Abstract
Histamine is an important allergic mediator and studies have defined roles for both histamine 1 and 4 receptors (H1R and H4R) in allergic airway inflammation. Here, we show that histamine is necessary to generate IL-4-driven eosinophilic inflammation, as histamine deficient mice cannot generate eosinophilic lung inflammation in response to intratracheal IL-4 and exogenous histamine restores responsiveness. This is histamine 2 receptor (H2R) dependent since H2R KO mice fail to respond to IL-4, and a H2R agonist restores inflammation in HDC KO. Furthermore, alveolar epithelial cells require H2R in order to produce CCL24, an eosinophil recruitment factor, while H2R blockade reduces CCL24 production from WT cells. In an allergic inflammation model, H2R KO mice show significantly reduced eosinophilic inflammation and CCL24 expression. These data demonstrate a previously unidentified role for H2R in allergic inflammation and establishes a synergy between endogenous histamine and IL-4 that supports eosinophilic recruitment to the lung.
INTRODUCTION
IL-4 is critically important for Th2-associated immunity, including allergic inflammation. Even in the absence of IL-5, IL-9 and IL-13, IL-4 is capable of eliciting the characteristic Th2 immune responses, including eosinophilia and IgE production, during helminth infection (1). Transgenic overexpression of IL-4 in the lung generates profound inflammatory responses without affecting airway reactivity (2), which is largely IL-13 dependent (3). Similarly, local delivery of recombinant IL-4 leads to significant accumulation of inflammatory cells in the lung (4).
While recruitment of eosinophils to the lungs of mice during allergic responses is regulated by both CCL11 and CCL24 (5), CCL11 expression has been shown to be mainly IL-13 driven (6) and lung eosinophilia in CCL11 KO mice is only mildly affected (7). Conversely, CCL24 has a dominant role in promoting airway eosinophilia (7). Transgenic co-expression of CCL24 and IL-5 within the airway leads to chronic eosinophil-associated lung damage that mirrors severe asthma (8). During allergic airway responses or upon IL-4 overexpression, CCL24 is significantly upregulated (7, 9, 10) but the mechanisms controlling this local tissue response remain unclear.
During allergic responses, mast cells residing within tissues release a plethora of mediators capable of influencing inflammatory cell recruitment (11). One mediator is histamine, a highly diffusible bioactive molecule that exerts its biological functions via four receptors (H1R, H2R, H3R, and H4R) (12). While histamine is best known for its role in vasodilation and smooth muscle responses during immediate hypersensitivity, the histamine receptors also exert potent immunomodulatory influences. H4R has also been shown to regulate allergic sensitization, since H4R KO mice have defective dendritic cell priming of T cells and H4R blockade ameliorates allergic inflammation (13). We previously demonstrated that H1R on T cells is necessary for their recruitment to the lung and subsequent escalation of Th2-associated airway inflammation (14). In this H1R study, we demonstrated that exogenous delivery of IL-4 was sufficient to elicit inflammatory cell recruitment to the lungs of these mice since it bypassed the requirement for T cell migration.
Here we show here that exogenous delivery of IL-4 cannot generate similar responses in mice specifically lacking histamine, suggesting that histamine is necessary for IL-4-driven lung eosinophilia. We demonstrate that this is mediated by the H2R receptor and, using both in vitro and in vivo approaches, that this receptor is critical for production of the eosinophilic chemokine CCL24. Consequently, our data suggests that histamine modulates the local lung responsiveness to IL-4 via H2R, permitting CCL24 production and subsequent eosinophil recruitment. We postulate that inhibition of this receptor may be useful in the therapeutic treatment of allergy and Th2-associated diseases.
MATERIALS AND METHODS
Animals
Female C57BL/6 (4-8-week-old; Taconic Farms, Hudson, NY), HDC KO (from Dr Hiroshi Ohtsu (15)) and H2R KO (from Dr Takeshi Watanabe (16)) mice were housed under specific pathogen-free conditions and maintained on an OVA-free diet. All experiments were approved by the Northwestern University Animal Care and Use Committee.
