Abstract
Background/Aims
Dietary copper deficiency is associated with a variety of manifestations of the metabolic syndrome, including hyperlipidemia and fatty liver. Fructose feeding has been reported to exacerbate complications of copper deficiency. In this study, we investigated whether copper deficiency plays a role in fructose-induced fatty liver and explored the potential underlying mechanism(s).
Methods
Male weanling Sprague-Dawley rats were fed either an adequate copper or a marginally copper deficient diet for 4 weeks. Deionized water or deionized water containing 30% fructose (w/v) was also given ad lib. Copper and iron status, hepatic injury and steatosis, duodenum copper transporter-1(Ctr-1) were assessed.
Results
Fructose feeding further impaired copper status and led to iron overload. Liver injury and fat accumulation were significantly induced in marginal copper deficient rats exposed to fructose as evidenced by robust increased plasma aspartate aminotransferase (AST) and hepatic triglyceride. Hepatic carnitine palmitoyl-CoA transferase I (CPT I) expression was significantly inhibited, whereas hepatic fatty acid synthase (FAS) was markedly up-regulated in marginal copper deficient rats fed with fructose. Hepatic antioxidant defense system was suppressed and lipid peroxidation was increased by marginal copper deficiency and fructose feeding. Moreover, duodenum Ctr-1 expression was significantly increased by marginal copper deficiency, whereas this increase was abrogated by fructose feeding.
Conclusion
Our data suggest that high fructose-induced nonalcoholic fatty liver disease (NAFLD) may be due, in part, to inadequate dietary copper. Impaired duodenum Ctr1 expression seen in fructose feeding may lead to decreased copper absorption, and subsequent copper deficiency.
Keywords: Fructose, Copper deficiency, AST, Fatty acid synthase, Copper transporter
Introduction
Since the introduction of high-fructose corn syrup in 1967, per capita fructose consumption has steadily risen. The increased consumption of fructose temporally parallels the increased prevalence of obesity and the metabolic syndrome in the United States and worldwide [1, 2]. Recent studies suggest that high fructose intake may be an important risk factor for the development of NAFLD [3, 4].
The average American diet contains only marginal amounts of copper [5]. Dietary copper deficiency is associated with a variety of metabolic changes, including hypercholesterolemia, increased blood pressure, and glucose intolerance in rodents [6–8]. Recently, decreased hepatic copper concentrations were observed in NAFLD patients and were correlated with alterations in iron metabolism [9]. The mechanism underlying copper deficiency-associated hyperlipidemia is not fully understood. One potential mechanism is iron overload, which can occur secondary to copper deficiency [10]. Importantly, the source of dietary carbohydrate can play a critical role in the development of complications of copper deficiency. Extensive literature from the early 1980s showed that dietary fructose markedly enhanced the metabolic complications of copper deficiency in rodents [6, 11–13]. Research by Fields and co-workers showed that fructose-fed copper deficient male rats had lower hematocrits, but increased liver weight, cholesterol and triglycerides; whereas those fed starch had the least complications [6, 11, 12]. The mechanisms for these interactions are not well defined. Previous studies suggested that fructose-exacerbated copper deficiency might be due, in part, to impaired gut absorption of copper [14, 15].
Recent research showed that a significant proportion of NAFLD patients were copper deficient [9]. Heavy fructose consumption is a well-recognized risk factor for NAFLD. Given that excess dietary fructose exacerbates copper deficiency and copper deficiency induces fatty liver and hyperlipidemia, we hypothesized that the hyperlipidemia and fatty liver seen in high fructose-induced NAFLD is due, in part, to copper deficiency, and marginal dietary copper intake may be an initial component in the “two-hit” model of nonalcoholic steatohepatitis [35]. The present study was conducted to determine whether marginal copper deficiency and chronic fructose consumption can act together to exacerbate liver injury and fat accumulation; whether fructose consumption further impairs copper status; and to explore potential underlying mechanism(s).
