Abstract
Surgeons want to perform a perfect total hip replacement (THR) with every operation. Human performance has limitations, especially when performing a mechanical operation in a biological environment. Recent suggested changes to improve outcomes have been large femoral heads and anterior incisions, but unfortunately neither has resulted in any scientific data that change has been effected. The scientific data does tell us that poor component positions and impingement are the source of increasing mechanical complications. Therefore, attempts to improve the surgeon’s performance by precise quantitative knowledge in the operating room have been used. We discuss robotic guided navigation as a solution. This technology provides predictable and reproducible results.
Keywords: Robot, Navigation, THR, Technique, MAKO platform, Robotic assisted total hip arthroplasty
Introduction
Fifty years of innovation by surgeons have improved Charnley’s original total hip replacement operation (THR) operation. Bone ingrowth cup and stem designs are predictable for fixation and are demonstrating longevity [1]. Outcome data of Charnley THR beyond 20 years shows cemented femoral fixation to be durable and overall survivorship of 70%–75% because of cup revisions [2]. Failures of THR before 20 years, both cemented and noncemented, occur primarily because of impingement and wear [3]. Design changes to avoid impingement, such as offset femoral stems and large femoral heads; and material changes, such as highly cross linked polyethylene, have reduced the threat of early failure from poor design and poor plastic.
Despite the substantial improvements, complications and early mechanical failures have increased in occurrence [4•]. Dislocation, periprosthetic fractures, excessive wear with metal-on-metal large head articulations because of poor component position and squeaking and fracture of ceramic articulations, again because of poor component position, have diminished results of THR. The readmissions of patients with THR are at an all-time high of 8.6% (nearly double from 10 years ago) even though mortality is the lowest ever, suggesting readmissions are not for medical reasons [5]. Bozic’s review of revision data confirms the predominant complications are mechanical with dislocation the most common cause of revision [4•]. Medical centers in both Boston and at Stanford University have reported outliers of cup inclination and anteversion of 50% or more suggesting one cause of the mechanical complications [6••, 7].
As a solution to dislocation, the use of the direct anterior exposure of the hip has been promoted by some implant companies to improve postoperative recovery even though there is not any scientific data to support this marketing position. Likewise, large heads of 36 mm and greater are suggested to protect against dislocation. A change to direct anterior incision by surgeons who have used a posterior approach increases complications [8]. The use of large heads has detracted from meticulous attention to component position, especially the acetabulum, because the surgeon assumes the large head protects against failure and provides better function [9]. With metal-on-metal large heads the consequence has been increased wear and cyst formation [10].
In 2011, the greatest weakness of the THR operation is our human performance as surgeons. The implants and materials we have today promote longevity. To improve survivorship at 20 years to 95% or better, the outliers in our technical accomplishment of reconstruction of the hip must be eliminated [6••, 7]. The operation must be performed so that the technique is predictable and reproducible for 100% of patients. As surgeons working only with our experience, intuition and instinct, we cannot help but make judgment errors—human errors—because we are performing a mechanical operation in a biological environment. This situation creates risk for unintended complications and the risks are enhanced when we use a standardized operation for variable hip anatomy. Assumption of 15° anteversion of the femoral stem and cup versions of 45° inclination and 15–20° anteversion are the standards we have used. But all scientific data in the past 15 years have proven hip anatomy is very individual, and arthritis occurs in most patients because the hip anatomy is abnormal with impingement of the hip causing cartilage destruction [11, 12, 13•]. Therefore, application of “normality” to the THR can cause impingement of the implants or bone.
As surgeons, during our operations, we cannot visualize the relationship of the acetabulum to the pelvis and to the functional axis of the body through its spinopelvic dynamics [12], nor can we visualize the inner contour of the femur that affects the anteversion of the cementless stem [13•]. The judgment errors made because of inaccurate information of anatomy may cause short term complications such as dislocation and impingement pain, which cause feelings of failure for the surgeon; or later complications of wear and loosening which are absolute failures for the patient. Today, every human endeavor that involves a device has used modern technology of computers to minimize the human errors. A computer can provide quantitative knowledge which changes qualitative judgment decisions to accurate and precise ones. This technology is the solution we surgeons can use to solve our intraoperative dilemmas.