Intratracheal IL-4-driven airway inflammation
5μg of BSA, carrier-free recombinant murine IL-4 (eBioscience, Franklin Lakes, NJ), histamine (Calbiochem, Rockland, MA), or dimaprit dihydrochloride (Tocris bioscience, Ellisville, MO) was administered intratracheally for 3 consecutive days. Mice were studied on day 4.
Primary ATII cell isolation
Aveolar Type II epithelial cells were isolated by Pulmonary Core B (Northwestern University, Chicago, IL), as previously described (17). Cells were cultured in DMEM with 10% FCS, 100U/ml penicillin and 100g/ml streptomycin and treated with 10ng/ml recombinant murine IL-4 (PeproTech) and 50μM hydrochloride ranitidine (Sigma Aldrich).
CCL24 ELISA
CCL24 was measured by ELISA (R&D Systems, Minneapolis, MN), according to the manufacturer’s instruction.
Allergic airway inflammation model
Mice received intraperitoneal injection of 10μg OVA (Grade VI; Sigma-Aldrich, St. Louis, MO) in alum (3 mg) or alum alone at days 0 and 14, followed by 20 minutes of aerosolized 1% OVA on days 21, 22, and 23 and studied on day 24.
Lung inflammation
Bronchial alveolar lavage (BAL) was collected by flushing lungs with 0.8 ml BAL fluid (10% FCS, 1mM EDTA, 1X PBS). BAL fluid was counted and cytospun onto slides and differential cell counts performed after staining with DiffQuik (Dade Behring, Neward, DE). Hematoxylin and eosin (H&E) and periodic acid shiff (PAS) histology was performed by Histo-Scientific Research laboratories (Mount Jackson, VA).
Real-time RT-PCR
Total RNA was isolated from 50-100 mg lung tissue using a RNeasy kit (Qiagen, Valencia, CA). cDNA was prepared using qScript cDNA Super Mix (Quanta, Gaithersburg, MD). Gene expression was determined as previously described (14, 18).
Statistics
Data are represented as mean ± SEM. Statistical significance was determined using Student t test. All analysis was done using GraphPad Prism Software (La Jolla, CA).
RESULTS AND DISCUSSION
Histamine is necessary for IL-4 induced eosinophil recruitment and CCL24 production
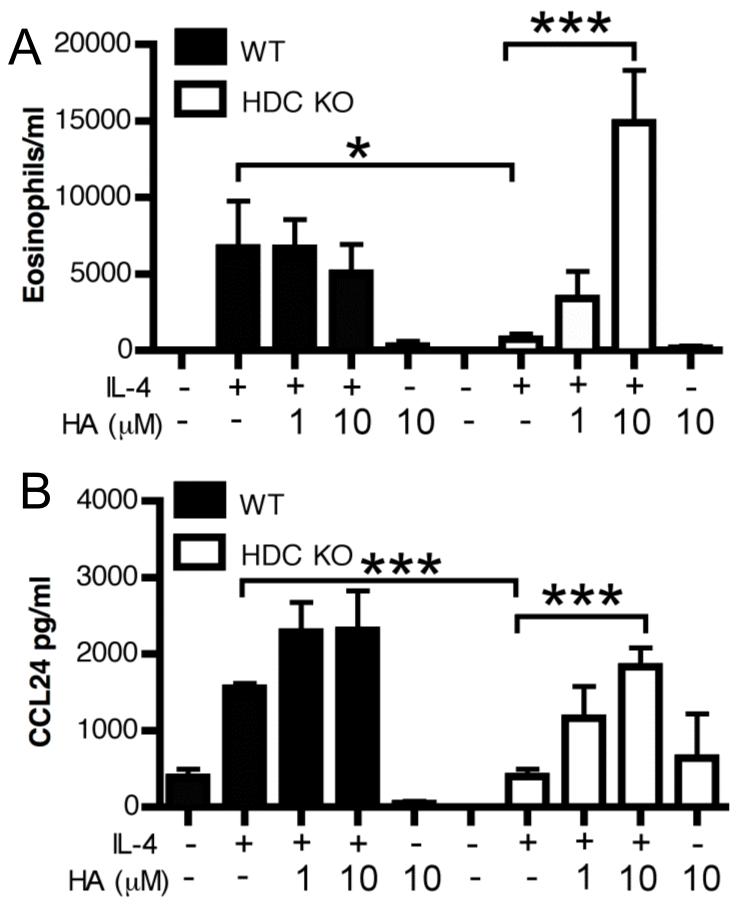
Since we previously showed that intranasal IL-4 could restore eosinophilic inflammatory responses in H1R KO mice (14), we wished to examine responses in the absence of histamine itself. In contrast to H1R KO mice, HDC KO mice failed to mount eosinophilic inflammation after IL-4 delivery (Figure 1A) and this could be restored with addition of exogenous histamine at the time of IL-4 administration. Mechanistically, histamine can directly promote eosinophil migration (19) while work using allergen challenge in patients also showed that histamine affects eosinophil migration indirectly by promoting local eotaxin expression (20, 21). Within the time frame we examined, histamine alone failed to induce