Abstract
The reverse shoulder arthroplasty emerged as a potential solution for those patients who could not be managed effectively with a conventional total shoulder arthroplasty. Grammont revolutionized the design by medializing and distalizing the center of rotation and utilizing a large convex glenoid surface and concave humeral component with a neck-shaft angle of 155°. This design has been highly successful in cuff deficient shoulders, and indications continue to broaden. Many mid-term studies have improved upon the early encouraging results. Long-term studies are starting to emerge, demonstrating good survivorship, but progressive functional and radiographic deterioration continue to be concerning. Careful patient selection and attention to appropriate technique are required to reduce the current high rate of complications. New prosthesis designs are continuing to develop to address some of these limitations.
Keywords: Reverse shoulder arthroplasty, Shoulder, Indications, Modern techniques in shoulder surgery
Introduction
Historically, shoulder surgery has aimed to restore or replicate the anatomy of the glenohumeral joint and the rotator cuff. The conventional total shoulder arthroplasty (TSA) achieves this goal and for many patients significantly improves function and reduces pain. The prosthesis is unconstrained and therefore relies on a well functioning rotator cuff to restore shoulder function. Attempts to achieve this goal in patients with large or massive cuff tears have achieved only limited success [1, 2]. This encouraged investigation into alternate prostheses to address cuff deficient shoulders.
The shoulder relies on intrinsic (rotator cuff) and extrinsic (deltoid being most critical) musculature for normal functioning. With large cuff tears, the normal force couple associated with forward elevation or attempted abduction is disrupted [3]. This results in unopposed deltoid contraction and a resultant force vector which superiorly displaces the humeral head toward the acromion and coracoacromial arch, and in some cases causes a “pseudoparalytic” shoulder. Over time with a massive tear, this action can produce permanent proximal migration of the humeral head with painful acromial erosion and/or glenohumeral arthritis.
Results of conventional total shoulder arthroplasty in this setting have been unsatisfactory due to eccentric glenoid loading and early failure. This mechanism of failure was described by Franklin et al. [4] who reported 50% glenoid loosening secondary to a “rocking horse” phenomenon. To avoid early glenoid loosening, hemiarthroplasty was considered the most appropriate prosthesis for rotator cuff tear arthropathy. Pain scores were generally improved, however concerns continued regarding the limited functional improvements in some patients and progressive glenoid erosion [5, 6].
Constrained prostheses were developed to better control the proximal migration in cuff deficient shoulders [7, 8]. These early designs allowed the humeral head to articulate within a constrained concave glenoid socket component. Despite some encouraging results, these prostheses had very high complication rates especially from component loosening secondary to interface stresses inherent to the constrained design [9]. Most of the early designs were abandoned. Semi-constrained designs with a glenoid hood to limit proximal migration suffered a similar fate.
As alternate designs were sought, the positions of the traditional “ball and socket” articulations were swapped and the concept of the reverse shoulder arthroplasty (RSA) evolved. Early designs, such as the Kessel [10] and Fenlin [11] prostheses, relied on preserving the centre of rotation lateral to the glenoid articulation. Unfortunately this resulted in similar problems with early glenoid loosening and failure due to glenoid component interface stresses [12].
In 1987, Professor Paul Grammont introduced a new reverse prosthesis with an improved biomechanical design [13]. The principles of his design were inherent prosthetic stability, convexity of the (weightbearing) glenoid component, glenosphere center at or within the glenoid neck, and a medialized and distalized center of rotation.
Stability
With rotator cuff insufficiency, the RSA design must prevent humeral proximal migration despite a proximally directed resultant joint force vector created by a dominant deltoid contraction. Conventional TSA has a shallow glenoid component which cannot resist proximal migration and dislocation if the force vector is greater than 30° from the centerline [14, 15]. The RSA reduces this risk by a semi-constrained design, with congruent articulating surfaces achieving concentric motion. A large deep humeral concave component with a non-anatomic neck-shaft angle of 155° further aids stability. Consequently, the resultant force vector can subtend an angle of at least 45° from the centerline without risk of dislocation [14, 15]. It should also be noted that the intrinsic stability of the prosthesis is not necessarily improved with larger prostheses. It relies on an increase in the ratio between the depth and diameter of the concave humeral component [16], amongst other factors.
Center of rotation at the scapular neck
Grammont’s design was different to previous RSA designs in it eliminated the glenosphere neck and subsequently medialized the centre of rotation to at or medial to the prosthesis-bone interface. The consequence was to reduce the shear forces and increase compressive forces across this interface, solving the early design problem of early loosening of the glenoid component. The inevitable consequence of eliminating the neck was that humeral adduction caused inferior impingement, thought to contribute to inferior scapular notching [17]. The clinical significance and long term impact of this phenomenon are still not clear.
