Abstract
With improvements in surgical techniques, implant design, and patient caremaps, surgeons have sought to accelerate early rehabilitation after total hip arthroplasty. Many authors have reported results of fundamentally similar protocols to achieve this end. These protocols focus on multi-modal pain management, early therapy, tissue-preserving surgical technique, and careful blood management. We present the implementation and results of such a protocol involving a different surgical approach, and highlight the published literature on this topic.
Keywords: Total hip arthroplasty, Rehabilitation, Accelerated mobilization protocol
Introduction
Total hip arthroplasty (THA) has become one of the most common and successful elective orthopedic procedures. Since its popularization in the late 1960s, surgeons have sought to improve the procedure on a number of different fronts. Cementless fixation of both acetabular and femoral components has proven reliable in early and long-term follow up. The greater range of implant modularity, including head size, head lengths, and stem offset options, has allowed more consistent recreation of hip geometry and has helped diminish the dislocation rate. Surgical approaches have evolved to be more tissue-sparing, generally leading to less early postoperative pain and less-restrictive precautions.
Coincident with the above improvements have come improvements in postoperative management. Traditional hospital stay after THA was quite long and the patients were severely restricted with regard to weightbearing, hip motion, and mobilization. Early mobilization is thought to decrease the incidence of known complications such as deep vein thrombosis (DVT), pulmonary embolism (PE), atelectasis, pneumonia, and urinary retention [1]. Early mobilization also improves patient satisfaction [2] and decreases the chance that the patient will require inpatient rehabilitation. While inpatient rehabilitation is appropriate for more debilitated patients, it does carry the risk of further exposure to nosocomial organisms and is an economic burden to the health care system. The ability to safely accelerate a patient’s recovery is dependent on coordinated preoperative, intraoperative, and postoperative management.
Herein we present one surgeon’s experience implementing an accelerated rehabilitation protocol for patients undergoing THA. As we will detail, the majority of the changes involved protocols for anesthesia, blood products, pain management, and therapy. The surgical approach, patient selection, and discharge criteria were not changed. The senior surgeon was already using a tissue-sparing technique for implantation [3]. Several such accelerated protocols have been published, and while many of the details may differ, the fundamental principles are similar. We are not attempting to compare this protocol to others, but merely to describe the rationale and implementation of the accelerated rehabilitation protocol and report the results. Our hypothesis is that combining tissue preserving techniques and uncemented implants with immediate mobilization on the day of surgery would decrease the length of stay without adverse effects on complications or readmissions.
Methods
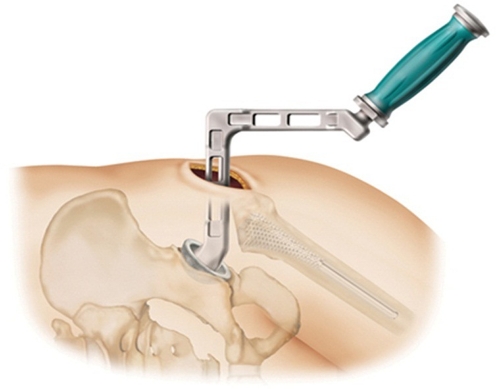
From March, 2010 to April, 2011, a total of 231 consecutive primary hips were replaced. Of these, 218 hips met the inclusion criteria of treatment using the superior capsulotomy surgical technique (Fig. 1) and patient age less than 80 years. No restrictions were placed on body mass index (BMI) or medical comorbidities. Mean age of the patients was 57.3 years (range 23.5–79.9 years), and 97 of the hip replacements were in men while 121 were in women.
Fig. 1.
Superior Capsulotomy Technique
Porous coated acetabular cups and proximally plasma-sprayed femoral components were used (Lineage Acetabular component, Renaissance ProFemur stem or Perfecta RS stem; Wright Medical Technology, Arlington, TN). These femoral components are distally-fluted, canal-filling, and proximally plasma-sprayed, and allow safe unrestricted weightbearing immediately after surgery. Tapered wedge stems were not used due to concerns about subsidence with immediate weightbearing [4]. All acetabular components were implanted using a patient-specific mechanical navigation device (Fig. 2, HipSextant, Surgical Planning Associates, Boston, MA) [5].