any inflammatory infiltration (Figure 1A). Conversely, while WT mice displayed a robust expression of the eosinophilic chemokine CCL24, this was ablated in the HDC KO mice and restored by exogenous histamine (Figure 1B). We were unable to detect significant upregulation of the other major eosinophilic chemokine CCL11 in any group (data not shown), concurrent with its proposed IL-13 dependence (6). Consequently, rather than directly promoting eosinophil migration, histamine serves to regulate the expression of the eosinophil chemoattractant signals upon IL-4 exposure in the lung tissue. Importantly, these findings suggest that there is a homeostatic role for histamine in facilitating responsiveness to IL-4 in WT animals, and this basal tone is lost in HDC KO mice. Since our previous work established that these effects of histamine were H1R-independent and H4R-mediated eosinophil migration has been shown to be mediated via CCL-16 (22), we next examined the role of H2R using H2R KO mice.
Figure 1. Endogenous histamine is necessary for IL-4 induced eosinophil recruitment and CCL24 production.
5 μg of IL-4 or BSA, with or without histamine (HA) were administered intratracheally to WT or HDC KO mice for 2 consecutive days and inflammation was determined on day 3. Eosinophils in the BAL fluid were identified using DiffQuick staining of cytospun BAL fluid cells (A). CCL24 levels were measured in BAL fluid by ELISA (B). Data represent mean ± SEM from 3 independent experiments (n = 3-7). *p < 0.05; *** p < 0.005 by Students t-test.
IL-4 mediated eosinophil recruitment to the lung requires H2R
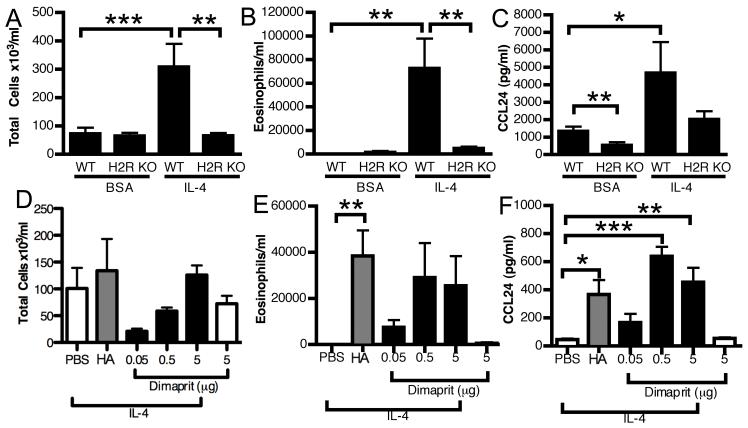
Similar to HDC KO mice, IL-4 was unable to induce eosinophilic inflammation in H2R KO mice (Figure 2B). CCL24 production was also impaired in H2R KO mice (Figure 2C). Furthermore, co-administration of IL-4 with an H2R agonist, dimaprit, was sufficient to increase both the eosinophil numbers (Figure 2E) and CCL24 production (Figure 2F) in the HDC KO. While the eosinophil numbers failed to reach statistical significance due to some variability (although all dimaprit-treated mice had eosinophils in the BAL fluid versus none of the PBS-treated), the CCL24 response was highly significant, supporting H2R being the critical histamine receptor in mediating the effects of histamine on eosinophilic lung responses. While the established role for H2R is in acid secretion in the stomach and the H2R KO mouse has abnormal gastric responses (16), H2R has a wide expression profile that includes both nonhematopoietic and hematopoietic cells (23). Since intratracheal administration of IL-4 acts locally to drive inflammation, we concluded that H2R was mostly likely necessary at the level of a tissue resident cell.
