Abstract
Objectives
To describe the association between two novel biomarkers, calprotectin and leucine-rich alpha glycoprotein-1 (LRG), and appendicitis in children.
Methods
This was a prospective, cohort study of children 3 to 18 years old presenting to a pediatric emergency department with possible appendicitis. Blood and urine samples were assayed for calprotectin and LRG via enzyme-linked immunosorbent assay. Final diagnosis was determined by histopathology or telephone follow-up. Biomarker levels were compared for subjects with and without appendicitis. Recursive partitioning was used to identify thresholds that predicted appendicitis.
Results
Of 176 subjects, mean age was 11.6 years (SD ±4.0 years) and 52% were male. Fifty-eight patients (34%) were diagnosed with appendicitis. Median plasma calprotectin, serum LRG, and urine LRG levels were higher in appendicitis versus non-appendicitis (p < 0.008). When stratified by perforation status, median plasma calprotectin and serum LRG levels were higher in non-perforated appendicitis vs. non-appendicitis (p < 0.01). Median serum LRG, urine LRG, and plasma calprotectin levels were higher in perforated appendicitis as compared to non-perforated appendicitis (p < 0.05). Urine calprotectin did not differ among groups. A serum LRG < 40,150 ng/ml, a urine LRG < 42 ng/ml, and a plasma calprotectin < 159 ng/ml, each provided a sensitivity and negative predictive value of 100% to identify children at low risk for appendicitis, but with specificities ranging from 23% to 35%. The standard white blood cell (WBC) count achieved 100% sensitivity at a higher specificity than both novel biomarkers.
Conclusions
Plasma calprotectin and serum/urine LRG are elevated in pediatric appendicitis. No individual marker performed as well as the WBC.
INTRODUCTION
Despite rapid increases in computed tomography (CT) utilization, pediatric appendicitis remains a challenging diagnosis, with 5% to 25% negative appendectomy, and 10% to 45% perforation rates.1–5 Novel biological markers for appendicitis represent a potential method to improve diagnostic accuracy. Through the use of advanced molecular techniques, several recent publications have identified proteins that are differentially expressed in the diseased appendix.6,7 These potential new biomarkers for appendicitis are markers of inflammation, as their up-regulation represents neutrophil differentiation, activation, or degranulation. With the risk of radiation exposure from CT, time delays resulting from CT use, and the cost of additional imaging, novel biomarkers for appendicitis may hold value as a means to stratify children with acute abdominal pain into clinical risk groups.8
Two of the novel biomarkers that may be clinically useful to assess for appendicitis are S-100:A8/A9 (also called calprotectin), and leucine-rich alpha glycoprotein-1 (LRG).7,9 Calprotectin is a protein complex consisting of S-100:A8 and S-100:A9. The two S-100 proteins are found within the cytoplasm of neutrophils and are released by neutrophils that are degranulating.10 Calprotectin is thought to have anti-microbial activity, likely through zinc chelation.10 LRG is a protein secreted by liver cells and by neutrophils undergoing differentiation.11 Although its exact function is not known, it is up-regulated in patients with acute inflammatory and bacterial conditions.12,13 Investigators recently described favorable test performance characteristics for serum calprotectin for diagnosing appendicitis in adult patients.9 Other investigators identified LRG as being selectively enriched in the urine of pediatric patients with appendicitis.7 Although promising, more research is needed to understand the potential clinical utility of these biomarkers. Therefore, in this pilot study, we aimed to determine the association between serum and urine levels of calprotectin and LRG and appendicitis in children with suspected appendicitis, and to identify calprotectin and LRG thresholds that could potentially be used to diagnose or exclude pediatric appendicitis.
METHODS
Study Design
We conducted a prospective, cross-sectional study in an urban, tertiary-care pediatric emergency department (ED) with approximately 50,000 visits per year. We obtained written informed consent from all parents and assent from children more than seven years of age. The study was approved by the local institutional review board.