Fig. 2.
a. Patient-specific planning of mechanical navigation of acetabular component orientation. b. Intraoperative use of patient-specific mechanical navigation device
The superior capsulotomy approach, as previously published, was used in all cases. This technique allows for both the femoral and the acetabular components to be placed with the patient in a lateral position through an incision in the superior capsule, posterior to the abductors and anterior to the short external rotators. The hip is not dislocated during surgery. Rather, the femur is prepared in situ through the top of the femoral neck, the neck is then transected, and the femoral head is excised en bloc. The acetabulum is prepared under direct vision using angled reamers, and the socket is placed with an offset inserter. The final construct is then reduced in situ. This technique avoids disruption of both anterior and posterior static and dynamic stabilizing structures. The abductors are retracted anteriorly, but are otherwise undisturbed. The technique requires an angled acetabular reamer and offset cup inserter, but otherwise can be used for any primary hip implant system, with or without modular necks. Due to the inherent stability conferred by the approach, as well as the consistency of cup placement afforded by the mechanical navigation system, patients are allowed unrestricted hip motion immediately after surgery. The senior author was using this approach and these components prior to implementation of rapid mobilization, and did not change any part of the procedure as part of the protocol.
The protocol included multimodal analgesia consisting of preoperative acetaminophen, celecoxib (400 mg po the day before and 200 mg the morning of surgery unless allergic or known renal disease), and oxycontin (10 mg po immediately before surgery unless allergic), acetaminophen (975 mg po immediately before surgery unless allergic or known renal disease), and intraoperative bupivicaine injection into the surgical site. General anesthesia was used in all cases. The bupivicaine injection (1/4% with epinephrine up to 1 cc/kg) and was directed solely into the gluteus maximus muscle where it had been bluntly split and into the subcutaneous tissues. No attempt was made to inject capsular or muscular structures surrounding the joint, in order to avoid inadvertent effects on the sciatic nerve. The injection did not contain toradol, corticosteroid, or narcotic. Postoperatively, the celecoxib and acetaminophen were continued on a scheduled basis, and patients were immediately started on oral narcotics (oxycodone unless allergic). Subcutaneous injection of narcotic was used for break-through pain as needed. No patient-controlled analgesia (PCA) pumps were used. Intravenous narcotics (fentanyl, dilaudid) were minimized in the PACU and were not used on the hospital floor. The general anesthetic protocol was left to the preference of the anesthesiologist with emphasis on the use of shorter acting agents. The use of pre-emptive antiemetics was also at the discretion of the anesthesiologist before and during surgery.
Patients were encouraged to donate autologous blood as far as possible (up to 30 days) before surgery. For those patients able to donate blood, pre-emptive autologous blood transfusion of all donated blood was started in the operating room regardless of hemoglobin levels measured pre- or intraoperatively. The pre-emptive autologous transfusion represented a significant change from prior practice. Patients without autologous blood were transfused according to traditional protocol. Red cell salvage reservoir was used in all surgeries, and typically yielded between 150 and 300 mL of blood for reinfusion. This had been in standard use prior to implementing the new protocol.
Urinary catheters are not used unless exceptional circumstances are present, whereas they were universally used under the previous protocol. Catheters are avoided to reduce the risk of urinary tract infections and to facilitate mobilization after surgery. Even patients with a history of radical prostatectomy and benign prostatic hypertrophy are not catheterized unless they fail to void post-operatively. Patients who have unusually complex or prolonged surgery may have a single catheterization at the completion of the procedure.
Maintenance intravenous fluids are discontinued as early as possible, both to encourage oral intake and to allow the patient to be free of all physical tethers that would impede mobilization.
Patients are transferred from the OR to the PACU and then to the hospital floor on a stretcher, not a hospital bed, as was the practice previously. The stretcher serves as a physical reminder to all caregivers that the patient will be mobilized early. Immediately upon arrival to the floor, patients stand in the hallway and walk to the hospital bed with assistance on both sides, whereas before this protocol they commonly would remain in bed until the following morning. Crutches are preferred over a walker whenever possible since teaching a transition from a walker to crutches can be obviated. In cases where physical therapy staff is not available, the nursing staff mobilizes the patient for the first time. For more senior or frail patients, standing and pivoting at the bedside is performed instead. Thereafter, patients are encouraged to get up with nursing or physical therapy staff from one to several times on the day of surgery and encouraged to walk to the bathroom to void.
