Abstract
Transplant tolerance, defined as indefinite allograft survival without immunosuppression, has been regularly achieved in laboratory mice but not in nonhuman primates or humans. In contrast to laboratory mice, primates regularly have high frequencies of alloreactive memory T cells (TMEMs) before transplantation. These TMEMs are poorly sensitive to conventional immunosuppression and costimulation blockade, and the presence of donor-reactive TMEMs in primates may account for their resistance to transplant tolerance protocols that have proven consistently effective in mice. We measured the frequencies of anti-donor TMEMs before and after transplantation in a series of rejecting and tolerant monkeys that underwent nonmyeloablative conditioning, short-term immunosuppression, and combined allogeneic kidney/cell transplantation. Transplants were acutely rejected in all the monkeys with high numbers of donor-specific TMEMs before transplantation. In contrast, long-term survival was observed in the recipients harboring lower frequencies of anti-donor TMEMs before transplantation. Similar amounts of TMEM homeostatic expansion were recorded in all transplanted monkeys upon hematopoietic reconstitution; however, only the tolerant monkeys had no expansion or activation of donor-reactive TMEMs after transplantation. These results indicate that the presence of high frequencies of host donor-reactive TMEMs before transplantation impairs tolerance induction to kidney allografts in this nonhuman primate model. Indeed, recipients harboring a low anamnestic reactivity to their donor before transplantation were successfully rendered tolerant via infusion of donor cells and short-term immunosuppression. This suggests that selection of allogeneic donors with low memory responses in recipients may be essential to successful transplant tolerance induction in patients.
INTRODUCTION
Enormous advances over the past 2 decades with nonselective immunosuppressive agents have greatly improved the early survival of allogeneic transplants in patients. Nevertheless, lifelong immunosuppression not only leads to treatment-related complications such as nephrotoxicity and increased susceptibility to cancer and infections but frequently does not prevent chronic rejection. Hence, there is a need for the design of more selective immune therapies in transplantation.
Transplantation tolerance, defined as lack of acute and chronic allograft rejection in the absence of ongoing immunosuppressive therapy (and intact immune reactivity to pathogens), is the holy grail of transplant immunologists. This goal has been achieved in some murine transplant models by induction of mixed hematopoietic chimerism (via donor bone marrow transplantation) (1–3) and T cell costimulation blockade (4–9). However, in primates, although these treatments reduce alloimmunity and improve allograft survival, they fail to consistently provide tolerance (10, 11). These observations emphasize the necessity of evaluating and refining in nonhuman primates the therapeutic protocols that have been defined in laboratory rodents before their clinical application. They also underscore the requirement for a better understanding of the mechanisms underlying allotransplant rejection versus acceptance in primates.
In contrast to laboratory mice, high numbers of alloreactive memory T cells (TMEMs) are present in nonhuman primates and humans before transplantation (4 to 8% and >40% of whole T cells in mice and primates, respectively) (12–14), presumably due to cross-reactivity from previous exposure to environmental, bacterial, or viral antigens. Indeed, self-MHC (major histocompatibility complex)/microbial peptide complexes mimicking allo-MHC/peptide complexes are known to sensitize some alloreactive T cells in rodents and humans (15–18). In addition, allospecific TMEMs are regularly generated after exposure to foreign MHC molecules during pregnancy, blood transfusion, or previous transplantation (19). TMEMs, including presumably alloreactive T cells, undergo peripheral homeostatic expansion in individuals rendered leukopenic due to irradiation or treatment with depleting antibodies, which are common procedures in clinical transplantation (20). These TMEMs are resistant to common immunosuppressive strategies including calcineurin inhibitors and costimulation blockade due to their low activation threshold, heightened proliferative capacity, rapid cytokine secretion, and ability to home to nonlymphoid tissues (21–24). It is likely that these allospecific TMEMs represent an essential element of the allograft rejection process in primates (25). It is noteworthy that alloreactive TMEMs induced in mice via microbial infection, skin allograft, or adoptive transfer invariably prevent transplant tolerance induction via mixed chimerism or costimulation blockade (26–29). These data suggest that the high frequency of alloreactive TMEMs found in unmanipulated primates may account for their resistance to tolerance induction, a hypothesis that has not yet been formally tested.
Here, we investigated the influence of pretransplant TMEM alloreactivity on tolerance to kidney allografts induced via combined donor cell infusion and short-term immunosuppression in cynomolgus monkeys. We show that long-term allograft survival in the absence of immunosuppression is favored in recipients displaying a low anamnestic response to their donors. This implies that long-term kidney allograft survival in the absence of immunosuppression may be achieved using current tolerance protocols in patients displaying a low anamnestic reactivity against their donor.