Study Setting and Population
From July 2009 to April 2010, children three to 18 years of age who presented to the ED with acute abdominal pain of less than 96 hours duration, and who were being evaluated for possible appendicitis, were considered for enrollment. We defined “possible appendicitis” as the treating physician choosing to obtain blood tests, radiological studies (CT and/or ultrasound [US]) or a surgical consultation for the purpose of diagnosing appendicitis. It is standard practice in our ED to obtain a white blood cell (WBC) count for all patients with suspected appendicitis. Radiological studies or surgical consultations are obtained at the discretion of the treating physician. We excluded patients with any of the following conditions: pregnancy, prior abdominal surgery (e.g. gastrostomy tube, abdominal hernia repair), chronic illness that potentially affected the gastrointestinal system (e.g. cystic fibrosis, inflammatory bowel disease, sickle cell anemia, chronic pancreatitis, diabetes, immune-suppression), or a medical condition that limited the conduct of an accurate history or physical examination (e.g. substantial language or developmental delay). We also excluded patients with radiologic studies (CT or US) of the abdomen performed prior to ED arrival, and those who had a history of abdominal trauma within the preceding seven days of ED evaluation. Once a patient was deemed eligible, the patient and family were approached for written informed consent and assent.
Study Protocol
We collected patient history and physical examination data via structured case report forms created specifically for this study. The treating pediatric emergency physicians (EPs) completed the forms prior to knowledge of any radiological study results (CT or US), if obtained. Patients’ medical records were abstracted to obtain data from laboratory, radiology, pathology, and operative reports. A single research assistant entered data into SPSS (Version 18.0, Chicago, IL); all data were double-checked for accuracy by one author. Enrollment occurred 24 hours a day, 7 days a week. We reviewed the daily ED admission log and electronic tracking system in order to identify potentially eligible patients who were not enrolled (i.e. missed). No formal sample size calculations were conducted, as the blood or urine levels of LRG or calprotectin by enzyme-linked immunosorbent assay (ELISA) have not been previously described for appendicitis.
Serum Collection
We obtained two additional blood samples (3 cc to 5 cc into a serum separator tube and 3 cc to 5 cc into a K+-EDTA plasma tube) in the ED. These additional samples were centrifuged within one hour of collection at a speed of 1300 × g for 10 minutes. During the hours of 09:00 to 16:00, trained laboratory technicians (located within a specialized laboratory of our hospital) then immediately divided the centrifuged serum sample into two aliquots and froze the samples at −80 °C. After 16:00 on weekdays and at all times on weekends, the spun serum samples were stored at 4 °C. The following business day, technicians divided each sample into two aliquots and froze the blood at −80C. According to the manufacturer’s instructions, the reproducibility of the calprotectin assay was partially dependent on careful handling and processing of the K+-EDTA plasma tube. Therefore, regardless of time of day or of week, after centrifugation, the plasma portion was removed from the K+-EDTA tube and transferred to a cryopreservation tube, taking care not to disrupt the red blood cell pellet. This sample was subsequently frozen at −80 °C. Samples were processed and assayed in accordance with published guidelines.14 The results for LRG or calprotectin were not made available to the treating clinician. The samples for the WBC count were obtained per standard procedure.
Urine Collection
Enrolled subjects provided a mid-stream clean catch urine sample, collected in a sterile cup (at least 5 cc). During the hours of 09:00 and 16:00, the urine samples were transported to a specialized laboratory within our hospital where trained laboratory technicians spun the urine and froze the samples at −80 °C. After 16:00 on weekdays and at all times on weekends, the urine samples were stored at 4 °C in a refrigerator located within our ED. The following business day, the urine samples were taken to the laboratory where they were processed as described for “serum collection.” Stability testing was conducted on urine samples kept at 4 °C for up to 48 hours, revealing little change in urine levels of LRG (data available upon request).
Testing for Calprotectin and LRG
Samples were thawed and assayed in batches. Quantification of calprotectin and LRG levels was performed via ELISA according to the manufacturer’s recommended procedures. For calprotectin analysis, samples were diluted (plasma 1:60, urine 1:10) in the manufacturer-supplied dilution buffer (Hycult Biotech, Uden, The Netherlands) so that results would be within the linear range of the assay. Similarly, for LRG analysis, serum samples were diluted 1:500 and urine samples were diluted 1:20 in the supplied dilution buffer (IBL America, Minneapolis, MN). Laboratory personnel were blinded to the diagnosis of enrolled patients.