DVT prophylaxis has not changed with rapid rehabilitation. All patients were treated with adjusted-dose warfarin for 6 weeks post-operatively with an INR goal of 1.7 to 3. All patients receive pneumatic compression to the contralateral limb during surgery and to both limbs in the PACU. Pneumatic compression is discontinued as soon as the patient has been mobilized.
Discharge criteria were unchanged, and focus mainly on safe independent mobilization, adequate pain control, medical stability, adequate bowel and bladder function, and safe hemoglobin levels. Length of stay (LOS), disposition, readmission, and complications were assessed. Length of stay was compared to a control group of X patients treated by the same surgeon using the same implants and surgical technique prior to implementation of the accelerated rehabilitation protocol.
Results
In the 218 hips replaced since implementation of the accelerated mobilization protocol, the mean length of stay was 1.65 days (+/−0.89, range 0–8 days). Comparatively, the control group of 209 primary hips performed prior to implementation of the protocol had a mean length of stay of 3.54 days (+/−1.05, range 2–13 days). In the accelerated mobilization group, LOS was 0 days for 1 patient (<1%), 1 day for 115 patients (50%), 2 days for 71 patients (31%), and more than 2 days in the remaining 31 patients (13%). 215 patients were discharged home (99%). Of the 3 patients transferred to rehabilitation, one had cerebral palsy and another had end stage renal disease, a mechanical heart valve, and a longstanding complete sciatic palsy on the contralateral side. One patient, discharged on post op day 3, was readmitted 3 weeks postop for a GI bleed in association with prolonged anti-inflammatory use prior to surgery. Another patient, discharged 4 days after surgery, was readmitted 8 months later for I&D for acutely increasing pain but with no evidence of infection. Otherwise, there were no readmissions, reoperations, dislocations, nerve injuries, or post-discharge blood transfusions.
Conclusions
The current peri-operative immediate mobilization protocol, combined with tissue-preserving, minimally invasive surgical techniques and implants designed to achieve immediate fixation, resulted in dramatic reduction in length of stay and a higher percentage of patients returning directly home compared to our historical experience. Complications did not occur in any of the patients that were discharged within 48 h of surgery. Current studies are underway to assess if pre-emptive autologous blood transfusion is a statistically significant independent variable associated with outcome.
Discussion
Accelerated rehabilitation after THA is not a new concept. Even standard current protocols, in which patients spend 3–4 days in the hospital after surgery, are substantially “accelerated” compared to what was normal 10 or 20 years ago. The definition of what constitutes an “early” discharge should also be made in the context of individual patients, as it is clear that younger, stronger patients will typically be ready for discharge sooner than elderly, frail patients. The development of consistent, safe early discharge protocols requires that barriers to discharge be addressed in a coordinated fashion, simplifying and speeding the early postoperative recovery for patients. Much of the credit for current accelerated rehabilitation protocols goes to Berger, who has published numerous articles outlining a successful strategy for performing outpatient THA [6]. Following his work, many other authors have reported success with similar protocols, although most have targeted shortened LOS rather than achieving true outpatient status for the surgery [7–9]. The main barriers to early discharge are patient expectations, pain and nausea control, blood management, and mobilization. Several published protocols exist, with different strategies to address each of the barriers. The principles of these protocols can be applied to any surgical approach in any institution. Ultimately, the goal is safe surgery with earlier independence for the patient.
Preoperative screening and education
Centers that have reported success with accelerated recovery have typically employed a comprehensive preoperative screening and education program involving the surgeon, anesthesiologist, internal medicine consultants, as well as therapists and discharge planners. Suboptimally managed medical conditions must be corrected before surgery. Risk stratification by the medical and anesthesia teams allows the early development of specific anesthetic plans for each patient, as well as identifying those patients who might be inappropriate for early discharge [1].
Preoperative education, either with printed materials, videos, or formal classes, helps the patient develop appropriate expectations for the perioperative period and develop a plan for any aid likely to be required postoperatively. Those patients targeted for early discharge need consistent reinforcement of the expected plan from all members of the preoperative team. Outpatient or home health physical therapy is arranged before surgery. Preoperative physical therapy helps educate patients on the use of assistive devices, which decreases the need for postoperative teaching [10, 11]. Results of preoperative therapy intended for range of motion or strengthening, however, have been mixed with regard to length of stay [12].