Medialization and distalization of the centre of rotation
The location of the center of rotation(COR) affects range of motion until impingement, deltoid tension, the lever arm of the deltoid as well as the torque at the baseplate-bone interface [18]. In a modern RSA with inferior baseplate positioning and the COR shifted from the humeral head center to glenosphere center, the lever arm length is approximately doubled, and as a consequence the efficacy of the deltoid for abduction is approximately doubled [15]. Furthermore, as a consequence of COR medialization, more anterior and posterior deltoid fibers can be recruited to improve abduction strength. The other consequence of doubling the deltoid lever arm length is that the same deltoid excursion produces a lesser arc of motion. Thus strength is reduced after approximately 90° of abduction [15].
Indications
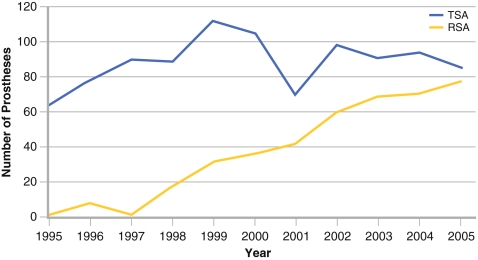
Indications for RSA have continued to evolve since the Grammont RSA was developed more than 20 years ago. Essential mechanical criteria for a successful RSA include having a functional deltoid and being able to achieve stable glenoid baseplate fixation. Initially, cuff tear arthropathy, massive cuff tear with arthritis and massive irreparable cuff tear were considered appropriate indications. The senior author (GW) has gradually changed his practice toward an increased use of the reverse prosthesis (Fig. 1). This was motivated by the poor results observed in some indications with unconstrained shoulder arthroplasties. By auditing outcomes at our institution, we demonstrated that the results of unconstrained prostheses were not satisfactory for certain indications. These included: (1) any kind of degenerative or inflammatory joint disease with cuff deficiency, (2) static humeral instabilities with associated glenohumeral arthritis, (3) primary or secondary arthritis with large or massive cuff tears or superior glenohumeral subluxation, (4) cuff tear arthropathy, (5) rheumatoid arthritis and (6) fracture sequelae or post-traumatic arthritis in cases of nonunion or severe malunion of the greater tuberosity.
Fig. 1.
Relative use of total and reverse shoulder arthroplasty at Centre Orthopédique Santy, Lyon, France from 1995 to 2005
This encouraged us to broaden our indications (Table 1). We now consider RSA in primary glenohumeral arthritis in three specific circumstances, depending on the age and motivation of the patient. These include: (1) static posterior subluxation with bi-concave glenoid (we have observed failures of unconstrained prostheses performed concomitantly with glenoid reconstruction), (2) fatty infiltration of infraspinatus and/or subscapularis greater than Goutallier stage two, and (3) associated cuff tear involving at least two tendons. We have also changed to the reverse prosthesis in fracture sequelae cases if the patient is older than 70 years. For the same reasons, three or four-part fractures in elderly patients with poor bone quality and very thin greater tuberosities were progressively more commonly treated with a reverse prosthesis. Similarly, patients with tumors necessitating tuberosity resection achieve better function with a RSA. These sometimes younger patients are warned that the prosthesis is only designed for a return to gentle activities of daily living, with no sports or strenuous activities allowed.
Table 1.
Summary of indications for reverse shoulder arthroplasty
| Cuff arthropathy |
| Massive irreparable cuff tear |
| Primary osteoarthritis (OA) with: |
| • an irreparable cuff tear greater than an isolated supraspinatus tear |
| • static posterior subluxation and biconcave glenoid (controversial) |
| • fatty infiltration of infraspinatus or subscapularis of Goutallier stage 2 or greater (controversial) |
| Sequelae of fracture |
| Displaced three or four part neck of humerus fractures in patients >75 years |
| Revision arthroplasty: |
| • Failed hemiarthroplasty or total shoulder arthroplasty with cuff deficiency |
| • In some cases to allow more reliable glenoid reconstruction with bone grafting |
| Tumors |
| Rheumatoid Arthritis |
RSA has also become our first option for revision of shoulder arthroplasty with cuff deficiency. In cases of revision TSA, when a glenoid reconstruction with bone graft is necessary (even if the cuff is still present), in our experience the RSA is a good solution. The metallic uncemented base plate allows a safer and better reconstruction than a cemented polyethylene component, with the base plate acting as a compression plate to stabilize the graft. The bone graft is under compressive load as opposed to shear forces in unconstrained prostheses, thereby enhancing graft healing.