RESULTS
Frequencies of alloreactive TMEMs in cynomolgus monkeys
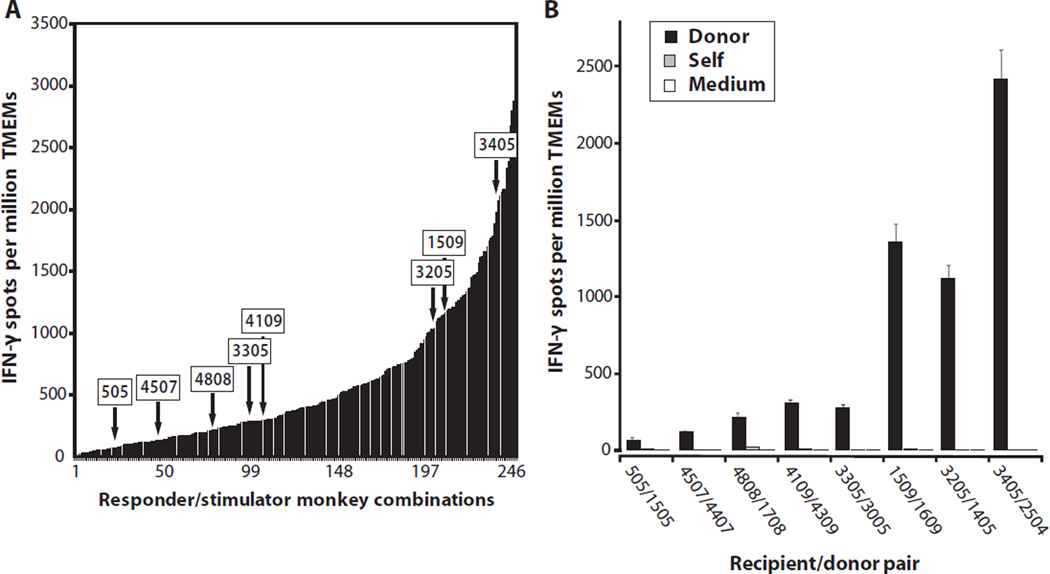
Peripheral blood TMEMs from a panel of individual cynomolgus monkeys were isolated by fluorescence-activated cell sorting (FACS) and stimulated in vitro with irradiated allogeneic stimulator cells (direct allorecognition) from each of a series of allogeneic monkeys. The numbers of cytokine-producing TMEMs were determined by enzyme-linked immunospot (ELISPOT) in each of 251 single responder/stimulator combinations. The magnitude of the memory interferon-γ (IFN-γ) alloresponse varied greatly among all the responder/stimulator combinations tested, ranging from 10 to 3624 cells per million TMEMs (Fig. 1A). Eight stimulator and responder combinations with various amounts of memory alloreactivity were selected as donor/recipient pairs for transplant experiments (arrows, Fig. 1, A and B).
Fig. 1.
Memory T cell (TMEM) responses before transplantation in cynomolgus monkeys. (A and B) Pretransplant frequencies of donor-reactive TMEMs in recipients. (A) The peripheral blood TMEMs from a panel of individual monkeys were isolated by FACS using CD95 and C28 markers and stimulated in vitro with irradiated allogeneic stimulator cells (direct allorecognition). The frequencies of IFN-γ−producing TMEMs were determined by ELISPOT in each of 251 responder/stimulator combinations. The arrows correspond to the eight monkey combinations selected for combined bone marrow/kidney transplantation. (B) Frequencies of IFN-γ–secreting TMEMs measured in the eight monkeys selected as recipients after stimulation with allogeneic APCs (from the allogeneic monkeys selected as donors) or control syngeneic (autologous) APCs or in the absence of APCs (medium).
Relationships between numbers of anti-donor TMEMs before transplantation or in either tolerant or rejecting recipients of kidney allografts
Eight monkeys selected for their distinct levels of donor-specific memory reactivity (Fig. 1B) were conditioned with the regimen shown in Fig. 2 (30) and received cells (bone marrow or spleen) and a kidney transplant from the same allogeneic donor. Immunosuppressive treatment with cyclosporine A was stopped 28 days after transplantation. The three recipients (M505, M4507, and M4808) with a low frequency of donor-specific TMEMs (<250 IFN-γ–producing cells per million TMEMs) displayed long-term allograft survival (Table 1). These monkeys became tolerant (graft survival >250 days, no chronic rejection) (Table 1). For the two monkeys that mounted an intermediate-level anamnestic response, M4109 became tolerant, whereas M3305 rejected its kidney allograft at day 140. These data suggest that other cells or factors could contribute to susceptibility to tolerogenesis in this model. The three other recipients, which displayed a high anti-donor pretransplant anamnestic reactivity (>1000 spots per million TMEMs), rejected their allografts acutely (60 to 90 days). The mean frequency of IFN-γ–producing TMEMs in the rejecting group was statistically significantly higher than that of the tolerant monkey group (one-tailed t test assuming equal variance, P = 0.023). All rejecting monkeys and one tolerant monkey lost donor chimerism by day 20 after transplantation, whereas chimerism was detectable for up to 50 days (M4808) and >50 days (M4507) in the two other tolerant animals. Anti-donor antibodies were detected in the serum of three out of four recipients with acute rejection, but only one tolerant monkey (M505) displayed alloantibodies.
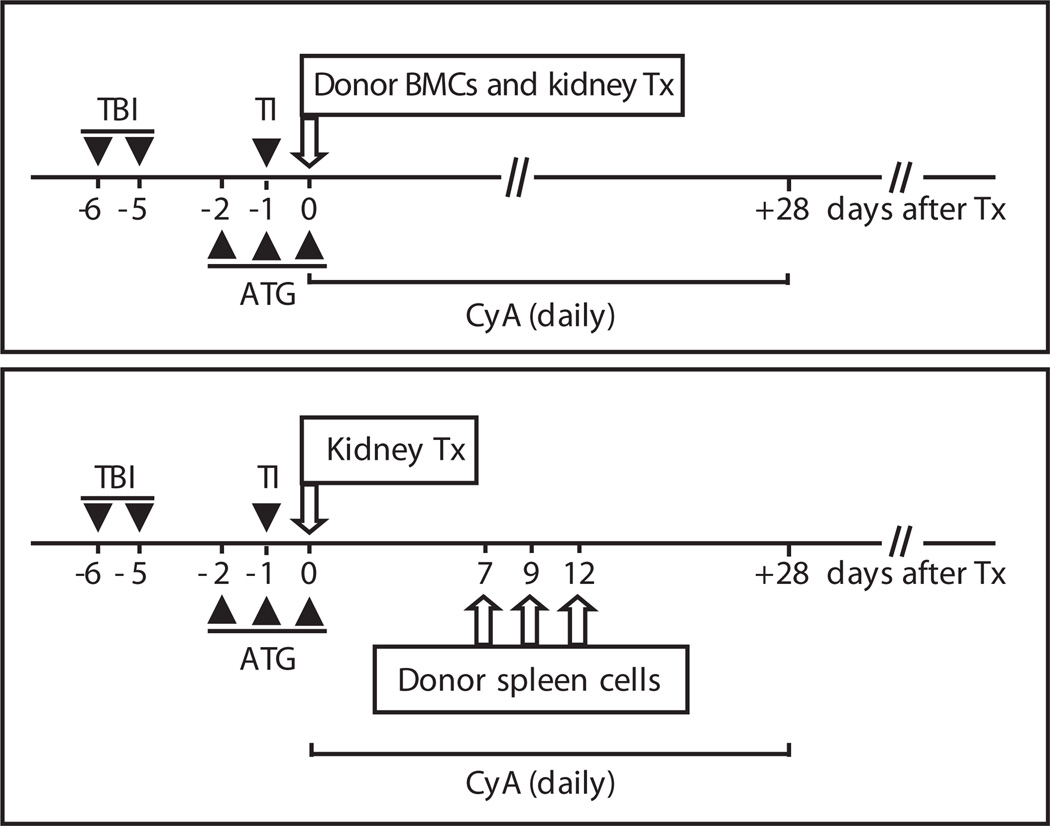
Fig. 2.
