]
- Byron KA, Varigos GA, Wootton AM. (1994). IL-4 production is increased in cigarette smokers. Clin Exp Immunol 95:333-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2002). Annual smoking-attributable mortality, years of potential life lost, and economic costs—United States, 1995-1999. MMWR Morb Mortal Wkly Rep 51:300-303 [PubMed] [Google Scholar]
- Chujo S, Okamoto S, Sunahara R, Adachi M, Yamada K, Hayashi H, et al. (2010). Cigarette smoke condensate extracts augment collagen-induced arthritis in mice. Int Immunopharmacol 10:1194-1199 [DOI] [PubMed] [Google Scholar]
- Chung KF. (2005). Inflammatory mediators in chronic obstructive pulmonary disease. Curr Drug Targets Inflamm Allergy 4:619-625 [DOI] [PubMed] [Google Scholar]
- Churg A, Dai J, Tai H, Xie C, Wright JL. (2002). Tumor necrosis factor-alpha is central to acute cigarette smoke-induced inflammation and connective tissue breakdown. Am J Respir Crit Care Med 166:849-854 [DOI] [PubMed] [Google Scholar]
- Cosio MG, Saetta M, Agusti A. (2009). Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med 360:2445-2454 [DOI] [PubMed] [Google Scholar]
- Cozen W, Diaz-Sanchez D, James Gauderman W, Zadnick J, Cockburn MG, Gill PS, et al. (2004). Th1 and Th2 cytokines and IgE levels in identical twins with varying levels of cigarette consumption. J Clin Immunol 24:617-622 [DOI] [PubMed] [Google Scholar]
- de Heens GL, van der Velden U, Loos BG. (2009). Cigarette smoking enhances T cell activation and a Th2 immune response; an aspect of the pathophysiology in periodontal disease. Cytokine 47:157-161 [DOI] [PubMed] [Google Scholar]
- Delima AJ, Karatzas S, Amar S, Graves DT. (2002). Inflammation and tissue loss caused by periodontal pathogens is reduced by interleukin-1 antagonists. J Infect Dis 186:511-516 [DOI] [PubMed] [Google Scholar]
- Demedts IK, Bracke KR, Van Pottelberge G, Testelmans D, Verleden GM, Vermassen FE, et al. (2007). Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 175:998-1005 [DOI] [PubMed] [Google Scholar]
- Ding YS, Zhang L, Jain RB, Jain N, Wang RY, Ashley DL, et al. (2008). Levels of tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons in mainstream smoke from different tobacco varieties. Cancer Epidemiol Biomarkers Prev 17:3366-3371 [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia JM, Hernandez JR, Martinez-Muniz MA, Sanchez-Antuna A, Marron MG, Monte C, et al. (1996). Airways reactivity, atopy and bronchoalveolar lavage in male smokers with airflow obstruction. Respiration 63:199-204 [DOI] [PubMed] [Google Scholar]
- Gaschler GJ, Zavitz CC, Bauer CM, Skrtic M, Lindahl M, Robbins CS, et al. (2008). Cigarette smoke exposure attenuates cytokine production by mouse alveolar macrophages. Am J Respir Cell Mol Biol 38:218-226 [DOI] [PubMed] [Google Scholar]
- Ginns LC, Ryu JH, Rogol PR, Sprince NL, Oliver LC, Larsson CJ. (1985). Natural killer cell activity in cigarette smokers and asbestos workers. Am Rev Respir Dis 131:831-834 [DOI] [PubMed] [Google Scholar]
- Green GM. (1968). Cigarette smoke: protection of alveolar macrophages by glutathione and cysteine. Science 162:810-811 [DOI] [PubMed] [Google Scholar]
- Green GM, Carolin D. (1967). The depressant effect of cigarette smoke on the in vitro antibacterial activity of alveolar macrophages. N Engl J Med 276:421-427 [DOI] [PubMed] [Google Scholar]
- Gualano RC, Hansen MJ, Vlahos R, Jones JE, Park-Jones RA, Deliyannis G, et al. (2008). Cigarette smoke worsens lung inflammation and impairs resolution of influenza infection in mice. Respir Res 9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Hirota T, Jodo AI, Doi S, Kameda M, Fujita K, et al. (2009). Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am J Respir Cell Mol Biol 40:368-374 [DOI] [PubMed] [Google Scholar]