Figure 2. H2R is necessary for IL-4 induced eosinophil recruitment and CCL24 production.
5 μg of IL-4 or BSA was administered intratracheally for 3 consecutive days to WT or H2R KO mice. On day 4, total cells (A), eosinophils (B) and CCL24 levels (C) in BAL fluid were determined. Data represent mean ± SEM from 3 independent experiments (n = 7-9). 5 μg of IL-4 or BSA was administered intratracheally for 3 consecutive days to HDC KO mice with or without histamine (10μM) or dimaprit and total cells (D), eosinophils (E) and CCL24 levels (F) in BAL fluid were measured on day 4. Data represent mean ± SEM from 3 independent experiments (n = 4-6). *p < 0.05; **p < 0.05; *** p < 0.005 by Students t-test.
H2R is critical for IL-4 induced CCL24 production by ATII cells
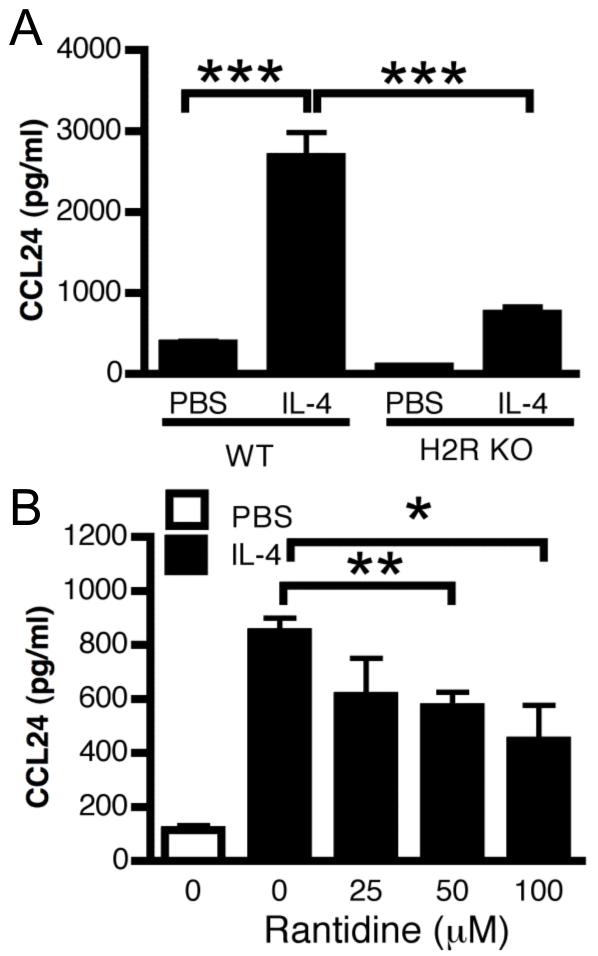
Airway smooth muscle cells express IL-4R but it has recently been shown that they do not contribute significantly to IL-4-induced inflammation (24). Therefore, we focused our interest on the lung epithelium. Alveolar Type II (ATII) cells have previously been shown to produce CCL24 in response to IL-4 (25). We initially determined that these cells also express high levels of H2R and that both WT and H2R KO cells express similar levels of IL-4R (data not shown). In response to IL-4, H2R KO ATII cells produced significantly less CCL24 than WT cells (Figure 3A). We further interrogated the role of H2R in CCL24 production using pharmacological blockade of H2R. WT ATII cells pre-treated with ranitidine 30 minutes prior to IL-4 treatment, demonstrated a dose-dependent significant reduction in CCL24 production (Figure 3B). Therefore, we propose that IL-4-driven eosinophilic inflammation is likely facilitated by endogenous histamine, acting via H2R on ATII cells within the lung to promote the generation of a CCL24 gradient. Interestingly, mast cells are commonly located in or around the airway epithelium and the frequency of intraepithelial mast cells is increased in asthmatics with a dominant Th2 phenotype (26). Therefore, this increased mast cell presence may serve to enhance the local tissue responsiveness to IL-4 by increasing local histamine levels.