Measures
The primary outcome was the presence or absence of appendicitis. The presence of appendicitis was determined by histopathology. Diagnosis of a perforated appendix was based upon review of the attending surgeon’s written post-operative diagnosis. For patients who did not have surgery, we determined the outcome by a follow-up telephone call 14 to 21 days following the index ED visit. If the family could not be reached, we conducted a review of the hospital electronic record system to assess for operations (i.e. appendectomy), hospitalizations, or ED visits during the follow-up period. Those assessing the outcome were masked to the biomarker levels.
Data Analysis
We conducted descriptive analyses for each biomarker, exploring ranges, means with standard deviations (SD), and medians with inter-quartile ranges (IQR). We assessed the association between each biomarker and the presence or absence of appendicitis (either non-perforated or perforated) with the Mann-Whitney U test, as the biomarker levels were asymmetrically distributed. Next, we constructed receiver operator characteristic (ROC) curves to explore the performance of each marker to predict appendicitis (perforated and non-perforated). These statistical analyses were conducted using SPSS (Version 18.0, Chicago, IL). We used recursive partitioning to identify biomarker thresholds that potentially maximized the sensitivity and specificity to “rule out” and “rule in” appendicitis by varying the cost for misclassification (CART 6.0, Salford Systems, San Diego, CA). We calculated 95% confidence intervals (CI) for the test characteristics of the individual biomarkers.
RESULTS
Study Population
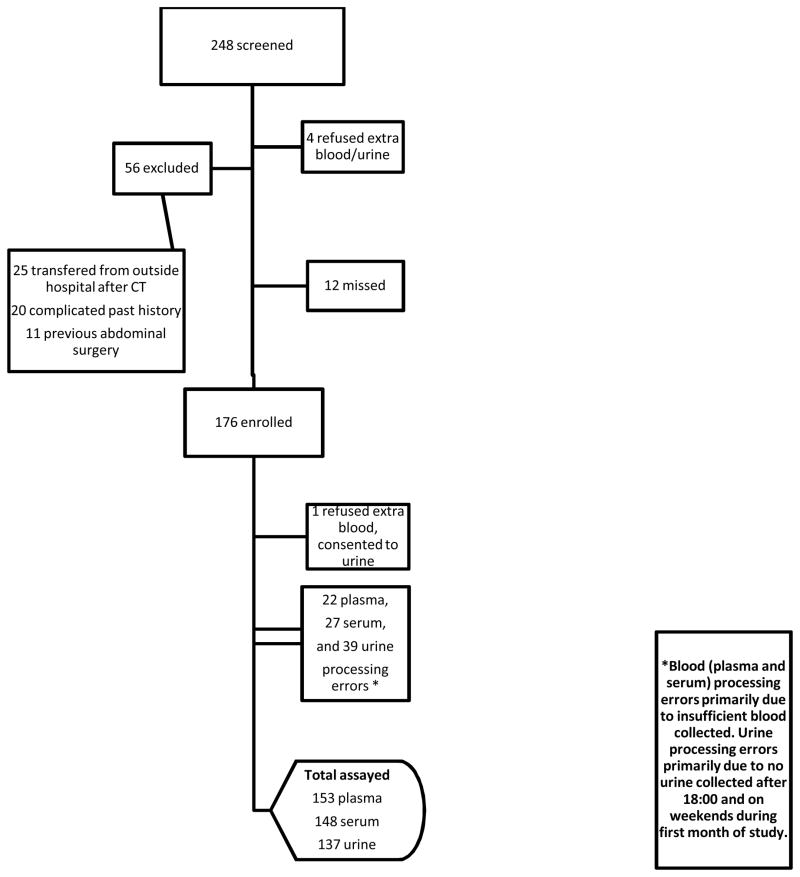
Over the 10-month study period, 248 patients 3 to 18 years of age presented to the ED and were considered for enrollment (i.e. possible appendicitis), of whom 192 were eligible for study participation and 176 were enrolled (92% capture rate). The number of blood and urine samples for analysis varied slightly. Of the 176 patients enrolled, plasma samples were obtained from 153 subjects, serum samples from 148, and urine from 137 (Figure 1). Most of the serum and plasma sample processing errors were due to insufficient quantities of blood. For the first four weeks of the study, urine samples were not collected after 18:00 or on the weekend. This resulted in the majority of missed urine samples (n = 39, coded as sample processing errors). Eligible patients who were not enrolled were slightly younger (mean age 9.6 years), had lower rates of abdominal imaging (68%), and lower rates of appendicitis (25%). However, these values did not differ significantly from the enrolled population.