- Harel-Meir M, Sherer Y, Shoenfeld Y. (2007). Tobacco smoking and autoimmune rheumatic diseases. Nat Clin Pract Rheumatol 3:707-715 [DOI] [PubMed] [Google Scholar]
- Heidrich J, Wellmann J, Heuschmann PU, Kraywinkel K, Keil U. (2007). Mortality and morbidity from coronary heart disease attributable to passive smoking. Eur Heart J 28:2498-2502 [DOI] [PubMed] [Google Scholar]
- Heliovaara M, Aho K, Aromaa A, Knekt P, Reunanen A. (1993). Smoking and risk of rheumatoid arthritis. J Rheumatol 20:1830-1835 [PubMed] [Google Scholar]
- Herr C, Beisswenger C, Hess C, Kandler K, Suttorp N, Welte T, et al. (2009). Suppression of pulmonary innate host defence in smokers. Thorax 64:144-149 [DOI] [PubMed] [Google Scholar]
- Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M. (2003). Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol Cell Biol 81:289-296; erratum in Immunol Cell Biol 81:499, 2003 [DOI] [PubMed] [Google Scholar]
- Huang MF, Lin WL, Ma YC. (2005). A study of reactive oxygen species in mainstream of cigarette. Indoor Air 15:135-140 [DOI] [PubMed] [Google Scholar]
- Huang QP, Zhong XN, Bai J, Qiu SL, Chen H, Zhang JQ. (2010). [Change of airway inflammation induced by Th1/Tc1 and the expression of T regulatory cells in smoking cessation rats]. Zhonghua Yi Xue Za Zhi 90:2552-2557 [article in Chinese]. [PubMed] [Google Scholar]
- Hutchinson D, Shepstone L, Moots R, Lear JT, Lynch MP. (2001). Heavy cigarette smoking is strongly associated with rheumatoid arthritis (RA), particularly in patients without a family history of RA. Ann Rheum Dis 60:223-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles KE, Dickinson DA, Wigley AF, Welty NE, Blank V, Forman HJ. (2005). HNE increases HO-1 through activation of the ERK pathway in pulmonary epithelial cells. Free Radic Biol Med 39:355-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isajevs S, Taivans I, Strazda G, Kopeika U, Bukovskis M, Gordjusina V, et al. (2009). Decreased FOXP3 expression in small airways of smokers with COPD. Eur Respir J 33:61-67 [DOI] [PubMed] [Google Scholar]
- Jha P, Ranson MK, Nguyen SN, Yach D. (2002). Estimates of global and regional smoking prevalence in 1995, by age and sex. Am J Public Health 92:1002-1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamma JJ, Nakou M, Baehni PC. (1999). Clinical and microbiological characteristics of smokers with early onset periodontitis. J Periodontal Res 34:25-33 [DOI] [PubMed] [Google Scholar]
- Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, et al. (2008). Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest 118:2771-2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kark JD, Lebiush M, Rannon L. (1982). Cigarette smoking as a risk factor for epidemic A(H1N1) influenza in young men. N Engl J Med 307:1042-1046 [DOI] [PubMed] [Google Scholar]
- Katz J, Caudle RM, Bhattacharyya I, Stewart CM, Cohen DM. (2005). Receptor for advanced glycation end product (RAGE) upregulation in human gingival fibroblasts incubated with nornicotine. J Periodontol 76:1171-1174 [DOI] [PubMed] [Google Scholar]
- Kirkham PA, Spooner G, Rahman I, Rossi AG. (2004). Macrophage phagocytosis of apoptotic neutrophils is compromised by matrix proteins modified by cigarette smoke and lipid peroxidation products. Biochem Biophys Res Commun 318:32-37 [DOI] [PubMed] [Google Scholar]
- Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. (2006). A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum 54:38-46 [DOI] [PubMed] [Google Scholar]