Conditioning and combined donor cell/kidney transplantation. The monkeys underwent total body irradiation (TBI) (1.5 Gy) at days −6 and −5 and thymic irradiation (TI) (7 Gy) at day −1. They also received ATG at days −2, −1, and 0 (50 mg/kg given intravenously). At day 0, they were transplanted with an allogeneic kidney and injected with bone marrow cells (BMCs) or spleen cells (3 × 108 to 4 × 108 cells/kg and 0.1 × 108 to 0.4 × 108 cells/kg, respectively) from the same donor, followed by a 28-day course of cyclosporine A (CYA) (15 mg/kg per day).
Table 1.
Memory T cell (TMEM) responses, chimerism, and kidney allograft rejection. The first two columns correspond to the identification numbers of the monkeys. The third column indicates the frequencies ± SD of donor-reactive TMEMs measured before transplantation in the recipient’s peripheral blood (number of anti-donor TMEMs producing IFN-γ per million TMEMs). The numbers correspond to the actual frequencies of donor-specific TMEMs. The fourth column (antibodies) indicates the presence of donor-specific antibodies (detection day indicated in parenthesis). The fifth column shows the duration of multilineage donor chimerism: 0 to 20 days after bone marrow transplantation (+), 20 to 50 days (++), and >50 days (+++). The last column shows the survival time of the kidney transplants (days) and the type of rejection (acute: acute rejection). nd, not determined.
| Recipient | Donor | IFN-γ TMEMs | Anti-donor antibodies | Chimerism | Rejection |
|---|---|---|---|---|---|
| 505 | 1505 | 70 ± 4 | +(d76) | + | Tolerant |
| 4507 | 4407 | 121 ± 9 | − | ++ | Tolerant |
| 4808 | 1708 | 218 ± 34 | − | + | Tolerant |
| 4109 | 4309 | 314 ± 18 | − | nd | Tolerant |
| 3305 | 3005 | 278 ± 28 | − | + | d140 (acute) |
| 1509 | 1609 | 1360 ± 106 | +(d82) | nd | d82 (acute) |
| 3205 | 1405 | 1125 ± 32 | +(d90) | + | d82 (acute) |
| 3405 | 2504 | 2422 ± 89 | +(d61) | + | d61 (acute) |
Homeostatic expansion of TMEMs in transplanted monkeys
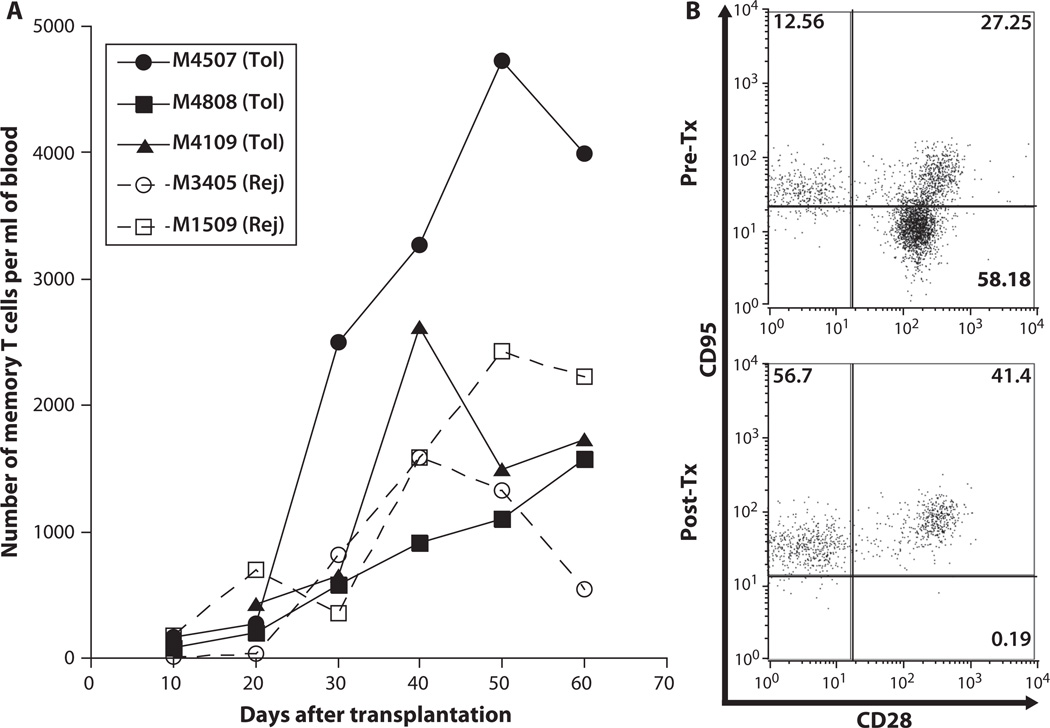
Homeostatic expansion of TMEMs found in vivo upon cell recovery after leukocyte depletion or irradiation has been associated with loss of chimerism and/or failure to achieve transplant tolerance in primates (20, 31). To test this, we compared the overall TMEM expansion in two monkeys that underwent acute rejection and three monkeys that became tolerant of their allograft. The homeostatic expansion of TMEMs was similar in the tolerant and rejecting monkeys (Fig. 3A). Actually, M4507, which belonged to the tolerant group, had the highest amount of TMEM expansion after transplantation. As shown in Fig. 3B, virtually all of the T cells replenishing the host after conditioning and transplantation displayed a surface memory phenotype (CD95+ T cells >98%) (32).