Materials and Methods
Animals
Male weanling Sprague-Dawley rats (35–45g) from the Harlan Laboratories (Indianapolis, IN) were fed (ad lib) a purified AIN-76 diet for laboratory rodents with defined copper content. The copper adequate rats received 6 mg/kg copper, and the experimental rats were fed with 1.6 mg/kg of copper for 4 weeks to achieve marginal copper deficiency. The animals were housed in stainless steel cages in a temperature and humidity controlled room with a 12:12h light-dark cycle. Animals had free access to either deionized water or deionized water containing 30% fructose (w/v). Fructose enriched drinking water was changed twice a week. All studies were approved by the University of Louisville Institutional Animal Care and Use Committee, which is certified by the American Association of Accreditation of Laboratory Animal Care.
Assessment of Copper and Iron Status
Plasma ceruloplasmin was measured on the basis of its oxidase activity [16]. Plasma copper was measured by graphite furnace atomic absorption spectrometer. Copper and iron content in the liver tissue was measured by inductively coupled plasma mass spectroscopy (ICP-MS). Plasma ferritin was determined by commercially available kit (ALPCO Diagnostics, Salem, NH).
Liver Enzyme Assay
Plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using commercially available kits (Infinity; Thermo Electron Corporation, Melbourne, Australia) based on a colorimetric method.
Plasma Biochemical Assays
The plasma biochemical assays were performed with commercially available kits: glucose, cholesterol (Infinity, Thermo Electron, Melbourne, Australia), non-esterified fatty acids (NEFA) (Waco Chemicals, Richmond, VA), insulin (Linco Research, St. Charles, MO), and monocyte chemoattractant protein-1 (MCP-1) (R&D Systems, Minneapolis, MN).
Assessment of Oxidative Stress
Hepatic superoxide production was evaluated by using dihydroethidium (DHE) (Invitrogen, Carlsbad, CA). Briefly, cryostat sections of liver were incubated with 5 µmol/L dihydroethidium at 37°C for 30 minutes. The red fluorescence from dihydroethidium was detected with fluorescence microscope. Hepatic lipid peroxidation was assessed by measuring malondialdehyde (MDA) and 4-hydroxyalkenals (HAE) using commercial kits (Oxford Biomedical Research, Oxford, MI). Reduced glutathione (GSH) and oxidized glutathione (GSSG) were determined by HPLC as described previously [17].
Histology and Immunohistochemistry
Formalin-fixed, paraffin-embedded liver sections were cut at 5 µm thickness, and stained with hematoxylin and eosin (H&E). Cryostat sections of liver tissue were stained with Oil Red O (Sigma-Aldrich, St. Louis, MO). Apoptotic hepatocytes were detected by the Terminal Deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) assay with an In Situ Apoptosis Detection Kit (Millipore, Billerica, MA).
For immunohistochemical analysis, paraffin-embedded duodenum sections were incubated with anti-Ctr-1 antibody (Santa Cruz Biotechnology, INC., Santa Cruz, CA) overnight at 4°C. Staining was visualized using the horseradish peroxidase-conjugated DAKO staining system (DAKO InVision, Carpenteria, CA). ). The area of positive staining was quantified using MetaMorph software (Universal Imaging Corporation, Downingtown, PA).
Hepatic Triglyceride Assay
Liver tissues were homogenized in ice-cold phosphate buffered saline. Hepatic total lipids were extracted with chloroform/methanol (2:1) according to the method described by Bligh and Dyer [18]. Hepatic triglyceride content was determined by commercially available kits (Infinity, Thermo Electron, Melbourne, Australia).
Western Blot
Equal amounts of protein extracted from liver homogenate were loaded on 8%–12% SDS-polyacrylamide gels and transferred to PVDF membrane (Millipore, Bedford, MA). Membranes were probed with primary antibody for copper, zinc-superoxide dismutase (Cu, Zn-SOD, SOD1), fatty acid synthase (FAS), CPT1, glutathione peroxidase (GPx)-1/2, GPx-4 (Santa Cruz Biotechnology, INC., Santa Cruz, CA), or β-actin (Cell Signaling Technology, INC., Danvers, MA), then incubated with the corresponding horseradish peroxidase-conjugated secondary antibody. Protein signals were visualized using the enhanced chemiluminescence system (Amersham Biosciences, Little Chalfont, UK). Band intensities were quantified using Image J software (http://rsb.info.nih.gov/ij/).