Nausea control
Postoperative nausea is a consistent source of patient dissatisfaction and a hurdle to mobilization and discharge. Multimodal nausea-control strategies have been shown to be effective. Pre-emptive antiemetics are administered either preoperatively or during surgery. Scopalomine patches are known to reduce nausea in patients who typically experience motion sickness, and may be beneficial for all patients in preventing nausea. Ondansetron, metoclopramide, and dexamethasone may be given intraoperatively with the same goal [3]. Use of neuraxial anesthesia is also generally associated with decreased postoperative nausea, although as in the case of the protocol presented above, general anesthetic protocols may also be tailored toward shorter-acting agents that limit nausea. Postoperatively, limiting narcotic usage, encouraging oral intake, and mobilizing the patient, as well as continuing as-needed anti-emetics, will minimize nausea [13].
Blood management
Patients with symptomatic postoperative anemia will have difficulty mobilizing and cannot be discharged. Implementation of an early discharge protocol thus requires aggressive blood management to ensure stable hemoglobin levels as early as possible. Requirement for transfusion after THA is closely linked to preoperative hemoglobin levels [9]. Patients who are anemic at baseline can benefit from preoperative erythropoietin, if there are no contraindications.
The next step is to minimize intraoperative and postoperative blood loss. Use of platelet rich plasma and bipolar cautery devices have both been touted for this purpose, but have yet to show convincing results. Tranexamic acid has been shown in multiple studies to decrease postoperative transfusion need. A variety of protocols for its use have been published, without an obvious advantage of one over the other. Tranexamic acid may be give intravenously or injected directly into the wound. It is relatively inexpensive and does not seem to increase the rate of DVT or PE [14]. Hypotensive anesthesia, regardless of the type of anesthetic selected, is efficacious in reducing intraoperative blood loss, although it does carry some risk of cerebral hypoperfusion and must be used only in patients at low risk for ischemic brain injury.
Red cell saver reservoir use is somewhat controversial. It is clearly indicated in cases of expected high blood loss, but does confer a significant additional cost, and the reinfused blood may not have the same oxygen-carrying capacity. Autologous blood donation is also controversial. Generally speaking, those patients who donate blood preoperatively are more likely to receive a transfusion, but less likely to receive banked blood. There is a small but real risk of clerical errors causing a transfusion complication [15]. The decision to recommend this should be based in part on expected blood loss, which in turn is dependent on anesthetic and surgical techniques. Studies are ongoing at this institution regarding the efficacy of autologous blood use.
Despite attempts to optimize preoperative hemoglobin levels, minimize blood loss, and reinfuse suctioned blood, some patients will still require transfusion. Multiple transfusion protocols exist in the literature, but to summarize for the purpose of earlier discharge, the hemoglobin levels should be checked earlier than they might be otherwise, and transfusion criteria should be less restrictive. If not combined with the above methods of avoiding transfusion, this would likely result in a higher transfusion rate.
Pain control
Multimodal pain management and preemptive nausea control have proven beneficial in early mobilization, increased patient safety, and higher patient satisfaction. Some of the published multimodal pain management work has been discredited, but it has become clear that treating operative pain with more than just narcotics, especially preemptively, decreases total narcotic use, narcotic-associated complications, and patient pain [16]. Patients are treated before surgery, typically in the preoperative holding area, with a long acting narcotic such as oxycodone SR and celecoxib [8] Contraindications to use of celecoxib include a history of coronary artery disease, renal insufficiency, or potentially a sulfa allergy. Medications intended for the treatment of neuropathic pain, including gabapentin and pregabalin, may be administered adjunctively. Multimodal pain management continues after surgery, and may include celecoxib or other non-steroidal anti-inflammatory medications, scheduled acetaminophen, and oral narcotics.
Traditionally, THA was performed almost exclusively under general anesthesia (GA). While this is still and excellent option for many patients, it does have known postoperative complications, including atelectasis, sore throat, and nausea. Additionally, the postoperative transition from GA to either intravenous or oral narcotics is often quite painful for the patient, resulting in the use of high narcotic dosages. High narcotic dosing is not only dangerous from the standpoint of respiratory depression, but also causes unpleasant side effects such as nausea, pruritis, constipation, and lethargy [17]. The use of shorter-acting agents, especially the intravenous narcotics used during the procedure, seems to diminish the postoperative side effects. Local anesthetic injection in the surgical site at the conclusion of the procedure is an option to smooth transition to oral analgesia, although the literature on local injections for this purpose is mixed [18].