Fig. 3.
Expansion of TMEMs after conditioning and transplantation. (A) Kinetics of posttransplant expansion of TMEMs measured in the peripheral blood of two recipients that underwent acute rejection (Rej) (dotted lines, open symbols) and three tolerant (Tol) monkeys (solid lines and symbols). (B) T cells from the peripheral blood were isolated and stained with anti-CD95 APC (DX2), anti-CD28 PE (CD28.2), and anti-CD3 PerCP (SP34-2) mAbs and analyzed via cytofluorometry. This plot shows the distribution of CD95−CD28+ (lower right: naïve T cells), CD95+CD28+ (upper right: central TMEMs), and CD95+CD28− (upper left: effector TMEMs), gated on CD3+ T cells. This plot is representative of the six monkeys tested individually before transplantation (Pre-Tx, top panel) and 50 days after transplantation (Post-Tx, lower panel). The variation of the percentages of T cell subsets between monkeys is shown in fig. S1. The numbers shown in each quadrant indicate the percentages of each T cell subset among T cells.
Expansion/activation of donor-reactive TMEMs in transplanted monkeys
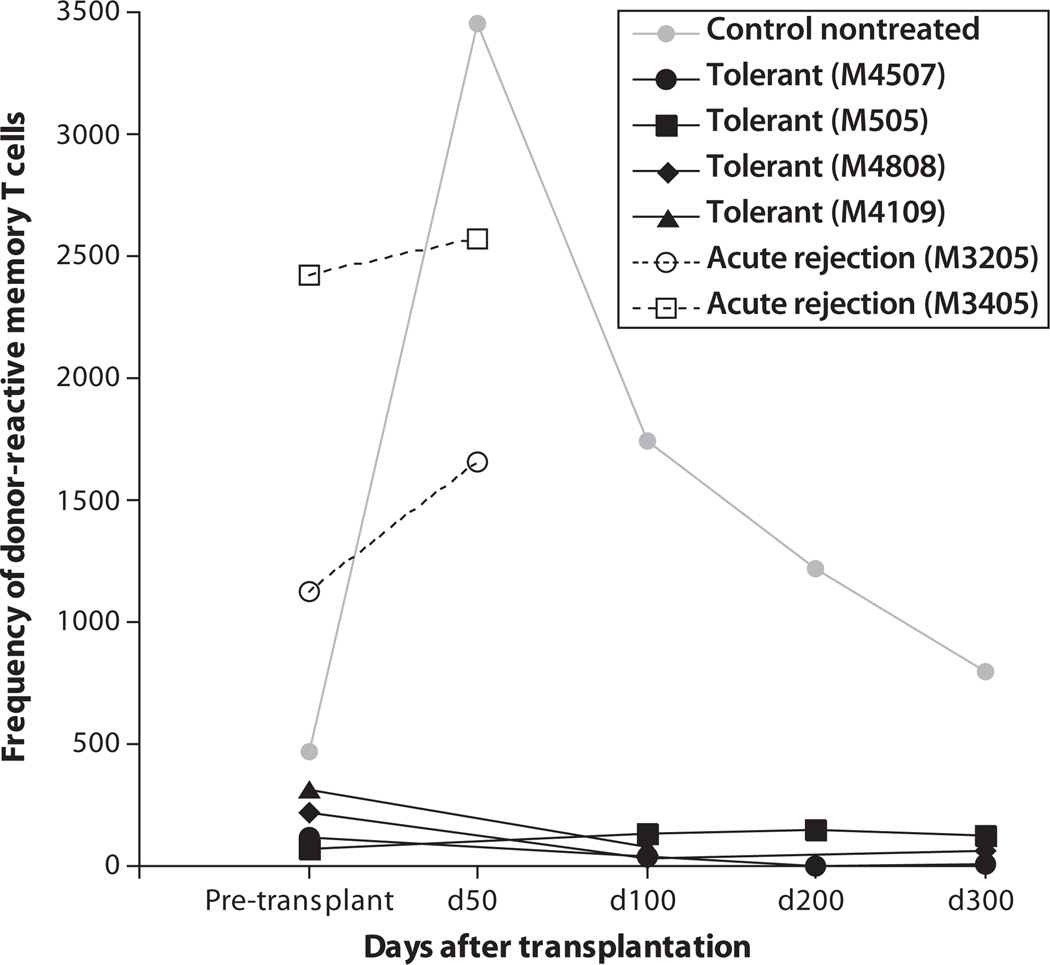
Next, we compared the kinetics and quantity of expansion of donor-reactive TMEMs activated after kidney transplantation in the four recipients displaying long-term transplant survival as well as two monkeys that rejected their kidney transplants in an acute fashion. First, the anamnestic donor-specific alloresponse was monitored in a control monkey that had received no treatment and rejected its kidney allograft within 6 days after transplantation. The pretransplant level of memory alloreactivity in this control monkey was intermediate (470 donor-specific IFN-γ–secreting cells per million TMEMs). After placement of the allogeneic kidney, we observed a massive expansion of donor-specific TMEMs that peaked at 50 days after transplantation (Fig. 4). The amount of memory alloreactivity was still high at 200 days after transplantation, long after rejection of the kidney allograft. At 300 days after transplantation, the frequency of donor-reactive TMEMs had almost returned to the pretransplantation level. In the rejecting monkeys (infused with donor cells) displaying a high memory alloreactivity before transplantation (M3205 and M3405), we observed some expansion of activated donor-specific TMEMs that secreted IFN-γ after transplantation (Fig. 4). It is noteworthy that the activation of a memory alloresponse in these monkeys was less pronounced than that observed in the control untreated monkey. This suggests that the immunosuppressive treatment had contained to some degree the posttransplant anamnestic alloresponse in the treated kidney recipients. In contrast, the tolerant monkeys displaying a low memory alloreactivity before transplantation showed no expansion of donor-specific TMEMs after transplantation (Fig. 4).