Figure 3. IL-4 induced CCL24 production by ATII cells is H2R dependent.
Primary mouse ATII cells were isolated and stimulated with 10ng of murine recombinant IL-4 for 48 hours. CCL24 levels were measured in supernatant from WT and H2R KO ATII cells (A) or from WT ATII cells treated with ranitidine (B). Data represent mean ± SEM from 3 independent experiments (n = 3-6). * p < 0.5; ** p < 0.05; *** p < 0.005 by Students t-test.
Paradoxically, our in vitro culture experiments did not receive exogenous histamine and yet H2R still regulated IL-4-driven CCL24 production. Histamine receptors are known to possess constitutive basal activity (27). This includes H2R, which ranitidine inhibits via inverse agonist activity (28). It has also been previously shown that histamine is present in cell culture media and required for optimal T cell activation in vitro (29). We determined that our FCS did contain histamine such that our cultures were receiving approximately 10-7M histamine (data not shown) but we were unable to eliminate this since the ATII cells failed to grow well in serum-free media. However, since our in vivo data using the HDC KO mice demonstrates that histamine is required for IL-4-driven eosinophila and CCL24 expression, we conclude that histamine is likely acting upon its receptor.
H2R is necessary for lung eosinophilia during allergic airway inflammation
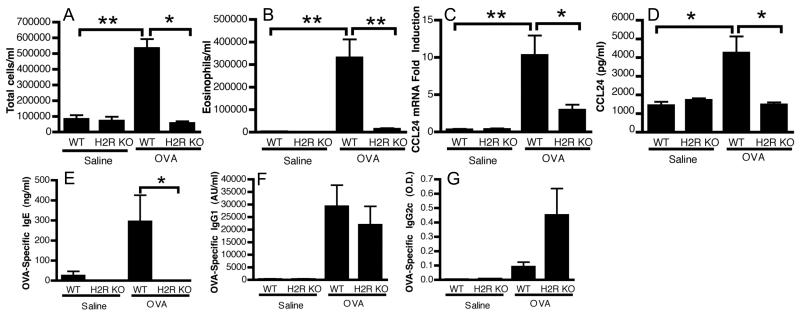
While we demonstrated a role for H1R in allergic inflammation (14), H2R has not been investigated. Using the same model of allergic airway inflammation we used in the H1R study (14), H2R KO mice showed reduced cellular infiltration in BAL fluid compared to WT (Figure 4A). This was not due to altered H1R expression since the relative expression of all histamine receptors in lung tissue from the H2R KO was similar to WT levels (data not shown). As predicted from our in vitro studies, this reduced cellular infiltrate was due largely to a significant reduction in eosinophils in the lungs of the H2R KO mice, with a concurrent reduction in CCL24 mRNA and protein production (Figure 4B-D). Additionally, we measured several other chemokine and cytokine levels, including eosinophil-associated IL-16, IL-5 and CCL5 (RANTES) (Supplemental Figure 1A-G). Rather than a general defect in expression of all responses, H2R KO mice displayed an apparent disregulation, with some mediators upregulated, similar to WT in response to challenge, while others were unchanged. Histology revealed substantial inflammation and mucus production in WT lung tissue that was significantly reduced in the H2R KO mice, which exhibited only minor perivascular inflammation (Supplemental Figure 1H). Quantification of PAS+ cells in the airway showed significantly fewer mucus expressing cells in H2R KO mice versus WT (data not shown). The presence of some inflammation and mucus production in the H2R KO mice does indicate some inflammatory responses occurred in the H2R KO mice, albeit significantly reduced. Furthermore, analysis of the OVA-specific immunoglobulins generated during sensitization showed an absence of specific IgE in H2R KO mice (Figure 4E) but competent production of IgG1 (Figure 4F) and IgG2c (Figure 4G), a similar pattern to that reported by Jutel et al. (30). They also demonstrated enhanced Th1 and Th2-associated OVA-specific recall responses from H2R KO splenocytes (30), a phenotype we have also observed (data not shown). Consequently, H2R KO mice do exhibit