Regional anesthesia has increasing been used for THA and can be useful in an accelerated rehabilitation protocol. This includes spinal anesthesia, indwelling epidural anesthesia, and lumbar plexus blocks. All of these offer a reliable operative anesthetic while also affording pain control postoperatively. More recently, spinal anesthetic that combines a local anesthetic with Duramorph has emerged as an attractive way to continue analgesia postoperatively without a long motor blockade [3], although it does require pulse oximetry monitoring due to risk of delayed respiratory depression. Indwelling nerve block catheters, typically blocking the lumbar plexus, may be continued at a low dose after discharge [4]. The low infusion rate affords analgesia without motor blockade, lessening fall risk. Ultimately, the anesthetic protocol must be tailored to the strengths of the anesthesia team and the specific needs of each patient, but all basic modalities can be adapted to an accelerated rehabilitation protocol.
Therapy
Early and aggressive therapy after surgery is an essential part of any accelerated rehabilitation protocol. Ideally, therapy will begin shortly after the patient arrives on the ward. This may be a dramatic departure from standard protocol, and requires buy-in from the therapy staff. This will likely require special communication between the surgeon and the therapy staff at first about the nature and purpose of the protocol. Patients may require several attempts at ambulation before meeting therapy criteria for discharge, so starting shortly after surgery, and repeating attempts multiple times each day, may be necessary to safely condense the in-hospital convalescence.
The therapy staff at a given hospital may be under-sized and limited in its ability to work with patients multiple times each day. Thus, it is beneficial to provide basic training to the nursing staff about assisting patients with ambulation, and empower them to do so.
Postoperative restrictions placed on the patient by the surgeon have a direct impact on speed of mobilization. Surgical approaches that require less-restrictive postoperative precautions are very helpful in this respect, as they decrease the anxiety of both the nursing staff and the patient with respect to mobilization. Additionally, choice of implants that allows no restriction in weightbearing may allow the patient to use a less-restrictive assistive device. This is especially helpful in patients with upper extremity weakness or pain. In the protocol presented above, there are no restrictions placed on the patient postoperatively, much as has been reported for two-incision THA and the various anterior approaches. That said, all approaches, including posterior approach, are amenable to accelerated rehabilitation protocols [19].
Conclusions
Multiple authors have reported success in speeding early recovery and discharge after THA. Consistent shortening of postoperative stay after THA requires coordinated efforts to address traditional barriers to discharge. These barriers include patient expectations, postoperative nausea and anemia, poor pain control, urinary retention, and difficulty mobilizing. There is considerable overlap of several of these areas, such that any one modification to an existing protocol is unlikely to result in a demonstratable difference in LOS. The combination of tissue-preserving surgery, multi-modal pain and nausea management, careful blood management, and early and aggressive therapy can yield consistent improvements in early recovery without adversely affecting outcomes.
Acknowledgements
To George Babikian, MD of Orthopedic Associates of Portland, Portland ME, and to Nora Bowne, RN of Maine Medical Center, Portland, ME.
Disclosure S. Wellman: none; A. Murphy: none; D. Gulczynski: none; S. Murphy: Consultant to Ceramtec, GmbH; patents and royalties from Wright Medical Technology, Inc.; holds stock/stock options in Surgical Planning Associates, Inc.