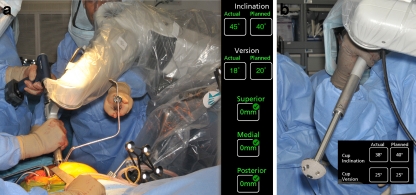
Nearly 3 years ago a surgeon research team (Lawrence Dorr, Richard ‘Dickey’ Jones, Mark Pagnano, and Robert Trousdale from Rochester Mayo Clinic plus Douglas Padgett and Amar Ranawat from Hospital for Special Surgery) set out to design an intraoperative technique which used computer technology to provide the surgeon with intraoperative quantitative knowledge that would enable a predictable, reproducible operation with every THR. We worked with the MAKO™ robotic guided navigation because of the accuracy of the technique and the fail safe mechanism against manual error of this technology (Fig. 1a–b). The operation could be personalized for each patient by a preoperative CT scan (Fig. 2a–b) and intraoperative registration of the patient’s anatomy. The tilt of the pelvis can be measured from the CT scan, as can the anteversion of the femur bone. The known pelvic tilt permits adjustment of the cup anteversion to the functional coronal plane of the patient’s body [14••]. The known anteversion of the femur gives an indication of stem anteversion, but stem anteversion may be different because of how the stem fits inside the bone [13•].
Fig. 1.
a The acetabulum is reamed with robotic guided navigation with the reamer constrained by a virtual haptic tunnel which prevents the surgeon from going off-line or too deep. The reamer stops if human error is made. The computer screen shows the angle of the reamer which only needs to be within 10° of desired numbers to create the correct hemisphere. The COR superiorly, medially, and anterior-posteriorly is defined and when the reamer achieves the correct COR the robot stops reaming. The robot is seen on the right-hand side of the figure and the surgeon’s hands hold the reamer. b The cup is connected to the robot and directed to the correct inclination and anteversion through a virtual haptic tunnel created by the robot. The computer screen will give the numbers achieved for inclination and anteversion and the haptic tunnel will not allow more than 5° variation from the plan
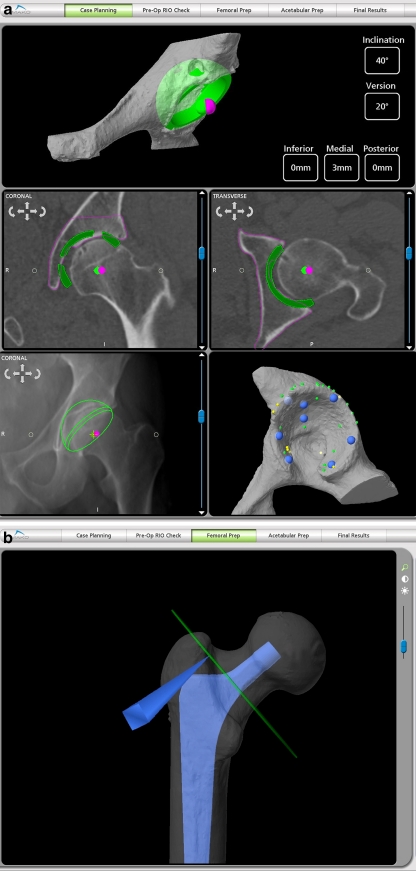
Fig. 2.
a Preoperative planning of the acetabular cup position on the CT scan allows restoration of the COR of the hip with the cup. Any difference between the COR of the cup (green dot) and the native hip (magenta dot) can be factored into the reconstruction of the femur. The depth of reaming is determined to create the COR position. The acetabular anteversion can be adjusted after the femoral anteversion is known (femur is prepared first). The CT planned cup position is then superimposed on the x-ray as seen in the lower left corner. The acetabular bony geometry of the CT scan is confirmed intraoperatively by registration as seen in the lower right hand corner. b The femoral neck cut is determined by restoring the femoral head center for the stem implant used. Differences between the stem COR and the bony femoral COR can be factored into the leg length and offset. The modular head desired can be selected according to any differences needed to correct leg length and offset. A defined level of neck cut (green line), with the stageline of the stem implanted at this level, will restore the leg length and offset the surgeon chooses during preoperative planning
Technical considerations
Preoperative CT scan A pre-operative CT scan is required for each patient undergoing THA with RIO (Makoplasty Hip Procedure). The CT scan does subject patients to three times the radiation of our usual hip plain radiographic series. We have never had a patient object because they understand the advantage of the surgeon knowing their unique anatomy which will allow their operation to be personalized for them. The patient specific virtual 3-D bone model of the pelvis and femur is created by the software and specific points are defined on the anatomy to help the software determine the patient’s position intra-operatively. The software accounts for the pelvic tilt by using the patient’s anterior/posterior pelvic tilt when lying supine on the CT table. All inclination and version numbers include this tilt, and so are on the radiographic plane of Murray (coronal plane) [15]. This implants the cup on the coronal (functional) plane of the body which is more correct than the anatomic plane.