Fig. 4.
Posttransplant activation/expansion of donor-specific TMEMs. TMEMs were collected from the peripheral blood of recipients before transplantation and at different time points after combined bone marrow/kidney transplantation. The frequencies of IFN-γ–producing TMEMs recognizing donor MHC antigens directly were determined by ELISPOT. The results are expressed as numbers of cytokine-producing cells per million TMEMs. Direct memory alloresponses were assessed in four tolerant recipients (solid lines and dots) and two acutely rejecting monkeys (dotted lines and open symbols). In addition, memory alloreactivity was monitored in a control recipient, which received no immunosuppressive and conditioning treatment before transplantation and underwent acute rejection within 6 days after the placement of an allogeneic kidney (gray line and symbols).
DISCUSSION
A number of approaches including mixed chimerism and T cell costimulation blockade are known to regularly achieve tolerance to organ allotransplants in laboratory mice (4, 5, 7–9, 33). However, transplant tolerance cannot be accomplished in mice whose T cells have been exposed to alloantigens or viral infections (12, 13, 26). Moreover, such resistance to tolerance induction can be extended to naïve mice via adoptive transfer of TMEMs collected from allosensitized mice (12, 13, 26). Therefore, the high frequency of TMEMs present in wild-caught monkeys before transplantation but not in laboratory mice could explain why current strategies have failed to consistently accomplish transplant tolerance in primates.
Here, TMEMs capable of recognizing an allogeneic MHC directly were detected in all the monkeys before any treatment. Among the eight monkeys studied after combined allogeneic donor cell and kidney transplantation, we found a statistically significant correlation between the frequencies of anti-donor TMEMs in recipients and the rejection or tolerance of kidney allografts (P = 0.023).
The preferential homeostatic expansion of T cells displaying memory markers after irradiation and leukocyte depletion has been described in mice and primates (20, 31, 34). Here, we show that homeostatic expansion of TMEMs after transplantation can be observed in both the rejecting and the tolerant monkeys, whereas the frequencies of donor-specific TMEMs increased in only the rejecting recipients. It is likely that many TMEMs undergoing homeostatic expansion represent cells that only transiently acquire a memory phenotype and that rapidly revert to a naïve phenotype as previously described by Bevan’s group (35). These unconventional TMEMs display surface memory markers but are not functional TMEMs. Therefore, as previously suggested by another study in transplanted monkeys treated with anti-CD154 antibodies, homeostatic T cell activation may not represent a major barrier to transplant tolerance induction in primates (36).
The treatment with immunosuppressive antibodies and calcineurin inhibitors failed to prevent the activation of alloreactive T cells only in the recipients with high pretransplant levels of donor-reactive TMEMs. It remains to be determined whether these TMEMs are responsible for the loss of donor hematopoietic chimerism in transplanted monkeys. These data imply that in tolerant monkeys, some mechanisms such as clonal deletion and/or T cell regulation had selectively impaired the expansion and activation of donor-reactive but not other TMEMs. It is important to note that although these tolerant monkeys display initially high levels of chimerism, they rapidly lose their donor hematopoietic chimerism despite the apparent lack of anti-donor T cell alloreactivity (11, 30, 37). These data suggest that failure to maintain durable mixed chimerism in these monkeys is not caused solely by the activation of some donor-specific TMEMs after transplantation.
In summary, our results reinforce the view that alloreactive TMEMs present in primates before transplantation prevent tolerance induction to allografts. However, our study shows that this is not an absolute rule in that a recipient displaying a low-level anamnestic reactivity against a particular donor can be successfully tolerized using our mixed chimerism protocol. We have recently reported that the amount of memory alloreactivity is extremely variable depending on the nature of the responder/stimulator pair considered (32). MHC matching between potential transplant recipients and donors was generally associated with a low anamnestic alloresponse (32). However, the contrary was not true because a significant number of individuals displayed a low memory reactivity to fully MHC gene disparate allogeneic monkeys (32). The mixed hematopoietic chimerism strategy has actually been successful in achieving tolerance in patients recipient of a fully or haplo-matched allogeneic kidney transplant (38, 39). The amounts of memory alloreactivity in these patients remain to be investigated. Our results underscore the need suggested by recent studies from Kirk’s group (25, 40, 41) for the development of immune therapies capable of blocking or eliminating these TMEMs before tolerance induction in primates. Alternatively, our results suggest that selection of donor/recipient pairs with low TMEM alloreactivity may be sufficient to resolve this problem and accomplish reliably tolerance induction to allogeneic organ and tissue transplants in patients.
MATERIALS AND METHODS
Animals
Male cynomolgus monkeys weighing 3 to 5 kg were used (Charles River Laboratories). Details of recipient/donor pair selection, host conditioning protocols, as well as clinical outcomes were previously reported (30).
