Abstract
Emerging evidence indicates that a small population of cancer cells is highly tumorigenic, endowed with self-renewal, and has the ability to differentiate into cells that constitute the bulk of tumors. These cells are considered the “drivers” of the tumorigenic process in some tumor types, and have been named cancer stem cells. Epithelial-mesenchymal transition (EMT) appears to be involved in the process leading to the acquisition of stemness by epithelial tumor cells. Through this process, cells acquire an invasive phenotype that may contribute to tumor recurrence and metastasis. Cancer stem cells have been identified in human head and neck squamous cell carcinomas (HNSCC) using markers such as CD133 and CD44 expression, and aldehyde dehydrogenase (ALDH) activity. The head and neck cancer stem cells reside primarily in perivascular niches in the invasive front where endothelial-cell initiated events contribute to their survival and function. In this review, we discuss the state-of-the-knowledge on the pathobiology of cancer stem cells, with a focus on the impact of these cells to head and neck tumor progression.
Keywords: Oral cancer, Tumorigenesis, Epithelial-mesenchymal transition, EMT, Self-renewal, Stemness, Perivascular niche, Squamous cell carcinoma, Angiogenesis
Introduction
Head and neck cancer is a major health problem throughout the world. In 2008, 263 900 new cases of head and neck cancer were diagnosed, and 128 000 deaths related to this malignancy have occurred worldwide.1 In the United States alone, there were 49 260 new cases and 11 480 deaths that were attributed to head and neck cancer in 2010.2 The standard of care for patients with head and neck squamous cell carcinomas (HNSCC) includes platinum-based chemotherapeutic drugs, surgery, and radiotherapy.3 However, the 5-year survival rate for these patients has remained in the range 50–60% for the last 3 decades.4 It is becoming increasingly evident that an improvement in the survival of head and neck cancer patients will require deeper understanding of the mechanisms underlying the initial steps of the tumorigenic process, as well as the strategies employed by cancer cells to disseminate to local lymph nodes and distant sites. Recent studies on the pathobiology of HNSCC have led to the discovery of a small population of cancer cells that is highly tumorigenic, capable of self-renewal, and behave as tumor progenitor cells.5 Such behavior is consistent with the features of cancer stem cells (CSC). Notably, cancer stem cells appear to play a major role in tumor recurrence and metastatic spread, common causes of the high morbidity and ultimately the death of the majority of patients with HNSCC. Therefore, targeted elimination of these cancer stem cells has been considered a new conceptual framework for head and neck cancer treatment. This review discusses the putative role of stem cells in tumorigenesis, the biological process that leads to the acquisition of stem cell properties, and the potential impact of the cancer stem cell hypothesis to the management of patients with head and neck cancer.
Cancer stem cells
According to the developmental status, physiological stem cells can be classified as embryonic or adult stem cells. Embryonic stem cells are derived from the inner mass of the mammalian blastocyst, have the ability to differentiate into cells of all three germ layers, and develop to all tissues and organs of the organism.6,7 In contrast, adult stem cells are undifferentiated cells with more limited self renewal and a differentiation potential that is more restricted to cell types of the tissue from where they are found. Adult stem cells play a major role in tissue homeostasis and regeneration. Stem cells also play a major role in the biology of several diseases, including cancer.8,9 Cancer stem cells are functionally defined as a subset of tumor cells that exhibit the ability of self-renewal and multipotency, serving as progenitor cancer cells.9,10 In low attachment culture conditions, cancer stem-like cells tend to form spheroids, named orospheres (Figure 1). At least two different hypotheses have been proposed to explain the heterogeneity of tumor-initiating capacity of tumor cells, the cancer stem cell hypothesis9,11 and the clonal evolution hypothesis.12,13
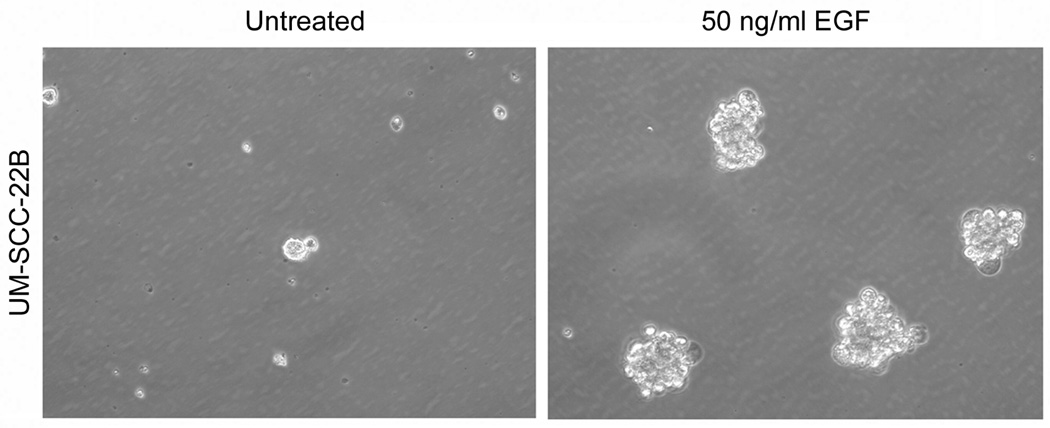
Figure 1.
Orosphere assay to study the acquisition of a cancer stem-like phenotype in vitro. UM-SCC-22B is a cell line derived from the metastatic lymph node of a patient with HNSCC in the hypopharynx. We have recently reported that UM-SCC-22B contains a sub-population of cells that exhibit cancer stem-like characteristics.160 Photomicrographs (200x) of UM-SCC-22B cells cultured with serum-free medium in ultra-low attachment plates in presence of 0 or 50 ng/ml EGF for 5 days. The formation of spheroid-like colonies containing at least 25 cells (named orospheres) was enhanced by EGF treatment. Growth of carcinoma cells in suspended spheres under low (or no serum) culture conditions has been considered indicative of acquisition of a stem-like phenotype in vitro.