Statistical Analysis
All data were expressed as mean ± SD (Standard Deviation) and analyzed by analysis of variance (ANOVA) followed by Newman Keuls’ Multiple Comparison Test and Student’s t- test. The interactions between copper and fructose were examined by two-way ANOVA. Differences at P < 0.05 were considered to be statistically significant.
Results
Effects of Marginal Copper Deficiency and Fructose Feeding on Liver, Body Weight, and Blood Metabolites
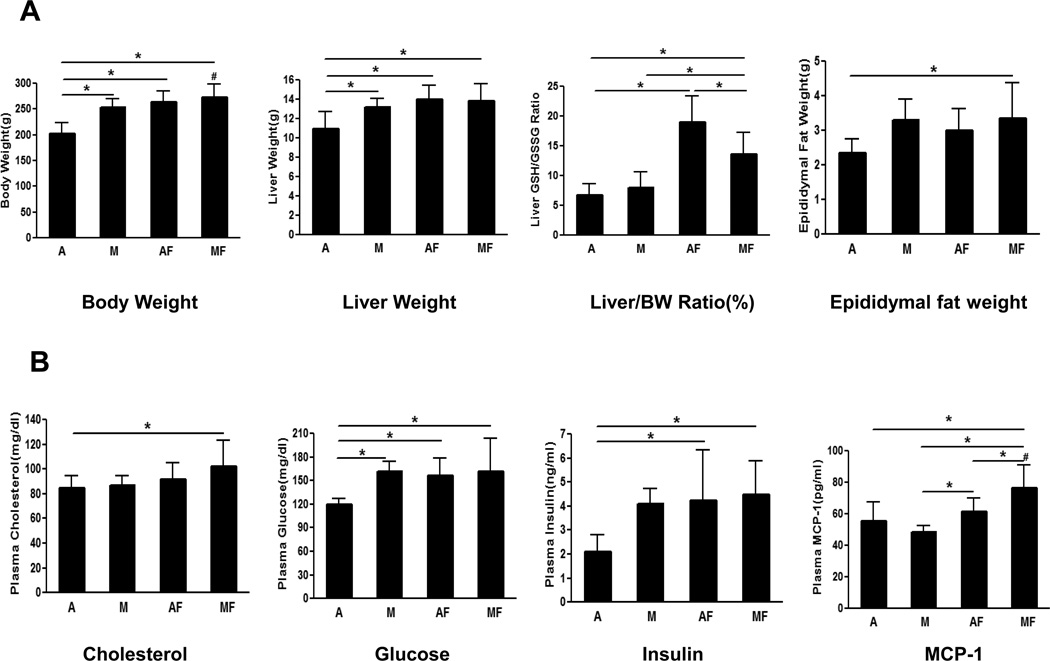
After 4 weeks of feeding, marginally copper deficient rats showed a significantly higher body weight compared to the adequate copper rats, and the body weight of adequate copper rats increased significantly with fructose feeding (Fig.1 A). Marginal copper deficiency as well as fructose feeding led to a significant increase of epididymal fat weight compared to controls (Fig.1 A). Plasma cholesterol was significantly increased in marginal copper deficient rats fed with fructose compared to the adequate copper rats. Both plasma glucose and insulin levels were elevated in marginal copper deficient rats and adequate copper rats fed fructose (Fig.1 B). High plasma glucose and high plasma insulin co-exist in marginal copper deficient rats fed with fructose, suggesting insulin resistance. In addition, fructose feeding and marginal copper deficiency synergistically increased plasma MCP-1 level compared to adequate copper rats (Fig.1 B).
Fig. 1. Characterization of marginal copper deficiency and fructose feedin.
(A) Body Weight (BW), liver Weight, liver/BW Ratio (%), Epididymal fat weight. (B) Plasma cholesterol, glucose, insulin, MCP-1. Data represent means ± SD (n=5–9). *p<0.05. #, interaction between copper and fructose is significant (p<0.05). A, adequate copper diet; M, marginal copper deficient diet; AF, adequate copper diet+30% fructose drinking; MF, marginal copper deficient diet+30% fructose drinking.