Bone marrow and kidney transplantation
The basic elements of our regimen are summarized in Fig. 2. In brief, these included total body irradiation [1.5 gray (Gy)], local thymic irradiation (7 Gy), intravenous anti-thymocyte globulin (ATG) (Atgam, Pharmacia and Upjohn) (50 mg/kg per day), or thymoglobulin (20 mg/kg, Genzyme) and kidney along with bone marrow (M505, M4507, M4808, M3305, M3205, and M3405) (3 × 108 to 4 × 108 cells/kg at day 0) or splenocyte (M4109 and M1509) (three injections of 0.1 × 108 to 0.4 × 108 cells at days 7, 9, and 12 after kidney transplantation) cell infusion (from the same donor), followed by a 1-month course of cyclosporine A (Novartis) (tapered from an initial dose of 15 mg/kg per day) to maintain therapeutic serum levels (>300 ng/ml). Cyclosporine A was discontinued on day 28 after transplant. Monkeys underwent heterotopic renal transplantation and bilateral nephrectomies under ketamine hydrochloride/diazepam anesthesia. In recipients treated with anti-CD154 monoclonal antibodies (mAbs) (h5C8, 20 mg/kg at days 0 and 2 and 10 mg/kg at days 5, 7, 9, and 12), ketorolac (1 mg/kg on days −1 and 0) was administered to prevent thrombosis, as previously described (42). In addition to this treatment, M4808 was treated with anti-CD20 mAbs (rituximab, 10 mg/kg given at days −14, −7, and 0). In the control monkey, which had received no immunosuppressive treatment, at the time of transplantation, one kidney had been left in situ with ureter ligated. At the time of rejection (day 6), continuity of the ureter’s native kidney was reestablished to allow continued study of the animal.
Flow cytometric analyses
Cell surface antigens were analyzed by multicolor flow cytometry. Peripheral blood mononuclear cells (PBMCs) were directly labeled with a combination of the following mAbs: CD3 PerCP (peridinin chlorophyll protein) (SP 34-2), CD4 PerCP (L-200), CD8 PerCP (RPA-T8), CD8 APC (allophycocyanin) (RPA-T8), CD95 FITC (fluorescein isothiocyanate) (DX2), CD95 APC (DX2), and CD28 PE (phycoerythrin) (CD28.2). All antibodies were purchased from BD Pharmingen. The fluorescence of the stained samples was analyzed with FACSCalibur and FACScan flow cytometers and CellQuest software (BD Immunocytometry Systems). Lymphocytes were gated on the forward and side light scatter, and 3000 to 5000 events were collected.
Isolation of TMEMs
PBMCs were incubated with mAbs anti-CD95 FITC (DX2) and anti-CD28 PE (CD28.2) for 15 min at +4°C, washed once, and resuspended in sort buffer [phosphate-buffered saline (PBS), 2% fetal calf serum (FCS)]. Cells were gated on lymphocytes and sorted into CD95−CD28+ naïve and CD95+CD28low/high memory populations with a FACSVantage cell sorter (BD Immunocytometry Systems) (32). The purity of sorted cells was consistently >95%.
Measurement of direct alloresponses by ELISPOT
ELISPOT plates (Millipore) were precoated with capture antibodies (5 µg/ml) against type IFN-γ cytokine (Mabtech) in PBS and stored overnight at 4°C. The plates were blocked for 1 hour with PBS containing 1% bovine serum albumin (BSA) (fraction V) (A1933, Sigma) followed by three washes in PBS. Responding cells (150 × 103) were added to each well of a 96-well ELISPOT plate in 100 µl of complete RPMI 1640 (Mediatech, Cellgro) supplemented with 10% pooled naïve monkey serum and l-glutamine, penicillin/streptomycin, and Hepes buffer (Invitrogen). The responding cells were cocultured with an equal number of irradiated stimulating allogeneic cells (150 × 103 cells per well) (43), or unstimulated in medium alone, or with phytohemagglutinin (PHA) at 1 µg/ml (Sigma). After a 48-hour incubation at 37°C, the plates were washed three times in PBS followed by another three washes in PBS/Tween (Sigma). Biotinylated detection antibodies (Mabtech) were added, and the plates were maintained at 4°C for an additional overnight incubation. After four washes with PBS/Tween, streptavidin–horseradish peroxidase conjugate in PBS/BSA (Dako #PO397) was added for at least 2 hours at room temperature, followed by an additional six washes. The development was performed with aminoethylcarbazole (10 mg/ml in N,N-dimethylformamide; D4254, Sigma) freshly prepared in 0.1 M sodium acetate buffer (pH 5) mixed with 30% H202. The resulting spots were counted with a computer-assisted ELISPOT image analyzer (CTL Inc.). The results of duplicate values were averaged (mean ± SD) and expressed as the frequency of cytokine-producing T cells per million of T cells plated minus medium.
Statistics
Statistical analyses were performed with StatView software (Abacus Concepts Inc.). P values were calculated with one-tailed t test assuming equal variance. A P value of <0.05 was considered statistically significant.
Pathology studies
Wedge renal biopsies were obtained whenever a rise in creatinine occurred and at 2- to 6-month intervals in animals with stable function (44). Biopsy, nephrectomy, and autopsy sections were stained with hematoxylin-eosin and periodic acid–Schiff stains and scored in coded samples with Banff 2003 and Collaborative Clinical Trials in Transplantation (CCTT) criteria (45, 46).
Acknowledgments
Funding: This work was supported by grants from the Massachusetts General Hospital Executive Committee on Research and National Institute of Allergy and Infectious Diseases grant U19 AI066705 to G.B., NIH grants 19 DK080652 and HL018446 to A.B.C., and NIH grants U191066705 and PO1HL18646 to J.C.M.
Footnotes
SUPPLEMENTARY MATERIAL
www.sciencetranslationalmedicine.org/cgi/content/full/3/86/86ra51/DC1
Fig. S1. Percentages of naïve and memory T cell subsets before and after transplantation.
Author contributions: All authors designed the study. O.N. and T.M. performed and analyzed most of the experiments, participated in their design, and analyzed the data. S.B., D.H.S., J.A., J.C.M., T.K., and A.B.C. performed the kidney transplantations and participated in the design of some of the experiments. R.B.C. and R.-N.S. performed histological evaluation of kidney transplants and provided critical suggestions and discussions. G.T. helped with statistical analyses, participated in the design of some of the experiments, and provided critical suggestions and discussions. G.B. conceived and supervised this study, was involved in the design and evaluation of all experiments, and wrote the manuscript with comments from coauthors.