Figure 1. Patient Flow Diagram.
*Blood (plasma and serum) processing errors primarily due to insufficient blood collected. Urine processing errors primarily due to no urine collected after 18:00 and on weekends during first month of study.
Clinical Characteristics and Outcomes
The clinical characteristics of the 176 enrolled patients are shown in Table 1. The mean age of enrolled patients was 11.6 years (SD ± 4.0 years) and 92 (52%) were male. Fifty-eight patients were diagnosed with appendicitis (34%), of whom 15 (25%) had a perforated appendix. Although those with appendicitis were more likely to be male, there was substantial overlap in the signs and symptoms of those with and without appendicitis. Of enrolled patients without appendicitis, the most common final diagnosis included non-specific abdominal pain, constipation, and gastroenteritis. We completed telephone follow-up on 107 (99%) of the 108 patients who did not undergo an operation; none had an appendectomy during the follow-up period. The medical records of the one patient lost to telephone follow-up revealed no further ED visits, operations, or hospitalizations within two months of enrollment.
Table 1.
Characteristics of Study Population
| Characteristic | No Appendicitis n = 118 | Appendicitis (perforated and non-perforated) n = 58 |
|---|---|---|
| Male, n (%) | 57 (48.3) | 35 (60.3) |
| Age in years, mean (SD) | 11.6 (± 4.1) | 11.8 (± 3.7) |
| Duration of abdominal pain < 24 hours, n (%) | 68 (55.9) | 37 (63.8) |
| Temperature, mean (SD) | 99.1 °F (± 1.5) | 99.0 °F (± 1.0) |
| Abdominal tenderness in right lower quadrant, n (%) | 113 (95.8) | 58 (100) |
| Abdominal CT imaging, n (%) | 94 (79.7) | 44 (75.9) |
CT = computed tomography
Biomarker Levels
Median levels of LRG (serum and urine) and calprotectin (plasma only) were statistically higher in patients with appendicitis as compared to those without appendicitis (Table 2). When biomarker levels were stratified by appendicitis status (non-appendicitis, non-perforated appendicitis, or perforated appendicitis), median blood levels for plasma calprotectin and serum LRG were higher in patients with perforated appendicitis as compared to those patients without perforation (p < 0.05), and higher in patients with non-perforated appendicitis as compared to those without appendicitis (p < 0.01) (Table 3). Urine LRG levels were also elevated in perforated appendicitis as compared to non-perforated appendicitis (p < 0.001). However, median urine LRG levels were not significantly elevated when comparing patients with non-perforated appendicitis to those without appendicitis. Last, urine calprotectin showed no statistical differences among groups.
Table 2.
Biomarker Levels: Appendicitis Compared to No Appendicitis
| Median Biomarker, ng/ml | No Appendicitis n = 118 | Appendicitis (Perforated and non-perforated) n = 58 | P value |
|---|---|---|---|
| Serum LRG | 53,593 (29,898–117,492) | 95,396 (67,198–144,734) | <0.001 |
| Urine LRG | 225.2 (46.5–1,442.8) | 683.5 (122.3–3,832.3) | 0.008 |
| Plasma calprotectin | 221.9 (147.3–329.8) | 330.1 (246.5–466.1) | <0.001 |
| Urine calprotectin | 10.3 (5.0–46.7) | 11.7 (5.5–31.3) | NS |
Median biomarker levels are presented with IQR (25%, 75%)
LRG = leucine-rich alpha glycoprotein-1
Table 3.