- Kode A, Yang SR, Rahman I. (2006). Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells. Respir Res 7:132; erratum in Respir Res 9:6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolappan C, Gopi PG. (2002). Tobacco smoking and pulmonary tuberculosis. Thorax 57:964-966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroening PR, Barnes TW, Pease L, Limper A, Kita H, Vassallo R. (2008). Cigarette smoke-induced oxidative stress suppresses generation of dendritic cell IL-12 and IL-23 through ERK-dependent pathways. J Immunol 181:1536-1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuschner WG, D’Alessandro A, Wong H, Blanc PD. (1996). Dose-dependent cigarette smoking-related inflammatory responses in healthy adults. Eur Respir J 9:1989-1994 [DOI] [PubMed] [Google Scholar]
- Laan M, Bozinovski S, Anderson GP. (2004). Cigarette smoke inhibits lipopolysaccharide-induced production of inflammatory cytokines by suppressing the activation of activator protein-1 in bronchial epithelial cells. J Immunol 173:4164-4170 [DOI] [PubMed] [Google Scholar]
- Lalla E, Lamster IB, Feit M, Huang L, Spessot A, Qu W, et al. (2000). Blockade of RAGE suppresses periodontitis-associated bone loss in diabetic mice. J Clin Invest 105:1117-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, et al. (2007). Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med 13:567-569 [DOI] [PubMed] [Google Scholar]
- Lindblad SS, Mydel P, Jonsson IM, Senior RM, Tarkowski A, Bokarewa M. (2009). Smoking and nicotine exposure delay development of collagen-induced arthritis in mice. Arthritis Res Ther 11:R88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Togo S, Al-Mugotir M, Kim H, Fang Q, Kobayashi T, et al. (2008). NF-kappaB mediates the survival of human bronchial epithelial cells exposed to cigarette smoke extract. Respir Res 9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, et al. (2007). TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol 25:193-219 [DOI] [PubMed] [Google Scholar]
- Lu LM, Zavitz CC, Chen B, Kianpour S, Wan Y, Stampfli MR. (2007). Cigarette smoke impairs NK cell-dependent tumor immune surveillance. J Immunol 178:936-943 [DOI] [PubMed] [Google Scholar]
- MacFarlane GD, Herzberg MC, Wolff LF, Hardie NA. (1992). Refractory periodontitis associated with abnormal polymorphonuclear leukocyte phagocytosis and cigarette smoking. J Periodontol 63:908-913 [DOI] [PubMed] [Google Scholar]
- Mahanonda R, Sa-Ard-Iam N, Eksomtramate M, Rerkyen P, Phairat B, Schaecher KE, et al. (2009). Cigarette smoke extract modulates human beta-defensin-2 and interleukin-8 expression in human gingival epithelial cells. J Periodontal Res 44:557-564 [DOI] [PubMed] [Google Scholar]
- Manzel LJ, Shi L, O’Shaughnessy PT, Thorne PS, Look DC. (2011). Cigarette smoke inhibition of the NF-{kappa}B-dependent response to bacteria in the airway. Am J Respir Cell Mol Biol 44:155-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Steinman RM. (2001). Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255-258 [DOI] [PubMed] [Google Scholar]
- Mian MF, Lauzon NM, Stampfli MR, Mossman KL, Ashkar AA. (2008). Impairment of human NK cell cytotoxic activity and cytokine release by cigarette smoke. J Leukoc Biol 83:774-784 [DOI] [PubMed] [Google Scholar]
- Mio T, Romberger DJ, Thompson AB, Robbins RA, Heires A, Rennard SI. (1997). Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. Am J Respir Crit Care Med 155:1770-1776 [DOI] [PubMed] [Google Scholar]
- Modestou MA, Manzel LJ, El-Mahdy S, Look DC. (2010). Inhibition of IFN-gamma-dependent antiviral airway epithelial defense by cigarette smoke. Respir Res 11:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris PD. (1995). Lifetime excess risk of death from lung cancer for a U.S. female never-smoker exposed to environmental tobacco smoke. Environ Res 68:3-9 [DOI] [PubMed] [Google Scholar]