Effects of Fructose Feeding on Copper and Iron Status
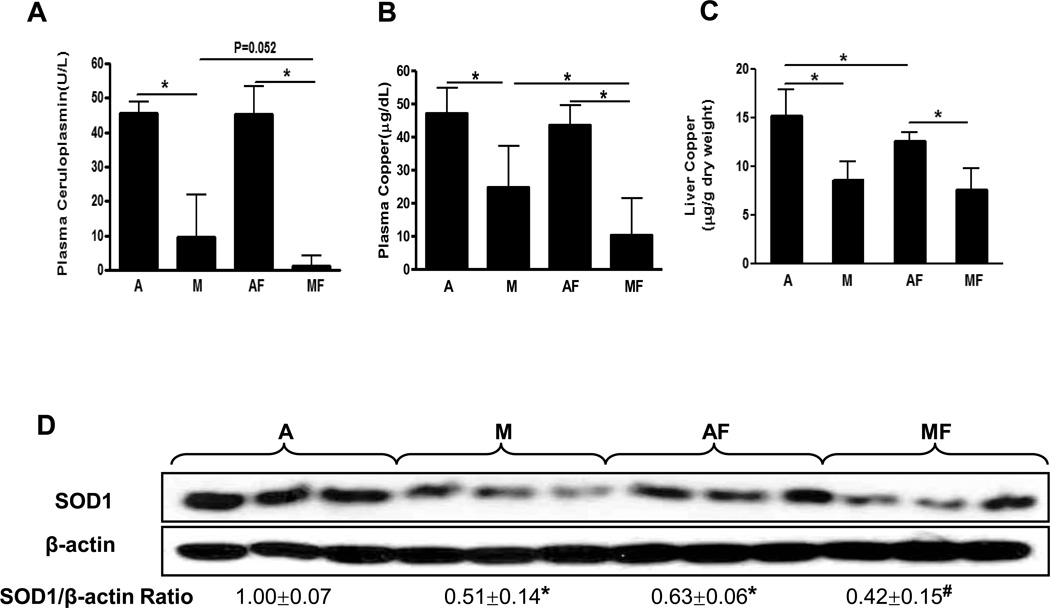
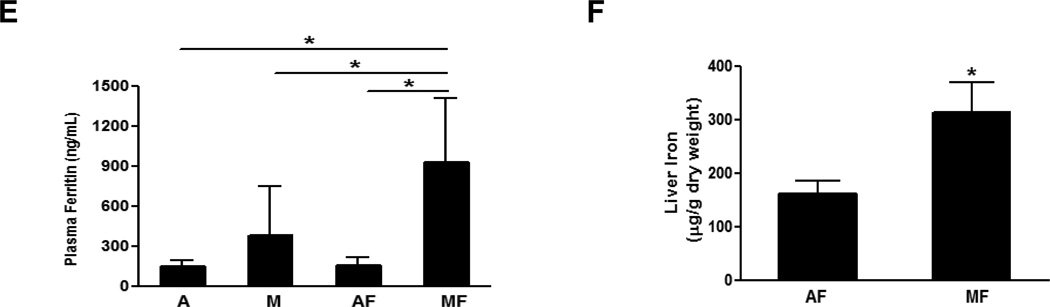
Plasma ceruloplasmin activity and plasma copper were significantly decreased in the rats fed with marginal copper deficient diet, and it was further decreased in marginal copper deficient rats fed fructose (Fig.2 A, B). Similarly, liver copper was decreased with fructose feeding in copper adequate rats, and further decreased in marginal copper deficient rats (Fig.2 C). An indirect indicator of copper status [19], hepatic SOD1 expression varied in relation to the changes in liver copper, as shown by Western Blot (Fig.2 D). The plasma ferritin, a marker of total body iron stores, was robustly increased in marginal copper deficient rats fed with fructose compared to controls (Fig.2 E). Consistent with the plasma ferritin, liver iron was also significantly increased in marginal copper deficient rats fed with fructose compared to control (Fig.2 F).
Fig. 2. Effect of fructose feeding on copper and iron status.