Competing interests: The authors declare that they have no competing interests.
REFERENCES
- 1.Sykes M, Sachs DH. Mixed allogeneic chimerism as an approach to transplantation tolerance. Immunol. Today. 1988;9:23–27. doi: 10.1016/0167-5699(88)91352-7. [DOI] [PubMed] [Google Scholar]
- 2.Wekerle T, Sykes M. Mixed chimerism as an approach for the induction of transplantation tolerance. Transplantation. 1999;68:459–467. doi: 10.1097/00007890-199908270-00001. [DOI] [PubMed] [Google Scholar]
- 3.Sykes M, Sachs DH. Bone marrow transplantation as a means of inducing tolerance. Semin. Immunol. 1990;2:401–417. [PubMed] [Google Scholar]
- 4.Pearson TC, Alexander DZ, Hendrix R, Elwood ET, Linsley PS, Winn KJ, Larsen CP. CTLA4-Ig plus bone marrow induces long-term allograft survival and donor specific unresponsiveness in the murine model. Evidence for hematopoietic chimerism. Transplantation. 1996;61:997–1004. doi: 10.1097/00007890-199604150-00002. [DOI] [PubMed] [Google Scholar]
- 5.Lakkis FG, Konieczny BT, Saleem S, Baddoura FK, Linsley PS, Alexander DZ, Lowry RP, Pearson TC, Larsen CP. Blocking the CD28-B7 T cell costimulation pathway induces long term cardiac allograft acceptance in the absence of IL-4. J. Immunol. 1997;158:2443–2448. [PubMed] [Google Scholar]
- 6.Larsen CP, Pearson TC. The CD40 pathway in allograft rejection, acceptance, and tolerance. Curr. Opin. Immunol. 1997;9:641–647. doi: 10.1016/s0952-7915(97)80043-x. [DOI] [PubMed] [Google Scholar]
- 7.Malm H, Corbascio M, Osterholm C, Cowan S, Larsen CP, Pearson TC, Ekberg H. CTLA4Ig induces long-term graft survival of allogeneic skin grafts and totally inhibits T-cell proliferation in LFA-1-deficient mice. Transplantation. 2002;73:293–297. doi: 10.1097/00007890-200201270-00024. [DOI] [PubMed] [Google Scholar]
- 8.Shirasugi N, Adams AB, Durham MM, Lukacher AE, Xu H, Rees P, Cowan SR, Williams MA, Pearson TC, Larsen CP. Prevention of chronic rejection in murine cardiac allografts: A comparison of chimerism- and nonchimerism-inducing costimulation blockade-based tolerance induction regimens. J. Immunol. 2002;169:2677–2684. doi: 10.4049/jimmunol.169.5.2677. [DOI] [PubMed] [Google Scholar]
- 9.Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4-Ig. Transplantation. 1994;57:1701–1706. [PubMed] [Google Scholar]
- 10.Kean LS, Gangappa S, Pearson TC, Larsen CP. Transplant tolerance in non-human primates: Progress, current challenges and unmet needs. Am. J. Transplant. 2006;6:884–893. doi: 10.1111/j.1600-6143.2006.01260.x. [DOI] [PubMed] [Google Scholar]
- 11.Ochiai T, Benichou G, Cosimi AB, Kawai T. Induction of allograft tolerance in nonhuman primates and humans. Front. Biosci. 2007;12:4248–4253. doi: 10.2741/2384. [DOI] [PubMed] [Google Scholar]
- 12.Adams AB, Pearson TC, Larsen CP. Heterologous immunity: An overlooked barrier to tolerance. Immunol. Rev. 2003;196:147–160. doi: 10.1046/j.1600-065x.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 13.Lakkis FG, Sayegh MH. Memory T cells: A hurdle to immunologic tolerance. J. Am. Soc. Nephrol. 2003;14:2402–2410. doi: 10.1097/01.asn.0000085020.78117.70. [DOI] [PubMed] [Google Scholar]
- 14.Valujskikh A, Lakkis FG. In remembrance of things past: Memory T cells and transplant rejection. Immunol. Rev. 2003;196:65–74. doi: 10.1046/j.1600-065x.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 15.Williams MA, Onami TM, Adams AB, Durham MM, Pearson TC, Ahmed R, Larsen CP. Cutting edge: Persistent viral infection prevents tolerance induction and escapes immune control following CD28/CD40 blockade-based regimen. J. Immunol. 2002;169:5387–5391. doi: 10.4049/jimmunol.169.10.5387. [DOI] [PubMed] [Google Scholar]
- 16.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J. Immunol. 2002;169:3686–3693. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 17.Brehm MA, Markees TG, Daniels KA, Greiner DL, Rossini AA, Welsh RM. Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections. J. Immunol. 2003;170:4077–4086. doi: 10.4049/jimmunol.170.8.4077. [DOI] [PubMed] [Google Scholar]
- 18.Burrows SR, Silins SL, Khanna R, Burrows JM, Rischmueller M, McCluskey J, Moss DJ. Cross-reactive memory T cells for Epstein-Barr virus augment the alloresponse to common human leukocyte antigens: Degenerate recognition of major histocompatibility complex-bound peptide by T cells and its role in alloreactivity. Eur. J. Immunol. 1997;27:1726–1736. doi: 10.1002/eji.1830270720. [DOI] [PubMed] [Google Scholar]
- 19.Bingaman AW, Farber DL. Memory T cells in transplantation: Generation, function, and potential role in rejection. Am. J. Transplant. 2004;4:846–852. doi: 10.1111/j.1600-6143.2004.00453.x. [DOI] [PubMed] [Google Scholar]
- 20.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, Markmann JF, Kassaee A, Rosengard BR, Hancock WW, Sayegh MH, Turka LA. Homeostatic proliferation is a barrier to transplantation tolerance. Nat. Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 22.Swain SL, Agrewala JN, Brown DM, Román E. Regulation of memory CD4 T cells: Generation, localization and persistence. Adv. Exp. Med. Biol. 2002;512:113–120. [PubMed] [Google Scholar]