Nowell proposed the clonal evolution hypothesis in 1976, stating that most neoplasms arise from a single cell, and that tumor progression results from acquired genetic variability within the original clone allowing sequential selection of more aggressive sub-lines.13 Tumor cell populations are apparently more genetically unstable than normal cells. Fearon and Vogelstein proposed a clonal evolution model for colon cancer, in which the progression from early adenoma to invasive carcinoma reflects the stepwise acquisition of mutations in specific cancer genes.14
Dick and collaborators provided early evidence for cancer stem cells using leukemia models. 10,11 They induced leukemia by transplanting human acute myeloid leukemia (AML) cells into non-obese diabetic severe combined immunodeficient (NOD/SCID) mice, and showed that primarily CD34+CD38- cells, but not CD34+CD38+ or CD34- cells, initiated leukemia. In addition, they showed that these progenitor cells could be serially transplanted into second recipients. Of note, serial transplantation in vivo has become accepted as an important criterion for the definition of cancer stem cells, and has been used experimentally as a means to propagate cells in an undifferentiated state. The Clarke laboratory unveiled the presence of cancer stem cells in solid tumors, i.e. breast cancer.8 In xenograft experiments, breast cancer cells sorted for CD44 and CD24 were transplanted into the mammary pads of NOD/SCID mice. These investigators observed that only the CD44+CD24- fraction initiated tumors, whereas 100-fold more CD44+CD24+ or CD44- cells did not. They did not find obvious morphologic and immunophenotypic distinctions between the tumorigenic and non-tumorigenic breast cancer cells. Notably, the CD44+DC24- cells showed evidence of self-renewal in serial transplantation studies. Since then, cancer stem cells were found in several other cancers, including head and neck,5 brain,15,16 lung,17,18 prostate,19 colorectal, 20,21 pancreas,22 liver,23 and melanoma.24
It is important to point out that the cancer stem cell hypothesis has been challenged by findings in some tumor types, as for example melanoma. The Morrison research group demonstrated that 25% of unselected melanoma cells are able to create tumors in immunodeficient mice, which is consistent with the stochastic tumorigenesis model.25 However, evidence to the contrary has also been seen. CXCR6 discriminated high tumorigenic from low-tumorigenic cells in melanoma models.26 In an independent study, CD271+ melanoma cells generated more tumors than CD271- cells.27 In head and neck cancer, several lines of evidence point to the function of a small group of cells with distinct tumorigenic potential. Seminal work by the Prince and colleagues revealed that CD44 expression discriminates a sub-population of progenitor cells.5 In a follow-up study, the Prince laboratory showed that aldehyde dehydrogenase (ALDH) activity also distinguishes a small group of highly tumorigenic cells.28 The ability to identify cancer stem cells was further enhanced by the combined use of both markers (ALDH and CD44) that revealed that 1–3% of the cells from primary human HNSCC are uniquely capable of generating tumors.29 Collectively, these studies suggest that the role of progenitor (stem-like) cells in the tumorigenic process is tumor-type and context dependent.
Development and cancer stem cells
In development, a highly orchestrated and hierarchical process is observed in which a stem cell progressively looses multipotency giving rise to restricted progenitor cells, which in turn differentiate into the cells that constitute the bulk of tissue or organ. In cancer, the cell of origin is the cell that receives the first oncogenic hit(s). A candidate cell of origin is the stem cell, which has the inherent potential of self-renewal and longevity, and therefore is more susceptible to acquired genetic or epigenetic changes that result in transformation. On the other hand, it is not clear if cancer stem cells originate solely from the transformation of normal stem cells. Cancer stem cells may also arise from restricted progenitors or differentiated cells that have acquired self-renewal properties as a consequence of genetic or epigenetic alterations.30 The plasticity of this system is exemplified by the observation that stem cells can derive from reprogramming of differentiated or somatic cells.31–33 In 2006, Takahashi and Yamanaka showed that Oct3/4, Sox2, c-Myc and Kif4 induce pluripotency in fibroblasts, generating “induced pluripotent stem (iPS)” cells.34 Transplantation of iPS cells into nude mice generates tumors that contain cells from all of the three germ layers. The same group also generated iPS cells from adult human fibroblasts.35 The Thomson research group showed that Oct4, Sox2, Nanog and Lin28 are sufficient to reprogram human somatic cells to pluripotent stem cells that exhibit the essential characteristics of embryonic stem cells.36 Of note, one of the hallmarks of cancer is the marked phenotypic, functional, proliferative and genetic heterogeneity of the cells.30,37 This suggests that the cell of origin is capable of generating a highly heterogeneous progeny.
It is becoming increasingly evident that the same pathways that are critical for physiological development also play a role in the early stages of tumorigenesis. For example, Wnt signaling is critical for embryonic development and controls homeostatic self-renewal.38 On the other hand, somatic mutations of the Wnt pathway are associated with the etiology of several tumors, including intestinal cancer.38,39 Mutations in adenomatous polyposis coli (APC) in crypt stem cells have been clearly associated with neoplastic transformation.40 Barker and colleagues identified that Lgr5-cells located at crypt bottom as stem cells that function as cells-of-origin of intestinal cancer.40,41 Another example is the transcription factor Sox2, which is essential to maintain the pluripotent phenotype in embryonic stem cells.42 However, Sox2 efficiently generates iPS cells 34,36 and is amplified in lung and esophageal squamous cancers.43,44 And finally, it is clear that the Notch1 signaling pathway plays a major role in embryogenesis, as demonstrated by the observation that homozygous mutant embryos died before 11.5 days of gestation.45 Conversely, Notch signaling is required for the generation and self-renewal of cancer stem cells in several tumor types, including colon cancer.46 Interestingly, it has been recently demonstrated that Notch1 mutations are frequently found in HNSCC,47,48 suggesting a potential role for this pathway in the biology of cancer stem cells and in the etiology of head and neck cancer. Collectively, these studies suggest that there are important lessons to be learned from developmental studies that could help identifying processes that result in the malignant transformation of epithelial cells and head and neck cancer initiation.
EMT and cancer stem cells
Epithelial-mesenchymal transition (EMT) is the process that allows a polarized epithelial cell to assume a mesenchymal cell phenotype, which is characterized by enhanced motility and invasiveness.49 EMT plays a critical role in embryogenesis, and is involved in several pathologies, including fibrosis49 and cancer.50–53 An example of this process in physiological settings is the ovarian epithelium that undergoes an EMT-like process during postovulatory wound healing. In this case, EMT is induced by epidermal growth factor (EGF) and involves the activation of metalloproteases and ERK.54 Key features of EMT are summarized in Table 1.
Table 1.
Characteristics of normal epithelial and mesenchymal cells
| Epithelial cells | Mesenchymal cells | |
|---|---|---|
| Morphology | Cobblestone | Elongated |
| polarized | Non Polarized | |
| Behavior | Non-motile | Migratory |
| Non-invasive | Invasive | |
| Markers | E-cadherin | Vimentin |
| Desmoplakin | N cadherin | |
| Cytokeratin | Snail | |
A critical step in EMT is the loss of cell polarity. Three protein complexes (Par, Crumbs, Scribble) participate in establishing and maintaining apico-basal polarity in epithelial cells.55 Snail alters epithelial cell polarity by repressing the transcription of Crumbs3 and abolishing the localization of both Par and Crumbs complexes at the junctions.56 Another hallmark of EMT is the loss of E-cadherin, which appears to be correlated with tumor progression. The loss of E-cadherin is considered a crucial step in the progression of papilloma to invasive carcinoma,57 and is regulated by a number of transcription factors such as Snail,58,59 Twist,60 and ZEB1.61 The transcription factor Snail controls EMT by repressing E-cadherin expression.62 Increased Twist expression is found in metastatic breast cancer and is required for EMT and breast cancer metastasis.60 Importantly, tumors undergoing EMT acquire resistance to chemotherapy.63–65 Colorectal cancer-derived epithelial cell lines expressing EMT markers exhibit mesenchymal morphology and resistance to oxaliplatin.63 Twist mediates EMT in breast cancer cells and enhances resistance to paclitaxel.64 Notably, the deletion of Twist can partially reverse multidrug resistance in breast cancer cells.65 These data show that the acquisition of a mesenchymal phenotype correlates with increased invasiveness of tumor cells, leading to recurrence/metastasis and poor clinical prognosis.