antigen sensitization and so the lack of airway eosinophilia and IgE are not likely due to defects in antigen processing and presentation. In agreement, we determined that CD11c+ dendritic cells from H2R KO mice pulsed with OVA323-329 peptide induced similar proliferation of OTII CD4+ T cells to those from WT mice (data not shown). Jutel et al. proposed that the lack of IgE was due to the heightened levels of IFNγ However it has since been shown that IL-21, and not IFNγ is the critical suppressive cytokine of IgE generation during allergic sensitization to OVA (31). IL-21 expression levels were similar between WT and H2R KO mice (data not shown), suggesting this was not responsible for the differences seen in IgE. Instead, we hypothesized that H2R may be also directly impacting B cell responses, since class switching to IgE is critically dependent upon IL-4 signaling (32, 33). Using B cells cultured in vitro with LPS plus increasing concentrations of IL-4, we determined that H2R KO cells were significantly impaired in their ability to generate IgE (Supplemental Figure 2), despite similar levels of IL-4R expression (data not shown). Consequently, we conclude that H2R is directly regulating the production of IgE by influencing the ability of B cells to respond to IL-4. It is possible that the lack of responsiveness to IL-4 by cells other than the airway epithelium underlie the relatively suppressed inflammatory response seen in the ovalbumin model. However, further investigation is required to dissect the significance of H2R expression on other cell types and how their cumulative response to IL-4 shapes allergic airway inflammation. Overall, these results suggest that H2R facilitates IL-4-driven eosinophil recruitment and IgE production in vivo.
Figure 4. H2R is necessary for eosinophilic airway inflammation to antigen.
Sham (saline) or OVA/alum sensitized WT and H2R KO mice were challenged with aerosolized OVA and studied for inflammatory responses. Total cells (A) and eosinophil (B) counts in BAL fluid were determined. CCL24 protein levels in BAL fluid (C) and mRNA levels in lung tissue (D) were also measured. OVA-specific antibody levels in serum were measured by ELISA (E-G). Data represent mean ± SEM from 2 independent experiments (n = 5-6). *p < 0.05; ** p < 0.05 by Students t-test.
In conclusion, our data defines a requirement for histamine in facilitating responsiveness to IL-4. We are proposing that the endogenous low levels of histamine present during homeostasis are sufficient for this effect. While histamine can be sequestered from diet or produced by bacteria, the HDC KO mice used in our study were not maintained on a histamine deficient diet and so this basal histamine is most likely derived from host immune cells, which could include stored histamine from mast cells or basophils but also inducible producers such as dendritic cells (13). While we previously demonstrated that H1R is critical for regulating the migration of antigen-specific T cells to the lung (14), this regulation of tissue responsiveness to IL-4 functions via H2R. Therefore, we propose that histamine exerts its varied influences on both systemic and local inflammatory responses via different receptors that can be targeted for the therapeutic treatment of allergy and Th2-associated diseases.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr Hiroshi Ohtsu and Dr Takeshi Watanabe for providing mice and Dr Mendy Miller for her manuscript editorial input.