- 23.Lefrançois L. Development, trafficking, and function of memory T-cell subsets. Immunol. Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 24.Kalia V, Sarkar S, Gourley TS, Rouse BT, Ahmed R. Differentiation of memory B and T cells. Curr. Opin. Immunol. 2006;18:255–264. doi: 10.1016/j.coi.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Page AJ, Ford ML, Kirk AD. Memory T-cell-specific therapeutics in organ transplantation. Curr. Opin. Organ Transplant. 2009;14:643–649. doi: 10.1097/MOT.0b013e328332bd4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am. J. Transplant. 2002;2:501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 27.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, Pearson TC, Ahmed R, Larsen CP. Heterologous immunity provides a potent barrier to transplantation tolerance. J. Clin. Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams MA, Tan JT, Adams AB, Durham MM, Shirasugi N, Whitmire JK, Harrington LE, Ahmed R, Pearson TC, Larsen CP. Characterization of virus-mediated inhibition of mixed chimerism and allospecific tolerance. J. Immunol. 2001;167:4987–4995. doi: 10.4049/jimmunol.167.9.4987. [DOI] [PubMed] [Google Scholar]
- 29.Valujskikh A. The challenge of inhibiting alloreactive T-cell memory. Am. J. Transplant. 2006;6:647–651. doi: 10.1111/j.1600-6143.2005.01215.x. [DOI] [PubMed] [Google Scholar]
- 30.Kawai T, Sogawa H, Boskovic S, Abrahamian G, Smith RN, Wee SL, Andrews D, Nadazdin O, Koyama I, Sykes M, Winn HJ, Colvin RB, Sachs DH, Cosimi AB. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am. J. Transplant. 2004;4:1391–1398. doi: 10.1111/j.1600-6143.2004.00523.x. [DOI] [PubMed] [Google Scholar]
- 31.Stockinger B, Kassiotis G, Bourgeois C. Homeostasis and T cell regulation. Curr. Opin. Immunol. 2004;16:775–779. doi: 10.1016/j.coi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Nadazdin O, Boskovic S, Murakami T, O’Connor DH, Wiseman RW, Karl JA, Tuscher JJ, Sachs DH, Madsen JC, Tocco G, Kawai T, Cosimi AB, Benichou G. Phenotype, distribution and alloreactive properties of memory T cells from cynomolgus monkeys. Am. J. Transplant. 2010;10:1375–1384. doi: 10.1111/j.1600-6143.2010.03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elwood ET, Larsen CP, Maurer DH, Routenberg KL, Neylan JF, Whelchel JD, O’Brien DP, Pearson TC. Microchimerism and rejection in clinical transplantation. Lancet. 1997;349:1358–1360. doi: 10.1016/s0140-6736(96)09105-2. [DOI] [PubMed] [Google Scholar]
- 34.Bourgeois C, Stockinger B. T cell homeostasis in steady state and lymphopenic conditions. Immunol. Lett. 2006;107:89–92. doi: 10.1016/j.imlet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J. Exp. Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearl JP, Xu H, Leopardi F, Preston E, Kirk AD. CD154 blockade, sirolimus, and donor-specific transfusion prevents renal allograft rejection in cynomolgus monkeys despite homeostatic T-cell activation. Transplantation. 2007;83:1219–1225. doi: 10.1097/01.tp.0000259929.04596.d5. [DOI] [PubMed] [Google Scholar]
- 37.Murakami T, Cosimi AB, Kawai T. Mixed chimerism to induce tolerance: Lessons learned from nonhuman primates. Transplant. Rev. 2009;23:19–24. doi: 10.1016/j.trre.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fudaba Y, Spitzer TR, Shaffer J, Kawai T, Fehr T, Delmonico F, Preffer F, Tolkoff-Rubin N, Dey BR, Saidman SL, Kraus A, Bonnefoix T, McAfee S, Power K, Kattleman K, Colvin RB, Sachs DH, Cosimi AB, Sykes M. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: In vivo and in vitro analyses. Am. J. Transplant. 2006;6:2121–2133. doi: 10.1111/j.1600-6143.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 39.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DS, Hertl M, Goes NB, Wong W, Williams WW, Jr, Colvin RB, Sykes M, Sachs DH. HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weaver TA, Charafeddine AH, Agarwal A, Turner AP, Russell M, Leopardi FV, Kampen RL, Stempora L, Song M, Larsen CP, Kirk AD. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat. Med. 2009;15:746–749. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo DJ, Weaver TA, Stempora L, Mehta AK, Ford ML, Larsen CP, Kirk AD. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. Am. J. Transplant. 2011;11:22–33. doi: 10.1111/j.1600-6143.2010.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat. Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 43.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J. Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
- 44.Smith RN, Kawai T, Boskovic S, Nadazdin O, Sachs DH, Cosimi AB, Colvin RB. Four stages and lack of stable accommodation in chronic alloantibody-mediated renal allograft rejection in cynomolgus monkeys. Am. J. Transplant. 2008;8:1662–1672. doi: 10.1111/j.1600-6143.2008.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K. Antibody-mediated rejection criteria—an addition to the Banff 97 classification of renal allograft rejection. Am. J. Transplant. 2003;3:708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 46.Colvin RB, Cohen AH, Saiontz C, Bonsib S, Buick M, Burke B, Carter S, Cavallo T, Haas M, Lindblad A, Manivel JC, Nast CC, Salomon D, Weaver C, Weiss M. Evaluation of pathologic criteria for acute renal allograft rejection: Reproducibility, sensitivity, and clinical correlation. J. Am. Soc. Nephrol. 1997;8:1930–1941. doi: 10.1681/ASN.V8121930. [DOI] [PubMed] [Google Scholar]