Recent reports have suggested that EMT is involved in the acquisition of cancer stem cell properties.59,66–69 In a seminal publication, the Weinberg research group showed that human mammary epithelial cells undergoing EMT acquire stem cell properties, as demonstrated by the ability of CD4highCD24low cells to form mammospheres in vitro and tumors in vivo. CD44+CD24−/low cells possessing cancer stem-like properties can be generated from CD44lowCD24+ non-tumorigenic mammary epithelial cells through activation of the Ras/MAPK signaling pathway and induction of EMT.69 Furthermore, in nasopharyngeal carcinomas, miR200a regulates EMT and induction of stem-like characteristics by targeting E-cadherin repressor ZEB2 via β-catenin signaling.70 It induces stem-like traits, including CD133+ side population, sphere formation capacity, increased Oct4 and ALDH expression in tumor spheres and tumor tissues, and tumorigenicity in vivo.70
In head and neck cancer, Twist1 induces Bmi-1 (B-cell specific Moloney murine leukemia virus insertion site 1), which in turn downregulates E-cadherin. Bmi-1 has an essential role in the regulation of self-renewal of stem cells.71–74 Patients with high Twist1 and Bmi-1 tend have worst prognosis.75 Upregulation of Bmi-1 induced EMT and enhanced the motility and invasiveness of human nasopharyngeal cancer cells, whereas silencing endogenous Bmi-1 reversed EMT and reduced motility.76 Bmi-1 transcriptionally downregulated expression of tumor suppressor PTEN via direct association with PTEN locus, ablation of PTEN expression partially rescued the migratory/invasive phenotype of Bmi-1 silenced cells.76
It has been reported that hypoxia or overexpression of HIF-1a induces EMT and metastasis in head and neck cancer cells.77 HIF-1α regulates the expression of Twist by binding to the hypoxia-response element (HRE). Notably, siRNA-mediated repression of Twist in hypoxia or HIF1-α overexpression reversed EMT and metastasis.77 Co-expression of HIF-1α, Twist and Snail in human head and neck tumors correlates with metastasis and poor prognosis.77 Overexpression of TrkB, a 145-KDa receptor tyrosine kinase, results in EMT and enhances invasion of human HNSCC.78 Downregulation of TrkB suppressed tumor growth.78 ALDH+ cells from HNSCC cell lines showed enhanced invasion, a phenotype consistent with EMT, and spheroid formation.79 Cells in spheroids reveal high level of the stemness-related transcription factors Oct3/4, Sox2 and Nanog, upregulation of Snail, Twist, alpha-SMA and Vimentin, and downregulation of E-cadherin.79 Collectively, these studies suggest that EMT may play a role in the acquisition of stem-like properties in HNSCC, which may ultimately contribute to local invasion and metastatic spread frequently observed in patients with head and neck cancer (Figure 2).
Figure 2.
Cancer stem-like cells (ALDH+CD44+) inside a blood vessel in a primary human head and neck cancer. (A) Highly aggressive human primary HNSCC cells invade a blood vessel (H&E). (B,C) Close-up view of the area limited by a square in panel A, showing cancer cells inside a blood vessel with positive staining for ALDH1 (B) and CD44 (C), as determined by immunohistochemistry. Please note strong cytoplasmic staining for ALDH1 and typical cell membrane staining for CD44 in the cells located inside the blood vessel (arrows).
Stem cell niches
Niches are specialized local microenvironments where stem cells reside. They appear to contribute to the survival and stemness of stem cells.80 It has also been postulated that a niche should shown the capacity to take up and maintain newly introduced stem cells upon depletion.80 For example, the crypt bottom is considered the niche for stem cells in normal small intestine and colon.41 It is also the niche for stem cells in intestinal cancer.40 The perivascular niche is the microenvironment of preference of brain cancer stem cells.81 It prevents the apoptosis of brain cancer stem cells and maintains an adequate balance between self-renewal and differentiation.81 When brain cancer stem cells were implanted together with endothelial cells in immunodeficient mice, tumor growth was accelerated.81,82 This suggests that factors secreted by normal cells surrounding and infiltrating tumors may promote the growth and progression of tumors.83
In head and neck tumors, the vast majority of the stem cells are found within a 100 µm-radius of a blood vessel, suggesting the existence of a perivascular niche.29 Using the SCID mouse model of human tumor angiogenesis,84 it was observed that specific ablation of tumor-associated endothelial cells with an inducible Caspase-9 results in the decrease in the fraction of head and neck cancer stem cells.29 It is becoming increasingly evident that the molecular crosstalk between HNSCC and endothelial cells is mutually relevant.85,86 Tumor cell-secreted factors activate Stat3, AKT and ERK signaling and enhance the survival and angiogenic potential of endothelial cells.85 Whereas endothelial cell-secreted factors (e.g. IL-6, CXCL8) enhance the migration of tumor cells and protect them against anoikis.86 Notably, endothelial cell-secreted factors promote the survival and self-renewal of cancer stem cells in HNSCC via upregulation of Bmi-1 expression.29 These studies demonstrate the existence of a functionally relevant perivascular niche in head and neck cancer, and suggest that targeted disruption of the crosstalk between endothelial cells and cancer stem cells might be beneficial for the treatment of head and neck cancer patients.
Stem cell markers
It has been recognized that cancer stem cells share many features with physiological stem cells. This constitutes a major difficulty for experimental cancer stem cell research, as well as for the development of targeted therapies. A strategy commonly employed by investigators is the use of molecular markers for the identification of cancer stem cells. In general, these markers are not unique to cancer stem cells. Therefore, the current trend is to combine markers to achieve higher specificity. Also, it is becoming increasingly evident that the most appropriate combination of markers is tumor type-dependent. The following is a brief discussion of some (but not all) markers of that have been used to identify cancer stem cells), with an emphasis on markers that are relevant to HNSCC.
A) Oct3/4, Sox2, Nanog
The transcription factors Oct3/4,87,88 Sox289 and Nanog90,91 play essential roles in the maintenance of pluripotency and self-renewal of embryonic stem cells.92,93 They promote self-renewal by interacting with other transcription factors (Stat3, Hesx1, Zic3) and critical cell signaling molecules (TCF3, FGF2, LEFTY2).92,93 It has been recently reported that the lamina propria of human oral mucosa contains stem cells, as determined by Oct4, Sox2 and Nanog expression.94 After treatment with dexamethasone and implantation in immunodeficient mice, these stem cells form tumors composed of ectodermal and mesodermal tissues, such as cartilage, bone, fat, striated muscle and neural tissues.94 These are interesting findings, since tumors were generated by stem cells retrieved from normal tissues.
The expression level of Oct4, Sox2, and Nanog is higher in poorly differentiated tumors than in well differentiated breast cancers, glioblastomas, and bladder carcinoma.95 These transcriptional factors are also upregulated in spheroid forming cells (i.e. stem-like cells) sorted from human HNSCC,96 and correlate with the grade of oral squamous cell carcimonas.97 Collectively, these data indicate that cells that exhibit stem-like features in cancer express the transcriptional factors Oct4, Sox2, and Nanog. However, the usefulness of these factors for the sorting of cancer stem cells by flow cytometry and posterior culture or implantation in animals is hindered by the fact that they are not expressed in the cell membrane, and therefore would require cell permeabilization.