Results
Grammont’s reverse shoulder prosthesis, initially implanted for cuff tear arthropathy, has been shown to give good short to medium term results [19], reliably restoring overhead function and relieving pain [17, 20–22]. Significant functional improvements have been demonstrated in large cohorts. Wall et al. [23] investigated 240 RSAs at mean 40 months post operatively and demonstrated mean elevation of 137°, a Constant score improvement from 22.8 to 59.7 post operatively and a satisfaction rate over 93%. The improvements in active rotation are more limited, which are closely related to remaining cuff function, especially teres minor [24]. RSA combined with latissimus dorsi and teres major tendon transfer has been developed to improve external rotation and spatial control in patients with no functional teres minor and infraspinatus [25]. Early results are promising [26].
Massive cuff tear and cuff tear arthropathy
Several studies [23, 27] have demonstrated that cuff tear arthropathy and massive cuff tear are the most reliable indications for RSA. In the largest long term study, Favard et al. [28•] retrospectively reviewed 527 arthroplasties in 506 patients performed between 1985 and 2003. Survivorship free of revision was 89% at 10 years, with survivorship to a Constant score less than 30 of 72% at 10 years. Functional results started to decline after 8 years, which is similar to the findings in Guery’s study [29], which demonstrated deteriorating function and increasing pain after 6 years. Causes for this deterioration appear to be more complex than simply age related deltoid weakening. It may be related to minimal amounts of loosening which is not yet visible on radiographs [29], or posterior extension of the cuff tear to involve teres minor with loss of active external rotation in abduction. Further study is required to clarify the cause of this reduction in function at long-term follow-up. Based on these findings, RSA is still usually reserved for patients over the age of 70 years.
Rheumatoid arthritis
The results of unconstrained implants in patients with rheumatoid arthritis have been disappointing, largely secondary to rotator cuff dysfunction and proximal humeral migration. One long-term study demonstrated 100% proximal migration irrespective of cuff status at time of surgery [30]. We have previously advised against the use of RSA in rheumatoid arthritis due to concerns regarding infection and glenoid failure [29]. There is increasing evidence demonstrating good short and medium term outcomes [31–35]. In our series of 18 cases with minimum 2 year and average 3.8 year follow-up, Constant scores increased from 23 to 65, and 94% of cases were very satisfied or satisfied with the outcome of their surgery [31]. The major complication observed was an increased risk of intra and post-operative fracture (22%), involving the acromion, scapula spine, coracoid or greater tuberosity. We did not observe any infection or instability in our series, however, other studies have demonstrated a higher infection rate. One study [32] noted there was no negative correlation between disease severity and outcome. Inserting a smaller cemented stem is recommended to allow accurate positioning of the metaphyseal component without risking humeral fracture. We believe RSA is ideally suited to this population regardless of cuff integrity and can be performed safely, but still advise caution as the longer term results are unknown.
Acute proximal humerus fractures
Complex three and four part neck of humerus fractures frequently occur in elderly patients with poor quality bone and multiple underlying comorbidites. Hemiarthroplasty has commonly been recommended in the acute setting [36, 37]. Unfortunately, greater and lesser tuberosity complications can result in an unsatisfactory functional outcome [38, 39]. Despite anatomic positioning, bone grafting and comprehensive fixation, tuberosity resorption or displacement often occurs with consequent poor outcomes. Reverse shoulder arthroplasty may offer a more predictable result, without relying on tuberosity healing. Less intensive rehabilitation protocols allow earlier motion and return to function [40].
Multiple recent studies have considered the outcomes of RSA for acute proximal humeral fracture. Bufquin et al. [41] demonstrated satisfactory mobility and functional improvement in 43 consecutive patients with mean follow-up of 22 months, despite tuberosity migration in 19 (53%). Cazeneuve et al. [42] evaluated 36 patients with fractures with a mean follow-up of 6.6 years, expressing concern that Constant scores began declining over time. In the French Multicenter study [43], 15 cases from 457 were identified as indicated for fracture. The results of 11 at mean 46 months were compared with 139 hemiarthroplasties performed for fracture by the same authors. Mean elevation and Constant score differences were not statistically different, but the RSA group achieved a more consistent outcome. 50% of the hemiarthroplasty group did not achieve 90 degress elevation, compared with only 1 case (9%) in the RSA group. One RSA patient suffered a dislocation requiring revision.
In summary, the RSA in acute fractures has been shown to produce acceptable outcomes, which are comparable to hemiarthroplasty. Functional outcomes appear less dependent on tuberosity healing. In elderly patients with a predictably high tuberosity complication rate with hemiarthroplasty, RSA is likely to achieve a more reliable result, but longer-term follow-up studies are required.
Revision and fracture sequelae
RSA performed for revision of a failed shoulder arthroplasty or sequelae of a proximal humeral fracture is a technically challenging procedure, with few long term studies published. Wall et al. [23] investigated 28 patients with posttraumatic arthritis and 45 revision arthroplasties with 42 and 40 months follow-up respectively. Constant scores improved from 19.7 to 53 and 19.7 to 52.2, and elevation ranges were 115° and 118° respectively. These outcomes were significantly worse than the other groups studied, but represent a large functional gain, with 89% stating they were very satisfied or satisfied with the outcome. Failed hemiarthoplasties had similar improvements but lower baseline values when compared with failed TSAs. Complications of revision surgery (36.7%) were also significantly higher compared with primary surgery (13.3%). Boileau et al. [27] presented similar functional and subjective results, and a 42% revision rate for the revision group. Despite these rates, we believe that the complex nature of these patients justify the continued use of RSA.