- Mortaz E, Kraneveld AD, Smit JJ, Kool M, Lambrecht BN, Kunkel SL, et al. (2009a). Effect of cigarette smoke extract on dendritic cells and their impact on T-cell proliferation. PLoS One 4:e4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortaz E, Lazar Z, Koenderman L, Kraneveld AD, Nijkamp FP, Folkerts G. (2009b). Cigarette smoke attenuates the production of cytokines by human plasmacytoid dendritic cells and enhances the release of IL-8 in response to TLR-9 stimulation. Respir Res 10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Miyata M, Ohba T, Ando T, Hatsushika K, Suenaga F, et al. (2008). Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J Allergy Clin Immunol 122:1208-1214 [DOI] [PubMed] [Google Scholar]
- Nuorti JP, Butler JC, Farley MM, Harrison LH, McGeer A, Kolczak MS, et al. (2000). Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med 342:681-689 [DOI] [PubMed] [Google Scholar]
- Okamoto S, Adachi M, Chujo S, Yamada K, Akita K, Itoh S, et al. (2011). Etiological role of cigarette smoking in rheumatoid arthritis: nasal exposure to cigarette smoke condensate extracts augments the development of collagen-induced arthritis in mice. Biochem Biophys Res Commun 404:1088-1092 [DOI] [PubMed] [Google Scholar]
- Pace E, Ferraro M, Siena L, Melis M, Montalbano AM, Johnson M, et al. (2008). Cigarette smoke increases Toll-like receptor 4 and modifies lipopolysaccharide-mediated responses in airway epithelial cells. Immunology 124:401-411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JB, Johnson GK, Reinhardt RA, Dyer JK, Maze CA, Dunning DG. (1996). Nicotine effects on PGE2 and IL-1 beta release by LPS-treated human monocytes. J Periodontal Res 31:99-104 [DOI] [PubMed] [Google Scholar]
- Pessina GP, Paulesu L, Corradeschi F, Luzzi E, Tanzini M, Di Stefano A, et al. (1993). Production of tumor necrosis factor alpha by rat alveolar macrophages collected after acute cigarette smoking. Arch Immunol Ther Exp (Warsz) 41:343-348 [PubMed] [Google Scholar]
- Phaybouth V, Wang SZ, Hutt JA, McDonald JD, Harrod KS, Barrett EG. (2006). Cigarette smoke suppresses Th1 cytokine production and increases RSV expression in a neonatal model. Am J Physiol Lung Cell Mol Physiol 290:L222-L2231 [DOI] [PubMed] [Google Scholar]
- Proctor RN. (2004). The global smoking epidemic: a history and status report. Clin Lung Cancer 5:371-376 [DOI] [PubMed] [Google Scholar]
- Reynolds PR, Kasteler SD, Schmitt RE, Hoidal JR. (2011). RAGE signals through Ras during tobacco smoke-induced pulmonary inflammation. Am J Respir Cell Mol Biol 45:411-418 [DOI] [PubMed] [Google Scholar]
- Robays LJ, Lanckacker EA, Moerloose KB, Maes T, Bracke KR, Brusselle GG, et al. (2009). Concomitant inhalation of cigarette smoke and aerosolized protein activates airway dendritic cells and induces allergic airway inflammation in a TLR-independent way. J Immunol 183:2758-2766 [DOI] [PubMed] [Google Scholar]
- Robbins CS, Franco F, Mouded M, Cernadas M, Shapiro SD. (2008). Cigarette smoke exposure impairs dendritic cell maturation and T cell proliferation in thoracic lymph nodes of mice. J Immunol 180:6623-6628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetta M, Di Stefano A, Turato G, Facchini FM, Corbino L, Mapp CE, et al. (1998). CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 157(3 Pt 1):822-826 [DOI] [PubMed] [Google Scholar]
- Samet JM, Wipfli HL. (2010). Globe still in grip of addiction. Nature 463:1020-1021 [DOI] [PubMed] [Google Scholar]
- Shan M, Cheng HF, Song LZ, Roberts L, Green L, Hacken-Bitar J, et al. (2009). Lung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysema. Sci Transl Med 1:4ra10. [DOI] [PubMed] [Google Scholar]
- Silman AJ, Newman J, MacGregor AJ. (1996). Cigarette smoking increases the risk of rheumatoid arthritis. Results from a nationwide study of disease-discordant twins. Arthritis Rheum 39:732-735 [DOI] [PubMed] [Google Scholar]
- Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. (2010). HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 28:367-388 [DOI] [PubMed] [Google Scholar]