B) CD133
Human CD133 (prominin-1) is a glycosylated protein with five transmembrane domains and two large extracellular loops.98,99 It was initially characterized as a marker for hematopoietic stem cells.97,98 After that, CD133 was also found in epithelial cells100,101 and in somatic stem cells from neural tissues,102,103 prostate,104 and kidney.105 Interestingly, human CD133+ cells from granulocyte colony stimulating factor-mobilized peripheral blood were able to differentiate into endothelial cells, when cultured in pro-endothelial lineage condition.106 In brain tumors, CD133+ cells revealed properties of cancer stem cells,107,108 and in the intestine, this marker identified stem cells that were susceptible to neoplastic transformation.109
In human oral squamous cell carcinoma, CD133+ stem-like cells possess higher clonogenicity, invasiveness, and tumorigenesis as compared with CD133- cells.110 CD133+ cells are resistant to standard chemotherapy with paclitaxel.110 CD133 has been identified as a marker of cancer stem cells in the human laryngeal tumor Hep-2 cell line.111 In an in vivo study, CD133+ cells sorted from the Hep-2 cell line showed higher tumorigenic potential than CD133-or unsorted cells.112 Notably, CD44+ cancer stem-like cells expressed higher CD133 levels than CD44- cells in HNSCC.113 In laryngeal squamous cell carcinomas, Bmi-1 is highly enriched in CD133+ cells, induces the proliferation of these cells, and prevents apoptosis.114 The analysis of these studies reveals that CD133 is an useful cancer stem cell marker in HNSCC, and might serve as a putative biomarker to identify head and neck cancer patients that are resistant to conventional chemotherapy.
C) CD44
CD44 is a cell surface glycoprotein that functions as a receptor for hyaluronic acid (hyaluronan).115,116 CD44 has affinity with other ligands (e.g. osteopontin)117,118 and certain matrix metalloproteinases (MMPs).119 In 1991, a CD44 variant was found to be involved in the metastatic potential of tumor cells.120 As an adhesion molecule, CD44 provides a cell surface docking receptor that is necessary for MMP-9 activity.121 Localization of MMP-9 in the cell surface of keratinocytes depends on its interaction with CD44, allows for activation of TGF-β, and is required for the promotion of tumor invasion and angiogenesis.119 Interestingly, immunohistochemical staining showed that CD44 and MMP-9 co-localize in tumor cells at the invasive front,122 the area where stem cells are typically found in tumors.
CD44 was the marker used in the first description of cancer stem cells in a solid malignancy (i.e. breast cancer).8 In 2007, Prince and colleagues unveiled that a subpopulation of CD44+ cells presented cancer stem-like properties in HNSCC.5 They found that CD44+ cells could be serially passaged in vivo, consistently reproducing the original tumor. CD44+ cells expressed high levels of Bmi-1 (stemness marker) and possessed the capacity of self-renewal and differentiation. Since then, many studies have used CD44 as a marker of cancer stem cells in head and neck cancer models.29,123,124 However, one must take into account a report that showed that the expression of two variants of CD44 (i.e. CD44s, CD44v6) is found in the majority of the cells in head and neck tissue (including carcinomas), and that this marker by itself was not able to distinguish normal from benign or malignant epithelial cells from the head and neck region.125 CD44 is considered a predictive marker for local recurrence after radiotherapy in patients with larynx cancer.126 High levels of CD44, aldehyde dehydrogenase and phosphorylated Stat3 are found in high-grade HNSCC, and are indicative of poor prognosis.124 Also, a higher frequency of CD44+ cells was observed in HNSCC that recurred than in human tumors without recurrence.127 Collectively, these studies suggest a direct correlation between CD44 expression, cancer stem cells, and the aggressiveness of head and neck tumors.
D) ALDH
Aldehyde dehydrogenase (ALDH) enzymes constitute a family of intracellular enzymes that are involved in cell differentiation, detoxification and drug resistance via the oxidation of intracellular aldehydes.128–131 ALDH1 is required to converse retinol (vitamin A) to retinoic acid.132 ALDH1 is the prototypic member of the ALDH family and is highly expressed in human hematopoietic progenitors or hematopoietic stem cells.129,131,133 ALDH1 has been characterized as a marker of normal and malignant human mammary stem cells, and a prognostic marker for breast cancer being a strong predictor of metastasis and poor patient outcome.134,135 In primary non-small cell lung cancer (NSCLC), ALDH-positive cancer cells showed stem-like properties, including tumorigenesis, colony formation and self-renewal.136
Several studies have demonstrated that ALDH+ cells have a behavior that is consistent with cancer stem cells in head and neck tumors.28,29,79,137 ALDH+ cells from patients with HNSCC showed enhanced tumorigenesis and radioresistance when compared with ALDH- cells.137 Interestingly, knockdown of Snail decreased the expression of ALDH and inhibited cancer stem-like properties and the tumorigenicity of CD44+CD24-ALDH+ cells.137 In a study from the Prince laboratory, 500 ALDHhigh cells from primary HNSCC formed tumors in 24 out of 25 mice, while only 3 out of 37 mice transplanted with ALDHlow cells showed tumors.28 Another study showed that 1000 ALDH+CD44+ cells from primary human HNSCC formed tumors in 13 out of 15 mice, whereas 10 000 ALDH-CD44- cells resulted in only 2 tumors in 15 mice.29 The self-renewal of ALDH+CD44+ cells was confirmed by colony and spheroid formation.29 These studies demonstrated that ALDH by itself, or in combination with CD44, is capable of discriminating a sub-population of highly tumorigenic cells that exhibit features of cancer stem cells in HNSCC.
E) Side population
Another strategy that has been used extensively to identify highly tumorigenic cells is based on the ability of such cells to eliminate a DNA dye, Hoechst 33342.138 Side population (SP) cells are enriched in hematopoietic stem cells139 and identified from a bone marrow-derived cell population.138 These cells express high levels of ATP-binding cassette (ABC) transporter family members (e.g. MDR1, ABCG2) that allow for the efflux of Hoechst 33342 and other drugs.140,141 Using fluorescence-activated cell sorting (FACS), SP cells have been identified in normal tissues (e.g. skin,142 lung,143 brain,144 and liver145) and solid tumors (e.g. hepatocellular carcinoma,146 glioma,147 gastrointestinal cancer,148 ovarian carcinoma,149 neuroblastoma150 and breast cancer.150
In recent years, SP cells have been characterized in HNSCC as highly tumorigenic cells with stem-like phenotype.151–155 The fraction of SP cells tends to be high in metastatic and aggressive HNSCC cells.153 In head and neck cancer, SP cells express high levels of ABCG2,152,153,155 Bmi-1,152,155 CD44, and Oct4.152 These cells exhibit abnormal Wnt signaling and are highly invasive153 and chemoresistant.153,155 The identification of side populations is technically simple and does not rely on the relative binding efficiencies of antibodies. More research is necessary to demonstrate how it compares against antibody-based approaches to specifically distinguish highly tumorigenic head and neck cancer stem cells, from other cells of HNSCC that present low tumorigenicity.