Complications
Zumstein et al. [44•] reviewed the complication rates from 21 cohort studies with follow-up greater than 24 months. There were 188 complications in 782 cases (24%). The most common was instability (4.7%), followed by infection (3.8%). The rate of notching was 35%, but in this study, was considered a postoperative problem rather than a complication.
Notching
Notching of the scapular neck is a radiological finding related to abutment of the medial edge of the humeral component of a RSA. Nyffeler et al. [45] suggested polyethylene debris and osteolysis may play a role in its development. Notching rates vary widely in the available literature, from 0% to 96% [15, 17, 44•, 46–48]. Nevertheless, causal factors and clinical significance remain controversial.
Multiple large studies have investigated these issues. In an early cohort, Sirveaux et al. [17] suggested notching may be related to glenoid loosening and Simovitch et al. [48] reported that scapular notching was associated with a poorer clinical outcome. In contrast, Levigne et al. [46]noted that notching did not correlate with clinical outcome, but is more common in superior tilted and high positioned baseplates. In his long term study [28•], Favard et al. observed progressive radiographic changes and more frequent large notches with long-term follow-up, but no correlation with the Constant score.
In attempting to reduce notching, Nyffeler et al. [49] investigated the biomechanical relevance of baseplate positioning, concluding that placing the glenosphere distally significantly improved adduction and abduction range. Gutierrez et al. [50] simulated various design options, concluding that the most effective methods of avoiding adduction impingement was provided by a humeral neck-shaft angle of 130°, followed by inferior glenosphere position, a 10 mm lateral offset center of rotation, inferior tilt of the glenosphere and a 42 mm diameter glenosphere. Valenti et al. [47] recently published the results of 76 patients implanted with a new prosthesis with a center of rotation 8.5 mm lateralized, and a humeral neck cut of 135° built up to 155° with polyethylene. No notching and no glenoid loosening was reported at mean follow-up 44 months.
Another method of lateralizing the center of rotation is by use of bone autograft from the humeral head, the bony increase-offset or BIO-RSA [51]. This design has the benefit of lateralizing the prosthesis but maintaining the center of rotation within bone. At minimum 2 years, all grafts had healed, significant improvements in shoulder mobility were noted, and notching was observed in 9 of 42 cases. We also recommend BIO-RSA in cases with subscapularis insufficiency to improve prosthesis stability (Fig. 2).
Fig. 2.
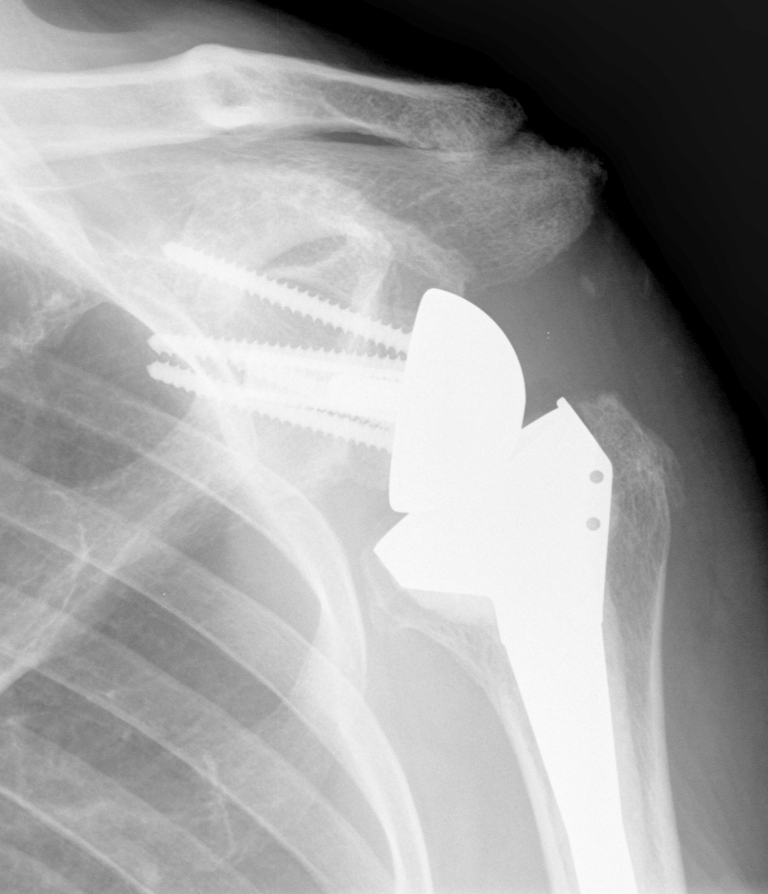
Postoperative x-ray of a 78 year old man who underwent a bony increased offset (BIO)-RSA for cuff tear arthropathy with subscapularis insufficiency
Infection
Infection is one of the most devastating complications of shoulder arthroplasty. The predisposing factors in RSA include a large subacromial potential space and a large prosthesis surface area. RSA infection rates range from 1% to 15% in published series [23, 44•, 52], with Zumstein’s systematic review reporting a deep infection rate of 3.8%. As expected, this was increased in the revision group (5.8% vs 2.9%). The common pathogens implicated are similar for both RSA and TSA, and include Propionibacterium acnes (P acnes), coagulase-negative Staphylococcus and Staphylococcus aureus [53]. P acnes, a gram-positive anaerobe, is thought to be a common organism in shoulder infection as it colonizes axillary sebaceous glands. Previously considered a contaminant, it is implicated as the pathogen in up to 75% of shoulder revision arthroplasty infections [54]. Diagnosis may be delayed as clinical signs are often subtle, inflammatory markers are often normal and cultures may not be positive for 2 weeks [55]. To improve diagnostic yield, aerobic and anaerobic cultures should be performed and incubation period prolonged (minimum 10 days) [55]. Management of an infected RSA is problematic due to patient age, previous surgery and the patient may not be medically suitable for further revision surgery. Therefore, often these patients end up with life-long suppressive antibiotics or resection arthroplasty.