Pre-operative planning The cup is positioned for depth of reaming in the acetabulum and the stem positioned by the correct neck cut of the femoral bony neck to reconstruct the leg length and offset. Of most importance, the center of rotation of the cup and of the femoral stem is determined (Fig. 2a–b).
Pelvic array placement If the operation is to be done in the lateral position, the patient is turned lateral, and secured with anterior and posterior supports for both pelvis and chest. With aseptic precaution, three threaded pins are inserted in the thickest portion of iliac crest (where bone grafts are taken) after making stab wounds with #15 blade. After the pins have been inserted, the pelvic attachment device is inserted onto the pins.The operative area, including the device, is covered with a betadine drape (Ioban 3 M). This base is used for attachment of the pelvic array (seen in the lower center of Fig. 1a).
Femoral registration Either an anterior or posterior exposure can be used for the operation. The femoral head is dislocated. Femoral registration requires insertion of two screws; one large screw for holding the femoral array and a smaller screw to be used to verify accuracy of registration (check point). The position for screw and check point is different for the posterior and the anterolateral approaches. For the posterior approach the large screw is inserted in the cortical bone at the junction of the intertrochanteric ridge and the lesser trochanter. The femoral check point (smaller screw) is placed just anterior to this in the greater trochanter and is verified by touching the probe to it. If the array screw becomes loose, the accuracy verification by the check point screw will show this. If this happens, the registration is no longer valid and final leg length and offset can be inaccurate.Femoral registration is accomplished by touching the probe to thirty two required points on the proximal femur as identified on the software and displayed on the computer screen. These points verify the anatomic geometry defined preoperatively by the CT scan. Ideally the femoral registration error should be less than 0.5 mm. Verification of the registration is done by touching the probe to the surface of the bone on 6–8 points of the proximal femur. If the registration error is more than 1 mm the verification fails and the surgeon must re-register the femur.
Femoral preparation After registration, the level of femoral bony neck cut is marked by touching the probe to the bone. The computer displays where the probe touches the bone in relation to the cut line on the screen (Fig. 2b). The cut level is marked with the bovie tip. The femur is prepared first to measure the anteversion of the femoral stem so that the cup anteversion can be adjusted to the stem anteversion and provide the correct combined anteversion of 25–45° [16]. The femur is prepared with broaches and with the final broach in place the anteversion is measured. The broach position in the femur allows the software to provide the millimeters of change in leg length and offset that will be obtained with a neutral head. This is accurate because the cup COR is known and will not change since the reaming accuracy of the acetabulum is controlled by the robot. With the final broach, the stem COR is known and is matched to the acetabular COR to give the leg length and offset reconstruction.
Acetabular registration The pelvic check point (screw) is inserted outside the acetabular cavity in the bone just superior to the posterior-superior acetabulum rim. The screw is directed away from acetabular surface. The probe is touched to the pelvic check point to verify the registration. Thirty two points on the acetabulum are identified by the software for the registration process and are touched using the probe to the bone surface. Verification is done by touching the probe to 8–10 points defined on the surface of the acetabulum (Fig. 2a). As with the femur, if the software displays a registration error of more than 1 mm the registration must be repeated.