Final thoughts and future directions
There are many factors that play a role in the study of the tumorigenic potential of cells. Among them, the immunological status of the host appears to have a direct impact on the efficacy of tumor initiation in murine experimental models. It has been reported that only 1 in one million acute myeloid leukemia (AML) cells generates a tumor when transplanted into NOD/SCID mice.156 However, the frequency of tumor-initiating cells is higher when these cells are transplanted into histocompatible mice. Indeed, 1 out of 10 lymphoma cells or AML cells can form tumors when injected into such mice.157 In melanoma, it was demonstrated that the frequency of tumor-initiating cells is less than 1 per 106 cells when transplanted into NOD/SCID mice.25 However, when melanoma cells were transplanted into highly immunocompromised NOD/SCID interleukin-2 receptor gamma chain null (Il2rg(−/−)) mice, the fraction of tumorigenic melanoma cells was increased by several orders of magnitude. In this case, 27% of unselected melanoma cells generated tumors.25 Collectively, these studies highlight the impact of the experimental model on the results of studies exploring the tumorigenic potential of cells. They constitute an important reminder that one should use caution while interpreting the results of laboratory studies involving the transplantation of human cells into murine hosts.
A critical issue that remains unanswered is what is the frequency of tumor-initiating cells in head and neck squamous cell carcinomas. The seminal publication by Prince and collaborators showed that CD44 expression distinguished a highly tumorigenic sub-population of cells (that behave as cancer stem cells) from another cell population that had low tumorigenic potential.5 A recent report revealed that tumor-initiating cells are rare (<1 in 2500 cells) in primary pancreas, lung, or head and neck tumors.158 And in a serial dilution assay, we observed that the transplantation of as low as 1 ALDH+CD44+ cell/SCID mouse consistently formed tumors (unpublished observations), while transplantation of 10,000 ALDH-CD44- generate tumors in only 13.3% of the mice.29 These studies, and many others not described in detail here,28,110,127,152 suggest that head and neck squamous cell carcinomas follow the cancer stem cell hypothesis. On the other hand, it was recently reported that all single-cell clones randomly isolated from certain HNSCC cell lines can form tumors when xenotransplanted to NOD/SCID mice.159 This study indicated that essentially any cell from HNSCC cell lines has the ability to form tumors. Further studies focused on the identification of the nature, frequency, and characteristics of the cells capable of generating HNSCC are certainly warranted.
The analysis of the existing literature suggests a hypothetical model for head and neck tumor progression (Figure 3). The crosstalk between HNSCC cells and other cells of the tumor microenvironment results in EMT, which enhances the motility of carcinoma cells and endows them with stem cell properties. The invasive phenotype of cells that have undergone EMT allows them to penetrate the lymphatic and/or angiogenic vasculature. And the highly tumorigenic nature of cancer stem cells enables some of them to initiate tumors in regional lymph nodes or in distant sites (e.g. lungs). According to this hypothetical model, patients with HNSCC might benefit from therapeutic strategies that inhibit EMT by blocking the crosstalk between tumor and stromal cells, or therapies that directly target the cancer stem cell.
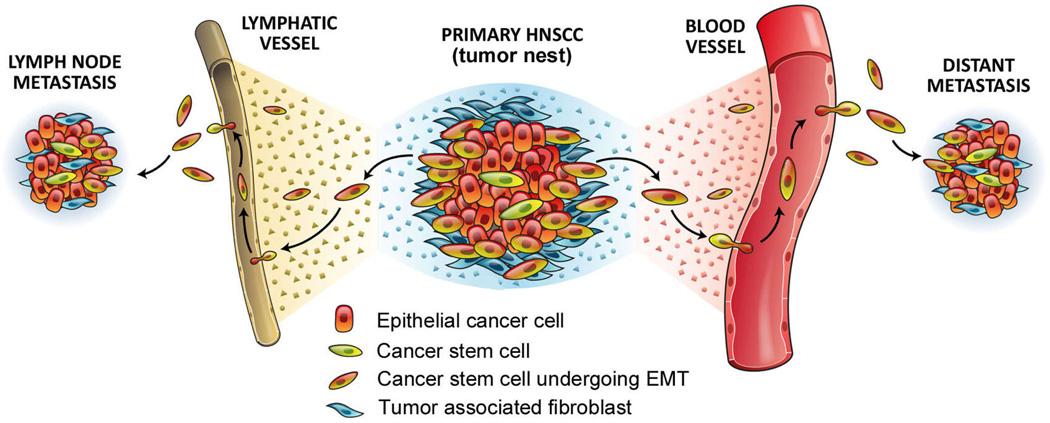
Figure 3.
Diagram depicting a putative model for the role of EMT and acquisition of cancer stem cell phenotype in the metastatic spread of HNSCC. The epithelial tumor is a complex organ that contains carcinoma cells, fibroblasts, immune cells, blood vessels, lymphatics, and a small population of cancer stem cells. Through EMT, cancer stem cells become invasive and acquire characteristics that enable them to metastasize to regional lymph nodes (through lymphatic vessels) and to distant sites, such as lungs (through blood vessels). Notably, it is unlikely that every single cancer stem cell has the ability to generate a viable metastasis.
In conclusion, the discovery that heterogeneous HNSCC tumor cells exhibit a spectrum of tumorigenic potentials has brought significant interest to the application of stem cell biology concepts to the understanding of the pathobiology of head and neck cancer. Much work remains to be done to more fully understand the biology of cancer stem cells in HNSCC. For example, whether cancer stem-like cells exist in premalignant lesions and what is their behavior and function through the multi-step process of disease progression remains largely unclear. However, what is clear is that the development of mechanism-based therapies for head and neck cancer will require deeper understanding of the biological processes that generate the cells that drive recurrence and metastatic spread. It is tempting to speculate that the combination of therapies aimed at debulking the tumor (e.g. surgery, conventional chemotherapy, radiotherapy) together with targeted therapies aimed at the elimination of the cancer stem cells might have a positive impact on the long-term outcome of patients with head and neck cancer in the future.