Instability
Instability is a common and devastating complication of the RSA, with dislocation rates reported between 0 and 30% [17, 22–24, 52, 56–61]. Zumstein’s recent systematic review [44•] of 782 RSAs revealed instability as the most common postoperative complication, with a mean incidence of 4.7%. Of those whose indication was revision of previous TSA or hemiarthroplasty, incidence was significantly higher at 9.8%. There are many proposed causes, but there is a lack of knowledge regarding the significance of each. These include number and nature of prior procedures, surgical approach, bone deficiency, subscapularis insufficiency, mechanical factors (deltoid tension, impingement) and uncommonly trauma [62].
The deltopectoral approach was used for 97.3% of all dislocations in Zumstein’s review [44•], suggesting the superolateral approach has a much lower rate of instability. Large anterior and inferior releases are required, including the subscapularis tendon and the glenoid insertion of the glenohumeral ligament. Edwards et al. [63] reviewed 138 RSAs performed via a deltopectoral approach. All seven dislocations (5.1%) occurred in patients with an irreparable subscapularis tendon, leading to the conclusion that an attempt to repair the subscapularis should be made in every case, even in the case of severe fatty infiltration, muscle atrophy, and bone loss.
Shortening of the humerus and failure to restore adequate tension of the deltoid contributes to instability in the presence of insufficiency of the normal anterior-inferior restraints. This is particularly important in cases of revision and fracture sequelae utilizing a deltopectoral approach. Guttierrez et al. [50] biomechanically validated this concern by examining the stability of various in vitro articulations, concluding that compressive force is the most significant intrinsic stability factor, followed by socket depth; with glenosphere size playing a much lesser role. Favre et al. [62] used a mechanical testing machine to assess the effect of component positioning in preventing anterior dislocation. They proposed that the humeral component version is the critical factor for intrinsic stability and that it should be implanted in neutral or slight anteversion. They also showed that the glenosphere version is much less significant and instability increases if implanted in more than 10° of retroversion. The most appropriate humeral version for function and stability continues to be debated. We have found version not to be correlated with risk of dislocation, however we routinely implant in 0° to 20° of retroversion. In cases of significant humeral bone loss, we recommend obtaining scaled contralateral radiographs to determine appropriate prosthesis height and minimize risk of dislocation [64].
Acromial insufficiency
Surprisingly good RSA results can be achieved despite preoperative acquired or congenital acromial pathology. Acromial insufficiency was identified in 41 (9%) of 457 RSAs implanted at five French centers between 1992 and 2003 [65]. 23 presented with an os acromiale, 17 had fracture or fragmentation of the acromion and one had a pseudarthrosis of the scapular spine. Despite deltoid induced acromial tilt in most patients, postoperative range of motion, Constant scores and subjective results were not diminished. In contrast, postoperative spine fractures (4 patients or 0.8% in this study), generally have poor results [65, 66]. They often occur in the first year, and results of attempted internal fixation have been unsatisfactory. We recommend early conservative treatment with an abduction splint for 6 weeks.