Biomarker Levels Stratified by Appendicitis Status
| Biomarker | No Appendicitis (n=116) | Non-Perforated Appendicitis (n = 43) | P value# | Perforated Appendicitis (n=15 ) | P value* |
|---|---|---|---|---|---|
| WBC (x 103/μL) | 10.93 (±5.7) | 15.8 (±3.8) | <0.001 | 16.8 (±4.4) | NS |
| Serum LRG (ng/ml) | 53,593 (29,897–117,492) | 84,763 (66,728–135,479) | 0.001 | 168,546 (71,497–202,579) | 0.05 |
| Urine LRG (ng/ml) | 225.2 (46.5–1,442.8) | 252.7 (106.7–2,547.5) | NS | 20,576.8 (1750.4–38,544.3) | <0.001 |
| Plasma calprotectin (ng/ml) | 221.9 (147.3–329.8) | 275.6 (238.4–378.4) | 0.01 | 490.4 (436.4–648.2) | <0.001 |
| Urine calprotectin (ng/ml) | 10.3 (5.0–46.7) | 11.0 (5.5–26.1) | NS | 36.4 (6.8–62.3) | NS |
Values are reported as mean (± SD) or median (25%–75% IQR)
P value of comparison of no appendicitis and those patients with non-perforated appendicitis
P value of comparison of non-perforated appendicitis to those patients with perforated appendicitis
LRG = leucine-rich alpha glycoprotein-1; WBC = white blood cell count; NS = not significant
Threshold Sensitivities and Specificities of Calprotectin and LRG
To assess biomarker accuracy, we constructed ROC curves and determined the area under the curve (AUC) for each of the biomarkers. The AUCs for serum LRG (0.69, 95% = CI 0.60 to 0.79), urine LRG (0.63, 95% CI = 0.52 to 0.73), and serum calprotectin (0.68, 95% CI = 0.59 to 0.79) were quite similar. In comparison, the AUC for the WBC was notably higher (0.82, 95% CI = 0.75 to 0.90). We next conducted recursive partitioning analyses to determine thresholds (cut-points) for each biomarker. Although a threshold for the novel biomarkers could be determined that provided 100% sensitivity to identify all children with appendicitis, the specificity at each threshold was low (Table 4). The WBC threshold at which sensitivity was 100% (8.85 × 103/μL) provided a higher specificity than any individual novel biomarker (Table 4). Finally, in an exploratory analysis we found that a combination of the WBC with either the serum LRG, urine LRG, or plasma calprotectin led to improved specificity (56%, 51%, 52%, respectively) for ruling out appendicitis over the WBC alone. Sensitivity was unchanged at 100%.
Table 4.
Threshold Values and Test Characteristics of Biomarkers to Identify Patients with Appendicitis
| Biomarker Level | Serum LRG < 40,150 ng/ml | Urine LRG < 42 ng/ml | Plasma Calprotectin < 159 ng/ml | WBC < 8.85 ×103/μL |
|---|---|---|---|---|
| Sensitivity | 100 (91–100) | 100 (91–100) | 100 (91–100) | 100 (92–100) |
| Specificity | 35 (26–44) | 23 (15–34) | 27 (19–37) | 42 (38–56) |
| NPV | 100 (89–100) | 100 (81–100) | 100 (85–100) | 100 (91–100) |
All values are % (95% CI)
WBC = white blood cell count; LRG = leucine-rich alpha glycoprotein-1; NPV = negative predictive value
DISCUSSION
Given the complexity of identifying appendicitis in children, clinicians have sought imaging and laboratory options to aid in the diagnosis. Investigators have studied an array of biological markers, including the WBC count, absolute neutrophil count, C-reactive protein, procalcitonin, and cytokines such as IL-6 and IL-8.15–18 None of these markers have displayed substantial discriminatory accuracy due to the overlap in inflammatory response between appendicitis and the numerous infections that stimulate an immune response.2,15,19,20 In the present study, we assessed two novel proteins whose increased expression is thought to represent increased neutrophil activity as a direct result of a focal inflammatory process. Although we found that calprotectin and LRG levels are increased in children with appendicitis and are low in those without appendicitis, neither individual marker was as accurate as the standard WBC.