Acknowledgments
We thank Dr. Thomas Carey for UM-SCC cell lines; Laura Hildebrand, Ana Carvalho, and Isabel Lauxen for the immunohistochemical images; and Chris Jung for his work with the medical illustrations. This work was supported by the Weathermax Foundation, University of Michigan Comprehensive Cancer Center; grant P50-CA97248 (University of Michigan Head and Neck SPORE) from the NIH/NCI; and grants R21-DE19279 and R01-DE21139 from the NIH/NIDCR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no conflict of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345(26):1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the Unite States: a site-specific analysis of the SEER database. Int J Cancer. 2005;114(5):806–816. doi: 10.1002/ijc.20740. [DOI] [PubMed] [Google Scholar]
- 5.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104(3):973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evan MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 7.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratoclarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78(12):7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 11.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoanq T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 12.Campbell LL, Polyak K. Breast tumor heterogeneity: cancer stem cells or clonal evolution? Cell Cycle. 2007;6(19):2332–2338. doi: 10.4161/cc.6.19.4914. [DOI] [PubMed] [Google Scholar]
- 13.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 14.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 15.Singh SK, Clarke ID, Terasaki M, Boon VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–28. [PubMed] [Google Scholar]
- 16.Singh SK, Hawkins C, Clark ID, Squir JA, Bayani L, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 17.Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67(10):4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 18.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 19.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumourigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 21.Dalerba P, Dylla SJ, Part IK, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104(24):10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13(2):153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Fang D, Nguyen TK, Leishear K, Finko K, Kulp AN, Hotz S, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65(20):9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 25.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumor formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taghazideh R, Noh M, Huh YH, Ciusani E, Sigalotti L, Maio M, et al. CXCR6, a newly defined biomarker of tissue-specific stem cell asymmetric self-renewal, identifies more aggressive human melanoma cancer stem cells. PLoS One. 2010;5(12):e15183. doi: 10.1371/journal.pone.0015183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, Belloni B, et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71(8):3098–3119. doi: 10.1158/0008-5472.CAN-10-3997. [DOI] [PubMed] [Google Scholar]
- 28.Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, et al. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010;32(9):1195–1201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, et al. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010;70(23):9969–9978. doi: 10.1158/0008-5472.CAN-10-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 31.Wilmut I, Schnieke AE, Mcwhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 32.Tata M, Takahama Y, Abe K, Nakatsuji N, Tada T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr Biol. 2001;11(19):1553–1558. doi: 10.1016/s0960-9822(01)00459-6. [DOI] [PubMed] [Google Scholar]
- 33.Cowan CA, Atienza J, Melton DA, Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369–1373. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Yu JY, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourtget J, Frane JL, Tian S, et al. Induced pluripotent stem cells lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 37.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3(12):895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 38.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Reya T, Clevers H. Wnt signaling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 40.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 41.Barker N, van Es JH, Kuipers J, Kujala P, van den Bom M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 42.Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, et al. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280(26):24731–24737. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- 43.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41(11):1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan P, Kadara H, Behrens C, Tang X, Woods D, Solis LM, et al. Sex determining region Y-Box 2 (SOX2) is a potential cell-lineage gene highly expressed in the pathogenesis of squamous cell carcinomas of the lung. PLoS One. 2010;5(2):e9112. doi: 10.1371/journal.pone.0009112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swiatek PJ, Lindsell CE, del Amo FF, Weinmaster G, Gridley T. Notch1 is essential for post-implantation development in mice. Genes Dev. 1994;8(6):707–719. doi: 10.1101/gad.8.6.707. [DOI] [PubMed] [Google Scholar]
- 46.Sikandar SS, Pate KT, Anderson S, Dizon D, Edwards RA, Waterman ML, et al. NOTCH signaling is required for formation and self-renewal of tumor-initiating cells and for repression of secretory cell differentiation in colon cancer. Cancer Res. 2010;70(4):1469–1478. doi: 10.1158/0008-5472.CAN-09-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011 Jul 28; doi: 10.1126/science.1206923. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011 Jul 28; doi: 10.1126/science.1208130. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112(12):1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transition in early development. Mech Dev. 2003;120(11):1351–1383. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 52.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmed N, Maines-Bandiera S, Quinn MA, Unger WG, Dedhar S, Auersperg N. Molecular pathways regulating EGF-induced epithelial-mesenchymal transition in human ovarian surface epithelium. Am J Physiol Cell Physiol. 2006;290(6):C1532–C1542. doi: 10.1152/ajpcell.00478.2005. [DOI] [PubMed] [Google Scholar]
- 55.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27(55):6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 56.Whiteman EL, Liu CJ, Fearon ER, Margolis B. The transcription factor snail represses Crumbs3 expression and disrupts apico-basal polarity complexes. Oncogene. 2008;27(27):3875–3879. doi: 10.1038/onc.2008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 58.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in the epithelial tumour cells. Nat Cell Biol. 2000;2(2):84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 59.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 61.Sánchez-Tilló E, Lázaro A, Torrent R, Cuatrecasas M, Vaquero EC, Castells A, et al. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene. 2010;29(24):3490–3500. doi: 10.1038/onc.2010.102. [DOI] [PubMed] [Google Scholar]
- 62.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 63.Yang AD, Fan F, Camp ER, van Buren G, Liu W, Somcio R, et al. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res. 12(14):2006. 4147–4153. doi: 10.1158/1078-0432.CCR-06-0038. [DOI] [PubMed] [Google Scholar]
- 64.Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion and resistance to paclitaxel. Cancer Res. 2007;67(5):1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- 65.Li QQ, Xu JD, Wang WJ, Cao XX, Chen Q, Tang F, et al. Twist1-mediated adriamycin-induced epithelial-mesenchymal transition relates to multidrug resistance and invasive potential in breast cancer cells. Clin Cancer Res. 2009;15(8):2657–2665. doi: 10.1158/1078-0432.CCR-08-2372. [DOI] [PubMed] [Google Scholar]
- 66.Eastham AM, Spencer H, Soncin F, Ritson S, Merry CL, Stem PL, et al. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007;67(23):11254–11262. doi: 10.1158/0008-5472.CAN-07-2253. [DOI] [PubMed] [Google Scholar]
- 67.Ullmann U, In’t Veld P, Gilles C, Sermon K, De Rycke M, Van de Velde H, et al. Epithelial-mesenchymal transition process in human embryonic stem cells cultured in feeder-free conditions. Mol Hum Reprod. 2007;13(1):21–32. doi: 10.1093/molehr/gal091. [DOI] [PubMed] [Google Scholar]
- 68.Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69(7):2887–2895. doi: 10.1158/0008-5472.CAN-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3(8):e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xia H, Cheung WK, Sze J, Lu G, Jiang S, Yao H, et al. miR-200a regulates epithelial-mesenchymal to stem-like transition via ZEB2 and p-catenin signaling. J Biol Chem. 2010;285(47):36995–37004. doi: 10.1074/jbc.M110.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spivakov M, Fisher AG. Epigenetic signatures of stem-cell identity. Nat Rev Cancer. 2007;8(4):263–271. doi: 10.1038/nrg2046. [DOI] [PubMed] [Google Scholar]