References
- 1.Johanson NA, Lachiewicz PF, Lieberman JR, Lotke PA, Parvizi J, Pelegrini V, et al. Prevention of symptomatic pulmonary embolism in patients undergoing total hip or knee arthroplasty. J Am Acad Orthop Surg. 2009;17:183–196. doi: 10.5435/00124635-200903000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Mancuso CA, Salvati EA, Johanson NA, Peterson ME, Charlson MA. Patients’ expectations and satisfaction with total hip arthroplasty. J Arthroplast. 1997;12:387–396. doi: 10.1016/S0883-5403(97)90194-7. [DOI] [PubMed] [Google Scholar]
- 3.Murphy SB, Ecker TM, Tannast M. Total hip arthroplasty performed using conventional and navigated tissue-preserving techniques. Clin Orthop Relat Res. 2006;453:160–167. doi: 10.1097/01.blo.0000246539.57198.29. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs CA, Christensen CP. Progressive subsidence of a tapered, proximally coated femoral stem in total hip arthroplasty. Int Orthop. 2009;33:917–922. doi: 10.1007/s00264-008-0583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steppacher SD, Kowal JH, Murphy SB. Improving cup positioning using a mechanical navigation instrument. Clin Orthop Relat Res. 2011;469:423–428. doi: 10.1007/s11999-010-1553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger RA, Sanders SA, Thill ES, Sporer SM, Della Valle C. Newer anesthesia and rehabilitation protocols enable outpatient hip replacement in selected patients. Clin Orthop Relat Res. 2009;467:1424–1430. doi: 10.1007/s11999-009-0741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorr LD, Thomas DJ, Zhu J, Dastane M, Chao L, Long WT. Outpatient Total Hip Arthroplasty. J Arthroplast. 2010;25:501–506. doi: 10.1016/j.arth.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Lombardi AV, Berend KR, Adams JB. A rapid recovery program: early home and pain free. Orthopedics. 2010;33:656. doi: 10.3928/01477447-20100722-38. [DOI] [PubMed] [Google Scholar]
- 9.Mears DC, Mears SC, Chelly JE, Dai F, Vulakovich KL. THA with a minimally invasive technique, multi-modal anesthesia, and home rehabilitation. Clin Orthop Relat Res. 2009;467:1412–1417. doi: 10.1007/s11999-009-0785-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas KM, Sethares KA. An investigation of the effects of preoperative interdisciplinary patient education on understanding postoperative expectations following a total joint arthroplasty. Orthop Nurs. 2008;27:374–381. doi: 10.1097/01.NOR.0000342428.74830.67. [DOI] [PubMed] [Google Scholar]
- 11.Pour AE, Parvizi J, Sharkey PF, Hozack WJ, Rothman RH. Minimally invasive hip arthroplasty: What role does patient preconditioning play? J Bone Joint Surg. 2007;89(9):1920–1927. doi: 10.2106/JBJS.F.01153. [DOI] [PubMed] [Google Scholar]
- 12.Hoogeboom TJ, Dronkers JJ, Ende CH, Oosting E, Meetern NL. Preoperative therapeutic exercise in frail elderly scheduled for total hip replacement: A randomized pilot trial. Clin Rehabil. 2010;24:901–910. doi: 10.1177/0269215510371427. [DOI] [PubMed] [Google Scholar]
- 13.Buvanendran A, Kroin JS, Tuman KJ, Lubenow TR, Elmofty D, Moric M, et al. Effects of perioperative administration of selective cyclooxygenase 2 inhibitor on pain management and recovery of function after knee replacement: A randomized controlled trial. J Am Med Assoc. 2003;290:2411–2418. doi: 10.1001/jama.290.18.2411. [DOI] [PubMed] [Google Scholar]
- 14.Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg. 2011;93B(1):39–46. doi: 10.1302/0301-620X.93B1.24984. [DOI] [PubMed] [Google Scholar]
- 15.Hatzidikis AM, Mendlick MR, McKillip T, Reddy RL, Garvin KL. Preoperative autologous donation for total joint arthroplasty: An analysis of risk factors for allogenic transfusion. J Bone Joint Surg. 2000;82:89–100. doi: 10.2106/00004623-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Maund E, McDaid C, Rice S, Wright K, Jenkins B, Woolacott N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: A systematic review. Br J Anaesth. 2011;106:292–297. doi: 10.1093/bja/aeq406. [DOI] [PubMed] [Google Scholar]
- 17.Horlocker TT, Kopp SL, Pagnano MW, Hebl JR. Analgesia for total hip and knee arthroplasty: A multimodal pathway featuring peripheral nerve block. J Am Acad Orthop Surg. 2006;14:126–135. doi: 10.5435/00124635-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Kehlet H, Andersen LO. Local infiltration analgesia in joint replacement: The evidence and recommendations for clinical practise. Acta Anaesthesiologica Scandanavica 2011, Epub ahead of print. [DOI] [PubMed]
- 19.Meneghini RM, Smits SA. Early discharge and recovery with three minimally invasive total hip arthroplasty approaches: A preliminary study. Clin Orthop Relat Res. 2009;467:1431–1437. doi: 10.1007/s11999-009-0729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]