Leucine-rich alpha glycoprotein-1 has been previously demonstrated to be elevated in patients with bacterial conditions.12 Investigators recently described elevated levels of LRG in the urine of patients with appendicitis.7 The protein is expressed by neutrophils undergoing differentiation, by the liver, and in high endothelial venules of the mesentery (such as the mesoappendix).7,21,22 Although LRG’s exact function is not known, it is thought to play a role in the activation and/or chemotaxis of neutrophils as they enter areas of inflammation. Our data suggest that LRG can be a sensitive but not specific marker for appendicitis, likely due to the array of inflammatory (especially bacterial) conditions that lead to LRG up-regulation. Conversely, low LRG levels in the blood or urine could potentially have clinical utility to identify a subset of patients at low risk for appendicitis.
Calprotectin has also been described as a marker of inflammation. In inflammatory conditions, the S-100:A8 and S100:A9 proteins are released by neutrophils, macrophages, and monocytes.23 Calprotectin is released at the site of a localized inflammatory process and elevated blood levels serve as a marker of increased neutrophil activity.24 Previous reports have described using calprotectin levels to monitor the degree of inflammation in juvenile rheumatoid arthritis and inflammatory bowel disease (IBD).23–25 For example, investigators recently demonstrated that fecal calprotectin levels could be used to assess the severity of mucosal inflammation in IBD and the response to treatment.26 In a recent study mainly of adults with possible appendicitis, calprotectin had an AUC of 0.71, sensitivity of 92.7% (95% CI = 80.6% to 97.5%), and specificity of 53.6% (95% CI = 45.3% to 61.3%) at a threshold of 20 units (units not defined in text).9 Our results revealed a similar overall AUC (0.68). However, we found that although low calprotectin levels could predict the absence of appendicitis, the specificity of the biomarker was low at this threshold (100% sensitivity and 27% specificity). Future studies will need to further explore the utility of calprotectin in combination with other biomarkers.
In addition to blood measurements, we assayed calprotectin and LRG in the urine. Urine assays have potential advantages as compared to serum/plasma measurements in that urine is usually less painful to obtain, certain proteins may be selectively concentrated in the urine, and urine assays have the potential for more widespread use and acceptance across settings (e.g. office and ED). Similar to a previously published article, we showed a relationship between the presence and absence of appendicitis and urine LRG levels.7 The renal threshold for LRG is not known, nor is it known whether LRG is selectively filtered into the urine. In addition, it is unclear why we did not detect a relationship between urine calprotectin and appendicitis; perhaps calprotectin is modified during passage through the renal collecting system, preventing its detection in the urine by our ELISA test kits.
It should be noted that we detected LRG and calprotectin in the urine (and serum) via a commercially available ELISA kit and not a western blot.7 The ELISA testing was performed in a clinical laboratory environment and provides quantitative results, whereas western blot is primarily used in a research setting and is semi-quantitative. Our ability to detect differential levels of the protein using ELISA is encouraging for future attempts to develop a rapid urine or serum assay.
LIMITATIONS
This was a single-center pilot study conducted to determine whether calprotectin or LRG warrant further investigation. Additional larger studies are needed to further understand whether these novel biomarkers provide marginal benefit when combined with clinical findings (e.g. as part of a prediction rule) and standard laboratory tests such as the WBC count and differential. Our preliminary exploratory analyses suggested that combining LRG or calprotectin with the WBC may be useful. Research should also assess the effect of dehydration on serum, plasma, and urine levels of these biomarkers, and determine the range of biomarker values in other disease conditions. If found to be clinically useful, more rapid measurement techniques must be developed rather than the greater than four hour processing time needed to complete testing in our research laboratory. We cannot exclude the possibility that our sample processing methods were not adequate to prevent degradation of assayed proteins in the blood or urine. However, we did adhere to published standards for biomarker processing, and used a special laboratory with particular expertise in biomarker discovery. Last, we based our final diagnosis on histopathology and operative reports for those who underwent an appendectomy rather than having each pathology specimen evaluated by a blinded pathologist.