- 72.Valk-lingbeek ME, Bruggeman SW, Van Lohuizen M. Stem cells and cancer; the polycomb connection. Cell. 2004;118(4):409–418. doi: 10.1016/j.cell.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 73.Widschwendter M, Fiegl H, Egle D, Mueller-Holzner E, Spizzo G, Weisenberger DJ, et al. Epigenetic stem cell signature in cancer. Nat Genet. 2007;39(2):157–158. doi: 10.1038/ng1941. [DOI] [PubMed] [Google Scholar]
- 74.Park IK, Morrison SJ, Clarke MF. Bmi-1, stem cells, and senescence regulation. J Clin Invest. 2004;113(2):175–179. doi: 10.1172/JCI20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, et al. Bmi-1 is essential in twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12(10):982–992. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 76.Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu LJ, et al. The polycomb group protein Bmi-1 repressed the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelia cells. J Clin Invest. 2009;119(12):3626–3636. doi: 10.1172/JCI39374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, et al. Direct regulation of TWIST by HIF-1α promotes metastasis. Nat Cell Biol. 2008;10(3):295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 78.Kupferman ME, Jiffar T, EI-Naggar A, Yilmaz T, Zhou G, Xie T, et al. TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma. Oncogene. 2010;29(14):2047–2059. doi: 10.1038/onc.2009.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen C, Wei Y, Hummel M, Hoffmann TK, Gross M, Kaufmann AM, et al. Evidence for epithelial-mesenchymal transition in cancer stem cells of head and neck squamous cell carcinoma. Plos One. 2011;06(1):e16466. doi: 10.1371/journal.pone.0016466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morrison SJ, Spradling AC. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132(4):598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 82.Yang ZJ, Wechsler-Reya RJ. Hit ‘em where they live: targeting the cancer stem cell niche. Cancer Cell. 2007;11(1):3–5. doi: 10.1016/j.ccr.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 83.TIsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 84.Nör JE, Peters MC, Christensen JB, Sutorik MM, Linn S, Khan MK, et al. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 2001;81(4):453–463. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Z, Neiva KG, Lingen MW, Ellis LM, Nör JE. VEGF-dependent tumor angiogenesis requires inverse and reciprocal regulation of VEGFR1 and VEGFR2. Cell Death Differ. 2010;17(3):499–512. doi: 10.1038/cdd.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neiva KG, Zhang Z, Miyazawa M, Warner KA, Karl E, Nör JE. Crosstalk initiated by endothelial cells enhances migration and inhibits anoikis of squamous cell carcinoma cells through STAT/Akt/ERK signaling. Neoplasia. 2009;11(6):583–593. doi: 10.1593/neo.09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95(3):379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 88.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24(4):372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 89.Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes De. 2003;17(1):126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chambers I, Colby D, Robretson M, Nichols J, Lee S, Tweedie S, et al. Functional expression cloning of nanog a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113(5):643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 91.Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113(5):631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 92.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122(6):947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, et al. The Oct4 and nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38(4):431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 94.Marynka-Kalmani K, Treves S, Yafee M, Rachima H, Gafni Y, Cohen MA, et al. The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cell. 2010;28(5):984–995. doi: 10.1002/stem.425. [DOI] [PubMed] [Google Scholar]
- 95.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated human tumors. Nat Genet. 2008;40(5):499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lim YC, Oh SY, Cha YY, Kim SH, Jin X, Kim H. Cancer stem cell traits in squamouspheres derived from primary head and neck squamous cell carcinomas. Oral Oncol. 2011;47(2):83–91. doi: 10.1016/j.oraloncology.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 97.Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, et al. Positive correlations of Oct-4 and nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008;14(13):4085–4095. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 98.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90(12):5002–5012. [PubMed] [Google Scholar]
- 99.Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, et al. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90(12):5013–5021. [PubMed] [Google Scholar]
- 100.Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci USA. 1997;94(23):12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Corbeil D, Roper K, Hellwig A, Tavian M, Miraglia S, Watt SM, et al. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem. 2000;275(8):5512–5520. doi: 10.1074/jbc.275.8.5512. [DOI] [PubMed] [Google Scholar]
- 102.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97(26):14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee A, Kessler JD, Read TA, Kaiser C, Corbeil D, Huttner WB, et al. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005;8(6):723–729. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shepherd CJ, Rizzo S, Ledaki I, Davies M, Brewer D, Attard G, et al. Expression profiling of CD133+ and CD133- epithelial cells from human prostate. Prostate. 2008;68(9):1007–1024. doi: 10.1002/pros.20765. [DOI] [PubMed] [Google Scholar]
- 105.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, et al. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166(2):545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95(10):3106–3112. [PubMed] [Google Scholar]
- 107.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–28. [PubMed] [Google Scholar]
- 108.Singh SK, Hawkins C, Clarke ID, Squire JA, Bavan J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 109.Zhu LQ, Gibson P, Currle DS, Tong Y, Richardson RJ, Bavazitov IT, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Q, Shi S, Yen Y, Brown T, Ta JQ, Le AD. A subpopulation of CD133(+) cancer stem-like cells characterized in human oral squamous cell carcinoma confer resistance to |chemotherapy. Cancer Lett. 2010;289(2):151–160. doi: 10.1016/j.canlet.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 111.Zhou L, Wei X, Cheng L, Tian J, Jiang JJ. CD133, one of the markers of cancer stem cells in Hep-2 cell line. Laryngoscope. 2007;117(3):455–460. doi: 10.1097/01.mlg.0000251586.15299.35. [DOI] [PubMed] [Google Scholar]
- 112.Wei XD, Zhou L, Cheng L, Tian J, Jiang JJ, Maccallum J. In vivo investigation of CD1323 as a putative marker of cancer stem cells in Hep-2 cell line. Head Neck. 2009;31(1):94–101. doi: 10.1002/hed.20935. [DOI] [PubMed] [Google Scholar]
- 113.Okamoto A, Chikamatsu K, Sakakura K, Hatsushika K, Takahashi G, Masuyama K. Expansion and characterization of cancer stem-like cells in squamous cells carcinoma of the head and neck. Oral Oncology. 2009;45(7):633–639. doi: 10.1016/j.oraloncology.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 114.Chen H, Zhou L, Dou T, Wan G, Tang H, Tian J. Bmi-1 ‘s maintenance of the proliferative capacity of laryngeal cancer stem cells. Head Neck. 2011;33(8):1115–1125. doi: 10.1002/hed.21576. [DOI] [PubMed] [Google Scholar]
- 115.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 116.Screaton GR, Bell MV, Jackson DC, Cornelis FB, Gerth U, Bell JI. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternative spliced exons. Proc Natl Acad Sci USA. 1992;89(24):12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weber GF, Ashker S, Cantor H. Interaction of CD44 and osteopontin as a potential basis for metastasis formation. Proc Assoc Am Phys. 1997;109(1):1–9. [PubMed] [Google Scholar]
- 118.Thalmann GN, Sikes RA, Devoll RE, Kiefer JA, Markwalder R, Klima I, et al. Osteopontin: possible role in prostate cancer progression. Clin Cancer Res. 1999;5(8):2271–2277. [PubMed] [Google Scholar]