Acetabular reaming The preoperative cup plan may need to have the desired anteversion of the cup changed after stem anteversion is known. The surgeon can ream by using multiple reamers to achieve the final socket size. However, the RIO Robotic Arm will also allow the surgeon to ream only once with the final planned size (Fig. 1a). If the surgeon starts reaming 3 mm or more below the planned cup size, s/he has the ability to orient the reamer in any direction; however, the reamer itself remains constrained to the plan so it cannot go “out of bounds” in the superior, medial or anterior-posterior directions. When reaming is started within 2 mm of the planned cup size, the surgeon must ream within +/− 10° of the planned cup position (constrained by virtual haptic tunnel). The reaming is line-to-line because of the precision of the reaming process (e.g. for 50 mm cup ream to 50 mm). For patients with hard bone or a sclerotic acetabulum, the acetabular rim is reamed 1 mm greater than the cup size (e.g. ream to 51 mm for 50 mm cup). The reaming is complete when the COR numbers for superior/inferior, medial/lateral, anterior/posterior are 0 and turn green. The 3D model of the bone will also illustrate the planned bone resection has been achieved when the green color of the acetabulum has been removed, showing white bone. Both methods confirm the surgeon has reached the established acetabular center of rotation. The bone model will turn red when the surgeon has reamed more than 0.5 mm past the plan. When the surgeon has reamed 1 mm past the plan in any direction the power drill will turn off.
Final cup placement The precision of the MAKO® robotic guided reaming creates such predictable accuracy that a trial cup is not needed. The porous shell is loaded onto the robotic arm and inserted in the acetabulum through a haptic tunnel which keeps the cup inclination and anteversion within 3° of the plan as the cup is implanted (Fig. 1b). After implantation of the cup the plastic liner is inserted. A standard liner should be used because the correct combined anteversion has been reconstructed so stability is insured and a hooded liner may cause impingement. After the liner is inserted the fit plane measurement can be done to confirm the cup position by touching the liner with the probe at five points. If the inclination and/or anteversion change by 4° or more the cup is likely loose, and screws should be inserted.
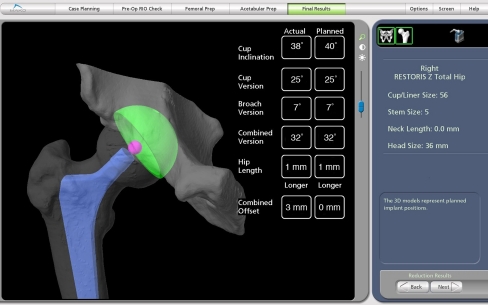
Final reduction and numbers The final stem is implanted and the trial head placed on the taper. The hip is reduced and the femoral array is inserted into the femoral screw. The computer screen displays the leg length and offset change compared to the planned change (Fig. 3). The adjustment to a shorter or longer head length is done as needed. This final computer screen shows quantitative numbers of the entire reconstruction compared to the planned reconstruction.
Fig. 3.
The final screen at completion of the reconstruction shows all the quantitative numbers for component position and biomechanical reconstruction with the final numbers compared to the preoperative plan
Conclusions
Impingement of the hip replacement, either implant or bone, can be avoided in all but the most flexible hips (hips with flexible spinopelvic dynamics as revealed by high preoperative anterior tilt of the pelvis of more than 15°) by correct combined anteversion of the stem and cup (safe zone 25–45°, 50° for flexible hips) to avoid component impingement; and correct center of rotation (COR) of the hip to avoid bone-on-bone impingement [3]. The COR is known after preoperative planning and will be achieved at surgery because of the precision of the robotic navigation (Fig. 2a). Knowing the COR of the acetabulum allows the correct femur bony neck cut to reconstruct the desired leg length and offset (Fig. 2b). In most hips it is beneficial to increase the offset up to 5 mm for clearance of the hip throughout the range of motion because the prosthetic head always has a smaller diameter than the bony head [17•, 18]. Accurate intraoperative quantitative knowledge of leg length and offset is immensely valuable to the surgeon.
Although radiographic measurement of leg length difference can be up to 6 mm without patient complications, the clinical measurement cannot exceed 2.5 mm, the difference because of radiographic rotation and magnification [19].
In summary, improvement of human performance for THR is the current most necessary innovation for this operation. Improving human performance in surgery will be done by machines in the OR just as it is in every other human endeavor outside of surgery. The MAKO™ robotic guided navigation gives precision of bone preparation of the femur and acetabulum (Fig. 3); it gives quantitative knowledge of component position and biomechanical reconstruction of leg length and offset; and it has a fail safe mechanism for acetabular preparation and cup implantation. For these reasons our surgical design team believes this technology is a valuable innovation for THR.