Conclusion
The RSA represents the most significant advance in shoulder arthroplasty in recent decades. It results in excellent pain relief and highly satisfactory functional improvements in patients for whom conventional arthroplasty has previously yielded less satisfactory outcomes. Indications continue to evolve and the number of prostheses implanted globally is rapidly expanding. Surgeons need to be aware not only of potential benefits but also of complications and ongoing concerns regarding the longevity of this prosthesis. As such, we typically recommend not implanting RSAs in patients less than 70 years old.
Acknowledgments
Disclosure No conflicts of interest relevant to this article were reported.
Contributor Information
Christopher J. Smithers, Phone: +61-2-99267178, FAX: +61-2-99266311, Email: smitherschris@yahoo.com
Allan A. Young, Phone: +61-2-94608888, FAX: +61-2-94606064, Email: youngadmin@sydneyshoulder.com.au
Gilles Walch, Phone: +33-437-530024, FAX: +33-437-530025, Email: walch.gilles@wanadoo.fr.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Neer CS, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232–1244. [PubMed] [Google Scholar]
- 2.Neer CS, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319–337. [PubMed] [Google Scholar]
- 3.Burkhart SS. Arthroscopic treatment of massive rotator cuff tears. Clinical results and biomechanical rationale. Clin Orthop Relat Res. 1991;267:45–56. [PubMed] [Google Scholar]
- 4.Franklin JL, Barrett WP, Jackins SE, et al. Glenoid loosening in total shoulder arthroplasty: association with rotator cuff deficiency. J Arthroplasty. 1977;3(1):39–46. doi: 10.1016/S0883-5403(88)80051-2. [DOI] [PubMed] [Google Scholar]
- 5.Neer CS. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1–13. [PubMed] [Google Scholar]
- 6.Marmor L. Hemiarthroplasty for the rheumatoid shoulder joint. Clin Orthop Relat Res. 1977;122:201–203. [PubMed] [Google Scholar]
- 7.Lettin AW, Copeland SA, Scales JT. The Stanmore total shoulder replacement. J Bone Joint Surg Br. 1982;64(1):47–51. doi: 10.1302/0301-620X.64B1.7068719. [DOI] [PubMed] [Google Scholar]
- 8.Linscheid RL, Colfield RH. Total shoulder arthroplasty: experimental but promising. Geriatrics. 1976;31(4):64–69. [PubMed] [Google Scholar]
- 9.Cofield RH. Status of total shoulder arthroplasty. Arch Surg (Chicago, Ill.: 1960) 1977;112(9):1088–1091. doi: 10.1001/archsurg.1977.01370090070014. [DOI] [PubMed] [Google Scholar]
- 10.Broström LA, Wallensten R, Olsson E, et al. The Kessel prosthesis in total shoulder arthroplasty. A five-year experience. Clin Orthop Relat Res. 1992;277:155–160. [PubMed] [Google Scholar]
- 11.Fenlin JM. Total glenohumeral joint replacement. Orthop Clin North Am. 1975;6(2):565–583. [PubMed] [Google Scholar]
- 12.Wretenberg PF, Wallensten R. The Kessel total shoulder arthroplasty. A 13- to 16-year retrospective followup. Clin Orthop Relat Res. 1999;365:100–103. doi: 10.1097/00003086-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Grammont P, Trouilloud P, Laffay JP, et al. Concept study and realization of a new total shoulder prosthesis. Rhumatologie. 1987;39:407–418. [Google Scholar]
- 14.Matsen FA, Boileau P, Walch G, et al. The reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2007;89(3):660–667. doi: 10.2106/00004623-200703000-00027. [DOI] [PubMed] [Google Scholar]
- 15.Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284–295. doi: 10.5435/00124635-200905000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Gutiérrez S, Luo Z, Levy J, et al. Arc of motion and socket depth in reverse shoulder implants. Clin Biomech. 2009;24(6):473–479. doi: 10.1016/j.clinbiomech.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Sirveaux F, Favard L, Oudet D, et al. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388–395. doi: 10.1302/0301-620X.86B3.14024. [DOI] [PubMed] [Google Scholar]
- 18.Hsu SH, Greiwe RM, Saifi C, et al. Reverse total shoulder arthroplasty—biomechanics and rationale. Oper Tech Orthop. 2011;21(1):52–59. doi: 10.1053/j.oto.2010.10.006. [DOI] [Google Scholar]
- 19.Baulot E, Chabernaud D, Grammont PM. Results of Grammont’s inverted prosthesis in omarthritis associated with major cuff destruction. Apropos of 16 cases [French] Acta Orthop Belg. 1995;61(Suppl 1):112–119. [PubMed] [Google Scholar]
- 20.Molé D, Favard L. Excentered scapulohumeral osteoarthritis [French] Rev Chir Orthop Reparatrice Appar Mot. 2007;93(6 Suppl):37–94. doi: 10.1016/S0035-1040(07)92708-7. [DOI] [PubMed] [Google Scholar]
- 21.Boileau P, Sinnerton RJ, Chuinard C, et al. Arthroplasty of the shoulder. J Bone Joint Surg Br. 2006;88(5):562–575. doi: 10.1302/0301-620X.88B5.16466. [DOI] [PubMed] [Google Scholar]