- 119.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 120.Günther U, Hofmann M, Rudy W, Reber S, Zöller M, Haussmann I, et al. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- 121.Desai B, Ma T, Zhu J, Chellaiah MA. Characterization of the expression of variant and standard ED44 in prostate cancer cells: Identification of the possible molecular mechanism of CD44/MMP9 complex formation on the cell surface. J Cell Biochem. 2009;108(1):272–284. doi: 10.1002/jcb.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sterz CM, Kulle C, Dakic B, Makarova G, Bottcher MC, Bette M, et al. A basal-cell-like compartment in head and neck squamous cell carcinomas represents the invasive front of the rumor and is expressing MMP-9. Oral Oncol. 2010;46(2):116–122. doi: 10.1016/j.oraloncology.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 123.Chikamatus K, Takahashi G, Sakakura K, Ferrone S, Masuyama K. Immunoregulatory properties of CD44+ cancer stem-like cells in squamous cell carcinoma of the head and neck. Head Neck. 2011;33(2):208–215. doi: 10.1002/hed.21420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen YW, Chen KH, Huang PI, Chen YC, Chiou GY, Lo WL, et al. Cucurbitacin I Suppressed stem-like property and enhanced radiation-induced apoptosis in head and neck squamous carcinoma-derived CD44+ALDH+ cells. Mol Cancer Ther. 2010;9(11):2879–2892. doi: 10.1158/1535-7163.MCT-10-0504. [DOI] [PubMed] [Google Scholar]
- 125.Mack B, Gires O. CD44s and CD44v6 expression in head and neck epithelia. PLoS One. 2008;3(10):e3360. doi: 10.1371/journal.pone.0003360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.de Jong MC, Pramana J, van der Wal JE, Lacko M, Peutz-Kootstra CJ, de Jong JM, et al. CD44 expression predicts local recurrence after radiotherapy in larynx cancer. Clin Cancer Res. 2010;16(21):5329–5338. doi: 10.1158/1078-0432.CCR-10-0799. [DOI] [PubMed] [Google Scholar]
- 127.Joshua B, Kaplan MJ, Doweck I, Pai R, Weissman IL, Prince ME, et al. Frequency of cells expressing CD44, a head and neck cancer stem cell marker: correlation with tumor aggressiveness. Head Neck. 2011 Feb 14; doi: 10.1002/hed.21699. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 128.Sophos NA, Vasiliou V. Aldehyde dehydrogenase gene superfamily: the 2002 update. TChem Biol Interact. 2003;143–144:5–22. doi: 10.1016/s0009-2797(02)00163-1. [DOI] [PubMed] [Google Scholar]
- 129.Magni M, Shammah S, Schiro R, Mellado W, Dalla-Favera R, Gianni AM. Induction of cyclophosphamide-resistance by aldehyde-dehydrogenase gene transfer. Blood. 1996;87(3):1097–1103. [PubMed] [Google Scholar]
- 130.Moreb J, Schweder M, Suresh A, Zucali JR. Overexpression of the human aldehyde dehydrogenase class 1 results in increased resistance to 4-hydroperoxycyclophosphamide. Cancer Gene Ther. 1996;3(1):24–30. [PubMed] [Google Scholar]
- 131.Chute JP, Muramoto GG, Whitesides J, Colvin M, Safi R, Chao NJ, et al. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci USA. 2006;103(31):11707–11712. doi: 10.1073/pnas.0603806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bhat PV, Samada H. Kinetic properties of the human liver cytosolic aldehyde dehydrogenase for retinal isomers. Biochem Pharmacol. 1999;57(2):195–197. doi: 10.1016/s0006-2952(98)00261-5. [DOI] [PubMed] [Google Scholar]
- 133.Kastan MB, Schlaffer E, Russo JE, Colvin OM, Civin CI, Hilton J. Direct demonstration of elevated aldehyde dehydrogenase in human hematopoietic progenitor cells. Blood. 1990;75(10):1947–1950. [PubMed] [Google Scholar]
- 134.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Charafe-Jauffret E, Ginestier C, Lovino F, Tarpin C, Diebel M, Esterni B, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16(1):45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sullivan JP, Spinola M, Dodge M, Raso MG, Behrens C, Gao B, et al. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on Notch signaling. Cancer Res. 2010;70(23):9937–9948. doi: 10.1158/0008-5472.CAN-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen YC, Chen YW, Hsu HS, Tseng LM, Huang PI, Lu KH, et al. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun. 2009;385(3):307–313. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 138.Goodell MA, Brose K, Paradis G, Gonner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183(4):1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Goodell MA, Rosenzweig M, Kim H, marks DF, DeMaria M, Paradis G, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3(12):1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 140.Hadnagy A, Gaboury L, Beaulieu R, Balicki D. SP analysis may be used to identify cancer stem cell populations. Exp Cell Res. 2006;312(19):3701–3710. doi: 10.1016/j.yexcr.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 141.Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008;268(1):1–9. doi: 10.1016/j.canlet.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 142.Larderet G, Fortunel NO, Vaigot P, Cegalerba M, Maltere P, Zobiri O, et al. Human side population keratinocytes exhibit long-term proliferative potential and a specific gene expression profile and can form a pluristratified epidermis. Stem Cells. 2006;24(4):965–974. doi: 10.1634/stemcells.2005-0196. [DOI] [PubMed] [Google Scholar]
- 143.Majka SM, Beutz MA, Hagen M, Izzo AA, Voelkel N, Helm KM. Identification of novel resident pulmonary stem cells: form and function of the lung side population. Stem Cells. 2005;23(8):1073–1081. doi: 10.1634/stemcells.2005-0039. [DOI] [PubMed] [Google Scholar]
- 144.Kim M, Morshead CM. Distinct populations of forebrain neural stem and progenitor cells can be isolated using side-population analysis. J Neurosci. 2003;23(33):10703–10709. doi: 10.1523/JNEUROSCI.23-33-10703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shimano K, Satake M, Okaya A, Kitanaka J, Kitanaka N, Takemura M, et al. Hepatic oval cells have the side population phenotype defined by expression of ATP-binding cassette transporter ABCG2/BCRP1. Am J Pathol. 2003;163(1):3–9. doi: 10.1016/S0002-9440(10)63624-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44(1):240–251. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 147.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer Stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA. 2004;101(3):781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hareguichi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, et al. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24(3):506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 149.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and mullerian inhibiting substance responsiveness. Proc Natl Acad Sci USA. 2006;103(30):11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hirschmann-Jax C, Foster AE, Wuff GG, Nuchtern JG, Jax TW, Gobel U, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004;101(39):14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang J, Guo LP, Chen LZ, Zeng YX, Lu SH. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res. 2007;67(8):3716–3724. doi: 10.1158/0008-5472.CAN-06-4343. [DOI] [PubMed] [Google Scholar]
- 152.Zhang P, Zhang Y, Mao L, Zhang Z, Chen W. Side population in oral squamous cell carcinoma possesses tumor stem cell phenotypes. Cancer Lett. 2009;277(2):227–334. doi: 10.1016/j.canlet.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 153.Song J, Chang I, Chen Z, Kang M, Wang CY. Characterization of side populations in HNSCC: highly invasive, chemoresistant and abnormal Wnt signaling. PLoS One. 2010;5(7):e11456. doi: 10.1371/journal.pone.0011456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wan GL, Zhou L, Xie M, Chen H, Tian J. Characterization of side population cells from laryngeal cancer cell lines. Head Neck. 2010;32(10):1302–1309. doi: 10.1002/hed.21325. [DOI] [PubMed] [Google Scholar]
- 155.Tabor MH, Clay MR, Owen JH, Bradford CR, Carey TE, Wolf GT, et al. Head and neck cancer stem cells: the side population. Laryngoscope. 2011;121(3):527–533. doi: 10.1002/lary.21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immumol. 2004;5:738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 157.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007:317–337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 158.Ishizawa K, Rasheed ZA, Karisch R, Wang Q, Kowalski J, Susky E, et al. Tumor-initiating cells are rare in many human tumors. Cell Stem Cell. 2010;7(3):279–282. doi: 10.1016/j.stem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cameron SR, Dahler AL, Endo-Munoz LB, Jabbar I, Thomas GP, Leo PJ, et al. Tumor-initiating activity and tumor morphology of HNSCC is modulated by interactions between clonal variants within the tumor. Lab Invest. 2010;90(11):1594–1603. doi: 10.1038/labinvest.2010.131. [DOI] [PubMed] [Google Scholar]
- 160.Campos M, Neiva K, Meyers K, Krishnamurthy S, Nör JE. Endothelial derived factors inhibit anoikis of head and neck cancer stem ells. Oral Oncol. doi: 10.1016/j.oraloncology.2011.09.010. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]