CONCLUSIONS
Plasma calprotectin and both serum and urine leucine-rich alpha glycoprotein-1 are elevated in children with appendicitis, and are low in those without appendicitis. The white blood cell count performed better than either new biomarker for the purpose of diagnosing appendicitis.
Acknowledgments
Funding: Supported by Grant Number UL1 RR024156 from the National Center for Research Resources (NICRR), a component of the National Institute of Health (NIH) and NIH Roadmap for Medical Research. In addition, Anupam Kharbanda received salary support through the Empire Clinical Research Program (New York State).
Footnotes
Prior Presentations: Presented in part at the annual meeting of Pediatric Academic Societies, Vancouver, Canada, May 2010
Conflicts of Interest: None of the authors have any financial disclosures or conflicts of interest to report. The funding agencies took no part in data analysis, interpretation, or manuscript preparation.
References
- 1.Flum DR, Morris A, Koepsell T, Dellinger EP. Has misdiagnosis of appendicitis decreased over time? A population-based analysis. JAMA. 2001;286(14):1748–53. doi: 10.1001/jama.286.14.1748. [DOI] [PubMed] [Google Scholar]
- 2.Bundy DG, Byerley JS, Liles EA, Perrin EM, Katznelson J, Rice HE. Does this child have appendicitis? JAMA. 2007;298(4):438–51. doi: 10.1001/jama.298.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Partrick DA, Janik JE, Janik JS, Bensard DD, Karrer FM. Increased CT scan utilization does not improve the diagnostic accuracy of appendicitis in children. J Pediatr Surg. 2003;38(5):659–62. doi: 10.1016/jpsu.2003.5017. [DOI] [PubMed] [Google Scholar]
- 4.Korner H, Sondenaa K, Soreide JA, et al. Incidence of acute nonperforated and perforated appendicitis: age-specific and sex-specific analysis. World J Surg. 1997;21(3):313–7. doi: 10.1007/s002689900235. [DOI] [PubMed] [Google Scholar]
- 5.Pena BM, Taylor GA, Lund DP, Mandl KD. Effect of computed tomography on patient management and costs in children with suspected appendicitis. Pediatrics. 1999;104(3 Pt 1):440–6. doi: 10.1542/peds.104.3.440. [DOI] [PubMed] [Google Scholar]
- 6.Murphy CG, Glickman JN, Tomczak K, et al. Acute appendicitis is characterized by a uniform and highly selective pattern of inflammatory gene expression. Mucosal Immunol. 2008;1(4):297–308. doi: 10.1038/mi.2008.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kentsis A, Lin YY, Kurek K, et al. Discovery and validation of urine markers of acute pediatric appendicitis using high-accuracy mass spectrometry. Ann Emerg Med. 2010;55(1):62–70. doi: 10.1016/j.annemergmed.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176(2):289–96. doi: 10.2214/ajr.176.2.1760289. [DOI] [PubMed] [Google Scholar]
- 9.Bealer JF, Colgin M. S100 A8/A9: potential new diagnostic aid for acute appendicitis. Acad Emerg Med. 2010;17:333–6. doi: 10.1111/j.1553-2712.2010.00663.x. [DOI] [PubMed] [Google Scholar]
- 10.Kumar RK, Yang Z, Bilson S, Thliveris S, Cooke BE, Geczy CL. Dimeric S100A8 in human neutrophils is diminished after phagocytosis. J Leukoc Biol. 2001;70(1):59–64. [PubMed] [Google Scholar]
- 11.O’Donnell LC, Druhan LJ, Avalos BR. Molecular characterization and expression analysis of leucine-rich alpha2-glycoprotein, a novel marker of granulocytic differentiation. J Leukoc Biol. 2002;72(3):478–85. [PubMed] [Google Scholar]