Footnotes
Supported by R01 AI072570
REFERENCES
- 1.Fallon PG, Jolin HE, Smith P, Emson CL, Townsend MJ, Fallon R, Smith P, McKenzie AN. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. 2002;17:7–17. doi: 10.1016/s1074-7613(02)00332-1. [DOI] [PubMed] [Google Scholar]
- 2.Rankin JA, Picarella DE, Geba GP, Temann UA, Prasad B, DiCosmo B, Tarallo A, Stripp B, Whitsett J, Flavell RA. Phenotypic and physiologic characterization of transgenic mice expressing interleukin 4 in the lung: lymphocytic and eosinophilic inflammation without airway hyperreactivity. Proc Natl Acad Sci U S A. 1996;93:7821–7825. doi: 10.1073/pnas.93.15.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walter DM, McIntire JJ, Berry G, McKenzie AN, Donaldson DD, DeKruyff RH, Umetsu DT. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001;167:4668–4675. doi: 10.4049/jimmunol.167.8.4668. [DOI] [PubMed] [Google Scholar]
- 4.Blaeser F, Bryce PJ, Ho N, Raman V, Dedeoglu F, Donaldson DD, Geha RS, Oettgen HC, Chatila TA. Targeted inactivation of the IL-4 receptor alpha chain I4R motif promotes allergic airway inflammation. J Exp Med. 2003;198:1189–1200. doi: 10.1084/jem.20030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–1310. doi: 10.1016/j.jaci.2007.03.048. quiz 1311-1302. [DOI] [PubMed] [Google Scholar]
- 6.Lewis CC, Aronow B, Hutton J, Santeliz J, Dienger K, Herman N, Finkelman FD, Wills-Karp M. Unique and overlapping gene expression patterns driven by IL-4 and IL-13 in the mouse lung. J Allergy Clin Immunol. 2009;123:795–804. e798. doi: 10.1016/j.jaci.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol. 2005;175:5341–5350. doi: 10.4049/jimmunol.175.8.5341. [DOI] [PubMed] [Google Scholar]
- 8.Ochkur SI, Jacobsen EA, Protheroe CA, Biechele TL, Pero RS, McGarry MP, Wang H, O’Neill KR, Colbert DC, Colby TV, Shen H, Blackburn MR, Irvin CC, Lee JJ, Lee NA. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol. 2007;178:7879–7889. doi: 10.4049/jimmunol.178.12.7879. [DOI] [PubMed] [Google Scholar]
- 9.Tumes DJ, Connolly A, Dent LA. Expression of survivin in lung eosinophils is associated with pathology in a mouse model of allergic asthma. Int Immunol. 2009;21:633–644. doi: 10.1093/intimm/dxp032. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann N, Hogan SP, Mishra A, Brandt EB, Bodette TR, Pope SM, Finkelman FD, Rothenberg ME. Murine eotaxin-2: a constitutive eosinophil chemokine induced by allergen challenge and IL-4 overexpression. J Immunol. 2000;165:5839–5846. doi: 10.4049/jimmunol.165.10.5839. [DOI] [PubMed] [Google Scholar]
- 11.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nature immunology. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 12.Dy M, Schneider E. Histamine-cytokine connection in immunity and hematopoiesis. Cytokine Growth Factor Rev. 2004;15:393–410. doi: 10.1016/j.cytogfr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Dunford PJ, O’Donnell N, Riley JP, Williams KN, Karlsson L, Thurmond RL. The histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ T cells. J Immunol. 2006;176:7062–7070. doi: 10.4049/jimmunol.176.11.7062. [DOI] [PubMed] [Google Scholar]
- 14.Bryce PJ, Mathias CB, Harrison KL, Watanabe T, Geha RS, Oettgen HC. The H1 histamine receptor regulates allergic lung responses. J Clin Invest. 2006;116:1624–1632. doi: 10.1172/JCI26150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, Tchougounova E, Hellman L, Gertsenstein M, Hirasawa N, Sakurai E, Buzas E, Kovacs P, Csaba G, Kittel A, Okada M, Hara M, Mar L, Numayama-Tsuruta K, Ishigaki-Suzuki S, Ohuchi K, Ichikawa A, Falus A, Watanabe T, Nagy A. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001;502:53–56. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi T, Tonai S, Ishihara Y, Koga R, Okabe S, Watanabe T. Abnormal functional and morphological regulation of the gastric mucosa in histamine H2 receptor-deficient mice. J Clin Invest. 2000;105:1741–1749. doi: 10.1172/JCI9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobbs LG. Isolation and culture of alveolar type II cells. Am J Physiol. 1990;258:L134–147. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- 18.Bryce PJ, Geha R, Oettgen HC. Desloratadine inhibits allergen-induced airway inflammation and bronchial hyperresponsiveness and alters T-cell responses in murine models of asthma. J Allergy Clin Immunol. 2003;112:149–158. doi: 10.1067/mai.2003.1616. [DOI] [PubMed] [Google Scholar]