Acknowledgments
Disclosures R. Tarwala: none; L. Dorr: stock/stock options in Mako and Total Joint Orthopedics.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Kim YH, Kim JS, Park JW, Joo JH. Comparison of total hip replacement with and without cement in patients younger than 50 years of age. J Bone Joint Surg [B] 2011;93B:449–455. doi: 10.1302/0301-620X.93B4.26149. [DOI] [PubMed] [Google Scholar]
- 2.Callaghan JJ, Forest EE, Olejniczak JP, Goetz DD, Johnston RC. Charnley total hip arthroplasty in patients less than 50 years old; a 20–25 year follow-up note. J Bone Joint Surg Am. 1998;80-A:704–714. doi: 10.2106/00004623-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Malik A, Maheshwari A, Dorr LD. Impingement with total hip replacement. J Bone Joint Surg Am. 2007;89(8):1832–1842. doi: 10.2106/JBJS.F.01313. [DOI] [PubMed] [Google Scholar]
- 4.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 5.Cram P, Lu X, Kaboli PJ, Vaughan-Sarrazin MS, Cai X, Wolf BR, Li Y. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty. JAMA. 2011;305(15):1560–1567. doi: 10.1001/jama.2011.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callanan MC, Jarrett B, Bragdon CR, Zurakowski D, Rubash HE, Freiberg AA, Malchau H. Risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop Relat Res. 2011;469:319–329. doi: 10.1007/s11999-010-1487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein NJ, Woolson ST, Jiori NJ. Acetabular component positioning using the transverse acetabular ligament: can you find it and does it help? Clin Orthop Relat Res. 2011;469:412–416. doi: 10.1007/s11999-010-1523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jewett BA, Collis DK. High complication rate with anterior total hip arthroplasties on a fracture table. Clin Orthop Relat Res. 2011;469:503–507. doi: 10.1007/s11999-010-1568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammerberg EM, Wan Z, Dastane M, Dorr LD. Wear and range of motion of different femoral head sizes. J Arthroplasty. 2010;25:839–843. doi: 10.1016/j.arth.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Hart AJ, Ilo K, Underwood R, Cann P, Henckel J, Lewis A, Cobb J, Skinner J. The relationship between the angle of version and rate of wear of retrieved metal-on-metal resurfacings. J Bone Joint Surg Br. 2001;93-B:315–320. doi: 10.1302/0301-620X.93B3.25545. [DOI] [PubMed] [Google Scholar]
- 11.Harris WH. The correlation between minor or unrecognized developmental deformities and the development of osteoarthritis of the hip. Instr Course Lect. 2009;58:257–259. [PubMed] [Google Scholar]
- 12.Lazennec JY, Boyer P, Gorin M, Catone Y, Rosseau MA. Acetabular anteversion with CT in supine, simulated standing, and sitting positions in a THA patient population. Clin Orthop Relat Res. 2011;469:1103–1109. doi: 10.1007/s11999-010-1732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorr LD, Wan Z, Malik A, Zhu J, Dastane M, Deshmane P. A comparison of surgeon estimation and computer tomographic measurement of femoral component anteversion in cementless total hip arthroplasty. J Bone Joint Surg Am. 2009;91(11):2598–2604. doi: 10.2106/JBJS.H.01225. [DOI] [PubMed] [Google Scholar]
- 14.Zhu J, Wan Z, Dorr LD. Quantification of pelvic tilt in total hip arthroplasty. Clin Orthop Relat Res. 2010;468(2):571–575. doi: 10.1007/s11999-009-1064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75(2):228–232. doi: 10.1302/0301-620X.75B2.8444942. [DOI] [PubMed] [Google Scholar]
- 16.Dorr LD, Malik A, Dastane M, Wan Z. Combined anteversion technique for total hip arthroplasty. Clin Orthop Relat Res. 2009;467(1):119–127. doi: 10.1007/s11999-008-0598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Little NJ, Busch CA, Gallagher JA, Rorabeck CH, Bourne RB. Acetabular polyethylene wear and acetabular inclination and femoral offset. Clin Orthop Relat Res. 2009;67:2895–2900. doi: 10.1007/s11999-009-0845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakalkale DP, Sharkey PF, Eng K, Hozack WJ, Rothman RH. Effect of femoral component offset on polyethylene wear in total hip arthroplasty. Clin Orthop Relat Res. 2001;388:125–134. doi: 10.1097/00003086-200107000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Dastane M, Dorr LD, Tarwala R, Wan Z. Hip offset in total hip arthroplasty: quantitative measurement with navigation. Clin Orthop Relat Res. 2011;469:429–436. doi: 10.1007/s11999-010-1554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]