- 22.Frankle M, Siegal S, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697–1705. doi: 10.2106/JBJS.D.02813. [DOI] [PubMed] [Google Scholar]
- 23.Wall B, Nove-Josserand L, O’Connor DP, et al. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476–1485. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 24.Boileau P, Watkinson DJ, Hatzidakis AM, et al. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 Suppl S):147S–161S. doi: 10.1016/j.jse.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Boileau P, Chuinard C, Roussanne Y, et al. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin Orthop Relat Res. 2008;466(3):584–593. doi: 10.1007/s11999-008-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boileau P, Rumian AP, Zumstein MA. Reversed shoulder arthroplasty with modified L’Episcopo for combined loss of active elevation and external rotation. J Shoulder Elbow Surg. 2010;19(2 Suppl):20–30. doi: 10.1016/j.jse.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Boileau P, Watkinson D, Hatzidakis A, et al. Neer award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527–540. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 28.• Favard L, Lévigne C, Nerot C, et al. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res. 2011;469(9):2469–75. A long term, large cohort study demonstrating functional survivorship does deteriorate over time with a break observed at 8 years. [DOI] [PMC free article] [PubMed]
- 29.Guery J, Favard L, Sirveaux F, et al. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for 5 to 10 years. J Bone Joint Surg Am. 2006;88(8):1742–1747. doi: 10.2106/JBJS.E.00851. [DOI] [PubMed] [Google Scholar]
- 30.Betts HM, Abu-Rajab R, Nunn T, et al. Total shoulder replacement in rheumatoid disease: a 16- to 23-year follow-up. J Bone Joint Surg Br. 2009;91(9):1197–1200. doi: 10.1302/0301-620X.91B9.22035. [DOI] [PubMed] [Google Scholar]
- 31.Young A, Walch G. Reverse shoulder arthroplasty in rheumatoid arthritis. J Bone Joint Surg Am. 2011, in press. [DOI] [PubMed]
- 32.Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2010;19(7):1076–1084. doi: 10.1016/j.jse.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 33.Lévigne C, Boileau P, Favard L, et al. Reverse shoulder arthroplasty in rheumatoid arthritis. In: Walch G, Boileau P, Molé, et al., editors. Reverse shoulder arthroplasty, clinical results, complications, revision. Montpellier: Sauramps Medical; 2006. pp. 165–178. [Google Scholar]
- 34.John M, Pap G, Angst F, et al. Short-term results after reversed shoulder arthroplasty (Delta III) in patients with rheumatoid arthritis and irreparable rotator cuff tear. Int Orthop. 2010;34(1):71–77. doi: 10.1007/s00264-009-0733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sperling JW, Cofield RH, Schleck CD, et al. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16(6):683–690. doi: 10.1016/j.jse.2007.02.135. [DOI] [PubMed] [Google Scholar]
- 36.Neer CS. Displaced proximal humeral fractures. II. Treatment of three-part and four-part displacement. J Bone Joint Surg Am. 1970;52(6):1090–1103. [PubMed] [Google Scholar]
- 37.Stableforth PG. Four-part fractures of the neck of the humerus. J Bone Joint Surg Br. 1984;66(1):104–108. doi: 10.1302/0301-620X.66B1.6693466. [DOI] [PubMed] [Google Scholar]
- 38.Antuña SA, Sperling JW, Cofield RH. Shoulder hemiarthroplasty for acute fractures of the proximal humerus: a minimum five-year follow-up. J Shoulder Elbow Surg. 2008;17(2):202–209. doi: 10.1016/j.jse.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 39.Boileau P, Krishnan SG, Tinsi L, et al. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):401–412. doi: 10.1067/mse.2002.124527. [DOI] [PubMed] [Google Scholar]
- 40.Wall B, Walch G. Reverse shoulder arthroplasty for the treatment of proximal humeral fractures. Hand Clin. 2007;23(4):425–430. doi: 10.1016/j.hcl.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Bufquin T, Hersan A, Hubert L, et al. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br. 2007;89(4):516–520. doi: 10.1302/0301-620X.89B4.18435. [DOI] [PubMed] [Google Scholar]
- 42.Cazeneuve JF, Cristofari D. The reverse shoulder prosthesis in the treatment of fractures of the proximal humerus in the elderly. J Bone Joint Surg Br. 2010;92(4):535–539. doi: 10.1302/0301-620X.92B4.22450. [DOI] [PubMed] [Google Scholar]
- 43.Sirveaux F, Navez G, Favard L, et al. Reverse prosthesis for acute proximal humerus fracture, the multicenter study. In: Walch G, Boileau P, Molé, et al., editors. Reverse shoulder arthroplasty, clinical results, complications, revision. Montpellier: Sauramps Medical; 2006. pp. 73–80. [Google Scholar]