- 19.O’Reilly M, Alpert R, Jenkinson S, Gladue RP, Foo S, Trim S, Peter B, Trevethick M, Fidock M. Identification of a histamine H4 receptor on human eosinophils--role in eosinophil chemotaxis. Journal of receptor and signal transduction research. 2002;22:431–448. doi: 10.1081/rrs-120014612. [DOI] [PubMed] [Google Scholar]
- 20.Menzies-Gow A, Ying S, Phipps S, Kay AB. Interactions between eotaxin, histamine and mast cells in early microvascular events associated with eosinophil recruitment to the site of allergic skin reactions in humans. Clin Exp Allergy. 2004;34:1276–1282. doi: 10.1111/j.1365-2222.2004.02014.x. [DOI] [PubMed] [Google Scholar]
- 21.Menzies-Gow A, Ying S, Sabroe I, Stubbs VL, Soler D, Williams TJ, Kay AB. Eotaxin (CCL11) and eotaxin-2 (CCL24) induce recruitment of eosinophils, basophils, neutrophils, and macrophages as well as features of early- and late-phase allergic reactions following cutaneous injection in human atopic and nonatopic volunteers. J Immunol. 2002;169:2712–2718. doi: 10.4049/jimmunol.169.5.2712. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama T, Kato Y, Hieshima K, Nagakubo D, Kunori Y, Fujisawa T, Yoshie O. Liver-expressed chemokine/CC chemokine ligand 16 attracts eosinophils by interacting with histamine H4 receptor. J Immunol. 2004;173:2078–2083. doi: 10.4049/jimmunol.173.3.2078. [DOI] [PubMed] [Google Scholar]
- 23.Jutel M, Blaser K, Akdis CA. Histamine receptors in immune regulation and allergen-specific immunotherapy. Immunol Allergy Clin North Am. 2006;26:245–259. doi: 10.1016/j.iac.2006.02.006. vii. [DOI] [PubMed] [Google Scholar]
- 24.Perkins C, Yanase N, Smulian G, Gildea L, Orekov T, Potter C, Brombacher F, Aronow B, Wills-Karp M, Finkelman FD. Selective stimulation of IL-4 receptor on smooth muscle induces airway hyperresponsiveness in mice. J Exp Med. 2011;208:853–867. doi: 10.1084/jem.20100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heiman AS, Abonyo BO, Darling-Reed SF, Alexander MS. Cytokine-stimulated human lung alveolar epithelial cells release eotaxin-2 (CCL24) and eotaxin-3 (CCL26) J Interferon Cytokine Res. 2005;25:82–91. doi: 10.1089/jir.2005.25.82. [DOI] [PubMed] [Google Scholar]
- 26.Dougherty RH, Sidhu SS, Raman K, Solon M, Solberg OD, Caughey GH, Woodruff PG, Fahy JV. Accumulation of intraepithelial mast cells with a unique protease phenotype in T(H)2-high asthma. J Allergy Clin Immunol. 2010;125:1046–1053. e1048. doi: 10.1016/j.jaci.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, Schunack W, Levi R, Haas HL. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacological reviews. 1997;49:253–278. [PubMed] [Google Scholar]
- 28.Smit MJ, Leurs R, Alewijnse AE, Blauw J, Van Nieuw Amerongen GP, Van De Vrede Y, Roovers E, Timmerman H. Inverse agonism of histamine H2 antagonist accounts for upregulation of spontaneously active histamine H2 receptors. Proc Natl Acad Sci U S A. 1996;93:6802–6807. doi: 10.1073/pnas.93.13.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noubade R, Milligan G, Zachary JF, Blankenhorn EP, del Rio R, Rincon M, Teuscher C. Histamine receptor H1 is required for TCR-mediated p38 MAPK activation and optimal IFN-gamma production in mice. J Clin Invest. 2007;117:3507–3518. doi: 10.1172/JCI32792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OA, Malolepszy J, Zak-Nejmark T, Koga R, Kobayashi T, Blaser K, Akdis CA. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001;413:420–425. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- 31.Shang XZ, Ma KY, Radewonuk J, Li J, Song XY, Griswold DE, Emmell E, Li L. IgE isotype switch and IgE production are enhanced in IL-21-deficient but not IFN-gamma-deficient mice in a Th2-biased response. Cellular immunology. 2006;241:66–74. doi: 10.1016/j.cellimm.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Lebman DA, Coffman RL. Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. J Exp Med. 1988;168:853–862. doi: 10.1084/jem.168.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, Doherty PC, Grosveld G, Paul WE, Ihle JN. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.