- Smelter DF, Sathish V, Thompson MA, Pabelick CM, Vassallo R, Prakash YS. (2010). Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. J Immunol 185:3035-3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth LJ, Starkey C, Vestbo J, Singh D. (2007). CD4-regulatory cells in COPD patients. Chest 132:156-163 [DOI] [PubMed] [Google Scholar]
- Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. (2010). Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 69:70-81 [DOI] [PubMed] [Google Scholar]
- Teng YT, Nguyen H, Gao X, Kong YY, Gorczynski RM, Singh B, et al. (2000). Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Invest 106:R59-R67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavanidis A, Vlachogianni T, Fiotakis K. (2009). Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int J Environ Res Public Health 6:445-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Toorn M, Slebos DJ, de Bruin HG, Leuvenink HG, Bakker SJ, Gans RO, et al. (2007). Cigarette smoke-induced blockade of the mitochondrial respiratory chain switches lung epithelial cell apoptosis into necrosis. Am J Physiol Lung Cell Mol Physiol 292:L1211-L1218 [DOI] [PubMed] [Google Scholar]
- Van Hove CL, Moerloose K, Maes T, Joos GF, Tournoy KG. (2008). Cigarette smoke enhances Th-2 driven airway inflammation and delays inhalational tolerance. Respir Res 9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassallo R, Tamada K, Lau JS, Kroening PR, Chen L. (2005). Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of Th-2 priming. J Immunol 175:2684-2691 [DOI] [PubMed] [Google Scholar]
- Vassallo R, Kroening PR, Parambil J, Kita H. (2008). Nicotine and oxidative cigarette smoke constituents induce immune-modulatory and pro-inflammatory dendritic cell responses. Mol Immunol 45:3321-3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters MJ, Paul-Clark MJ, McMaster SK, Ito K, Adcock IM, Mitchell JA. (2005). Cigarette smoke activates human monocytes by an oxidant-AP-1 signaling pathway: implications for steroid resistance. Mol Pharmacol 68:1343-1353 [DOI] [PubMed] [Google Scholar]
- Westman M, Saha S, Morshed M, Lampa J. (2010). Lack of acetylcholine nicotine alpha 7 receptor suppresses development of collagen-induced arthritis and adaptive immunity. Clin Exp Immunol 162:62-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witschi H. (2005). Carcinogenic activity of cigarette smoke gas phase and its modulation by beta-carotene and N-acetylcysteine. Toxicol Sci 84:81-87 [DOI] [PubMed] [Google Scholar]
- Yamin M, Holbrook EH, Gray ST, Harold R, Busaba N, Sridhar A, et al. (2008). Cigarette smoke combined with Toll-like receptor 3 signaling triggers exaggerated epithelial regulated upon activation, normal T-cell expressed and secreted/CCL5 expression in chronic rhinosinusitis. J Allergy Clin Immunol 122:1145-1153.e3 [DOI] [PubMed] [Google Scholar]
- Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, et al. (2006). Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol 291:L46-L57 [DOI] [PubMed] [Google Scholar]
- Yu H, Yang YH, Rajaiah R, Moudgil KD. (2011). Nicotine-induced differential modulation of autoimmune arthritis in the Lewis rat involves changes in interleukin-17 and anti-cyclic citrullinated peptide antibodies. Arthritis Rheum 63:981-991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon JJ, Grossi SG, Machtei EE, Ho AW, Dunford R, Genco RJ. (1996). Cigarette smoking increases the risk for subgingival infection with periodontal pathogens. J Periodontol 67(10 Suppl):1050S-1054S [DOI] [PubMed] [Google Scholar]
- Zhou L, Chong MM, Littman DR. (2009). Plasticity of CD4+ T cell lineage differentiation. Immunity 30:646-655 [DOI] [PubMed] [Google Scholar]
- Zivadinov R, Weinstock-Guttman B, Hashmi K, Abdelrahman N, Stosic M, Dwyer M, et al. (2009). Smoking is associated with increased lesion volumes and brain atrophy in multiple sclerosis. Neurology 73:504-510 [DOI] [PMC free article] [PubMed] [Google Scholar]