- 12.Bini L, Magi B, Marzocchi B, et al. Two-dimensional electrophoretic patterns of acute-phase human serum proteins in the course of bacterial and viral diseases. Electrophoresis. 1996;17(3):612–6. doi: 10.1002/elps.1150170333. [DOI] [PubMed] [Google Scholar]
- 13.Shirai R, Hirano F, Ohkura N, Ikeda K, Inoue S. Up-regulation of the expression of leucine-rich alpha(2)-glycoprotein in hepatocytes by the mediators of acute-phase response. Biochem Biophys Res Comm. 2009;382(4):776–9. doi: 10.1016/j.bbrc.2009.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rai AJ, Gelfand CA, Haywood BC, et al. HUPO Plasma Proteome Project specimen collection and handling: towards the standardization of parameters for plasma proteome samples. Proteomics. 2005;5(13):3262–77. doi: 10.1002/pmic.200401245. [DOI] [PubMed] [Google Scholar]
- 15.Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg. 2004;91(1):28–37. doi: 10.1002/bjs.4464. [DOI] [PubMed] [Google Scholar]
- 16.Kharbanda AB, Taylor GA, Fishman SJ, Bachur RG. A clinical decision rule to identify children at low risk for appendicitis. Pediatrics. 2005;116(3):709–16. doi: 10.1542/peds.2005-0094. [DOI] [PubMed] [Google Scholar]
- 17.Wu HP, Lin CY, Chang CF, Chang YJ, Huang CY. Predictive value of C-reactive protein at different cutoff levels in acute appendicitis. Am J Emerg Med. 2005;23(4):449–53. doi: 10.1016/j.ajem.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Yoon DY, Chu J, Chandler C, Hiyama S, Thompson JE, Hines OJ. Human cytokine levels in nonperforated versus perforated appendicitis: molecular serum markers for extent of disease? Am Surg. 2002;68(12):1033–7. [PubMed] [Google Scholar]
- 19.Eriksson S, Olander B, Pira U, Granstrom L. White blood cell count, leucocyte elastase activity, and serum concentrations of interleukin-6 and C-reactive protein after open appendicectomy. Eur J Surg. 1997;163(2):123–7. [PubMed] [Google Scholar]
- 20.Erkasap S, Ates E, Ustuner Z, et al. Diagnostic value of interleukin-6 and C-reactive protein in acute appendicitis. Swiss Surg. 2000;6(4):169–72. doi: 10.1024/1023-9332.6.4.169. [DOI] [PubMed] [Google Scholar]
- 21.Saito K, Tanaka T, Kanda H, et al. Gene expression profiling of mucosal addressin cell adhesion molecule-1+ high endothelial venule cells (HEV) and identification of a leucine-rich HEV glycoprotein as a HEV marker. J Immunol. 2002;168(3):1050–9. doi: 10.4049/jimmunol.168.3.1050. [DOI] [PubMed] [Google Scholar]
- 22.Weivoda S, Andersen JD, Skogen A, et al. ELISA for human serum leucine-rich alpha-2-glycoprotein-1 employing cytochrome c as the capturing ligand. J Immunol Methods. 2008;336(1):22–9. doi: 10.1016/j.jim.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frosch M, Strey A, Vogl T, et al. Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2000;43(3):628–37. doi: 10.1002/1529-0131(200003)43:3<628::AID-ANR20>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 24.Costa F, Mumolo MG, Bellini M, et al. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig Liver Dis. 2003;35(9):642–7. doi: 10.1016/s1590-8658(03)00381-5. [DOI] [PubMed] [Google Scholar]
- 25.Roseth AG, Aadland E, Grzyb K. Normalization of faecal calprotectin: a predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol. 2004;39(10):1017–20. doi: 10.1080/00365520410007971. [DOI] [PubMed] [Google Scholar]
- 26.Canani RB, Terrin G, Rapacciuolo L, et al. Faecal calprotectin as reliable non-invasive marker to assess the severity of mucosal inflammation in children with inflammatory bowel disease. Dig Liver Dis. 2008;40(7):547–53. doi: 10.1016/j.dld.2008.01.017. [DOI] [PubMed] [Google Scholar]