- 44.Zumstein MA, Pinedo M, Old J, et al. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146–157. doi: 10.1016/j.jse.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Nyffeler RW, Werner CML, Simmen BR, et al. Analysis of a retrieved delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86(8):1187–1191. doi: 10.1302/0301-620X.86B8.15228. [DOI] [PubMed] [Google Scholar]
- 46.Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925–935. doi: 10.1016/j.jse.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 47.Valenti P, Sauzières P, Katz D, et al. Do less medialized reverse shoulder prostheses increase motion and reduce notching? Clin Orthop Relat Res. 2011;469(9):2550–7. [DOI] [PMC free article] [PubMed]
- 48.Simovitch RW, Zumstein MA, Lohri E, et al. Predictors of scapular notching in patients managed with the delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89(3):588–600. doi: 10.2106/JBJS.F.00226. [DOI] [PubMed] [Google Scholar]
- 49.Nyffeler RW, Werner CML, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524–528. doi: 10.1016/j.jse.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 50.Gutiérrez S, Keller TS, Levy JC, et al. Hierarchy of stability factors in reverse shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):670–676. doi: 10.1007/s11999-007-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boileau P, O’Shea K, Moineau G, et al. Bony increased-offset reverse shoulder arthroplasty (BIO-RSA) for cuff tear arthropathy. Oper Tech Orthop. 2011;21(1):69–78. doi: 10.1053/j.oto.2010.11.003. [DOI] [Google Scholar]
- 52.Trappey GJ, O’Connor DP, Edwards TB. What are the instability and infection rates after reverse shoulder arthroplasty? Clin Orthop Relat Res. 2010;469(9):2505–11. [DOI] [PMC free article] [PubMed]
- 53.Beekman PDA, Katusic D, Berghs BM, et al. One-stage revision for patients with a chronically infected reverse total shoulder replacement. J Bone Joint Surg Br. 2010;92(6):817–822. doi: 10.1302/0301-620X.92B6.23045. [DOI] [PubMed] [Google Scholar]
- 54.Kelly JD, Hobgood ER. Positive culture rate in revision shoulder arthroplasty. Clin Orthop Relat Res. 2009;467(9):2343–2348. doi: 10.1007/s11999-009-0875-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dodson CC, Craig EV, Cordasco FA, et al. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J Shoulder Elbow Surg. 2010;19(2):303–307. doi: 10.1016/j.jse.2009.07.065. [DOI] [PubMed] [Google Scholar]
- 56.Gallo RA, Gamradt SC, Mattern CJ, et al. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg. 2011;20(4):584–590. doi: 10.1016/j.jse.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 57.Boulahia A, Edwards TB, Walch G, et al. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthop. 2002;25(2):129–133. doi: 10.3928/0147-7447-20020201-16. [DOI] [PubMed] [Google Scholar]
- 58.Delloye C, Joris D, Colette A, et al. Mechanical complications of total shoulder inverted prosthesis [French] Rev Chir Orthop Reparatrice Appar Mot. 2002;88(4):410–414. [PubMed] [Google Scholar]
- 59.Nove-Josserand L, Walch G, et al. Instability of the reverse prosthesis. In: Walch G, Boileau P, Molé, et al., editors. Reverse shoulder arthroplasty, clinical results, complications, revision. Montpellier: Sauramps Medical; 2006. pp. 247–260. [Google Scholar]
- 60.Werner CML, Steinmann PA, Gilbart M, et al. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476–1486. doi: 10.2106/JBJS.D.02342. [DOI] [PubMed] [Google Scholar]
- 61.Wilde L, Sys G, Julien Y, et al. The reversed Delta shoulder prosthesis in reconstruction of the proximal humerus after tumour resection. Acta Orthop Belg. 2003;69(6):495–500. [PubMed] [Google Scholar]
- 62.Favre P, Sussmann PS, Gerber C. The effect of component positioning on intrinsic stability of the reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(4):550–556. doi: 10.1016/j.jse.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 63.Edwards TB, Williams MD, Labriola JE, et al. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):892–896. doi: 10.1016/j.jse.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 64.Lädermann A, Williams MD, Melis B, et al. Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):588–595. doi: 10.1016/j.jse.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 65.Walch G, Mottier F, Wall B, et al. Acromial insufficiency in reverse shoulder arthroplasties. J Shoulder Elbow Surg. 2009;18(3):495–502. doi: 10.1016/j.jse.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 66.Hattrup SJ. The influence of postoperative acromial and scapular spine fractures on the results of reverse shoulder arthroplasty. Orthopedics 2010;33(5):302. [DOI] [PubMed]