(A) Plasma ceruloplasmin. (B) Plasma copper. (C) Liver copper. Data represent means ± SD (n=5–9). *p<0.05. (D) Hepatic SOD1 expression was determined by Western Blots. Optical density of band was quantified by ImageJ software. The ratio to β-actin was calculated by assigning the value from adequate copper diet controls as one. Data represent means ± SD (n=3). *p<0.05 versus A; #p<0.05 versus AF. (E) Plasma ferritin. (F) Liver iron. Data represent means ± SD (n=5–9). *p<0.05. A, adequate copper diet; M, marginal copper deficient diet; AF, adequate copper diet+30% fructose drinking; MF, marginal copper deficient diet+30% fructose drinking.
Dietary Marginal Copper Deficiency and Fructose Feeding Interact To Exacerbate Liver Injury and Accelerate Hepatic Fat Accumulation
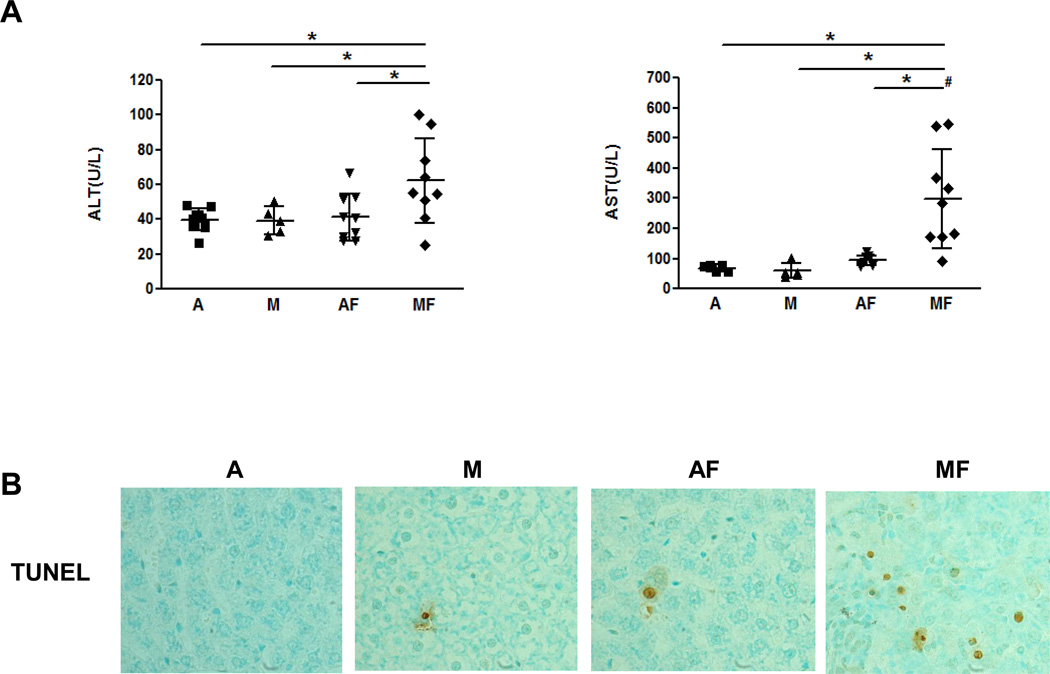
Liver injury was assessed by plasma levels of liver enzymes (ALT and AST). Both ALT and AST levels were not significantly changed in rats fed either adequate copper or marginal copper without fructose in drinking water. However, fructose feeding led to a significant increase in plasma ALT level by ≈50% in marginal copper deficient rats compared with copper adequate animals (Fig.3 A). Specifically, marginal copper deficiency and fructose feeding synergistically increased plasma AST level by approximately 5-fold and 3-fold compared to marginal copper deficiency rats and fructose-fed adequate copper rats, respectively. Consistent with the biochemical findings, obvious hepatocyte apoptosis was observed by TUNEL staining in marginal copper deficiency fructose fed animals (Fig.3 B).
Fig. 3. Effect of marginal copper deficiency and fructose feeding on liver injury.
(A) Plasma ALT and AST level. (B) Representative photomicrographs of TUNEL staining of liver section (400×). Data represent means ± SD (n=5–9). *p<0.05; #, interaction between copper and fructose is significant (p<0.05). A, adequate copper diet; M, marginal copper deficient diet; AF, adequate copper diet+30% fructose drinking; MF, marginal copper deficient diet+30% fructose drinking.
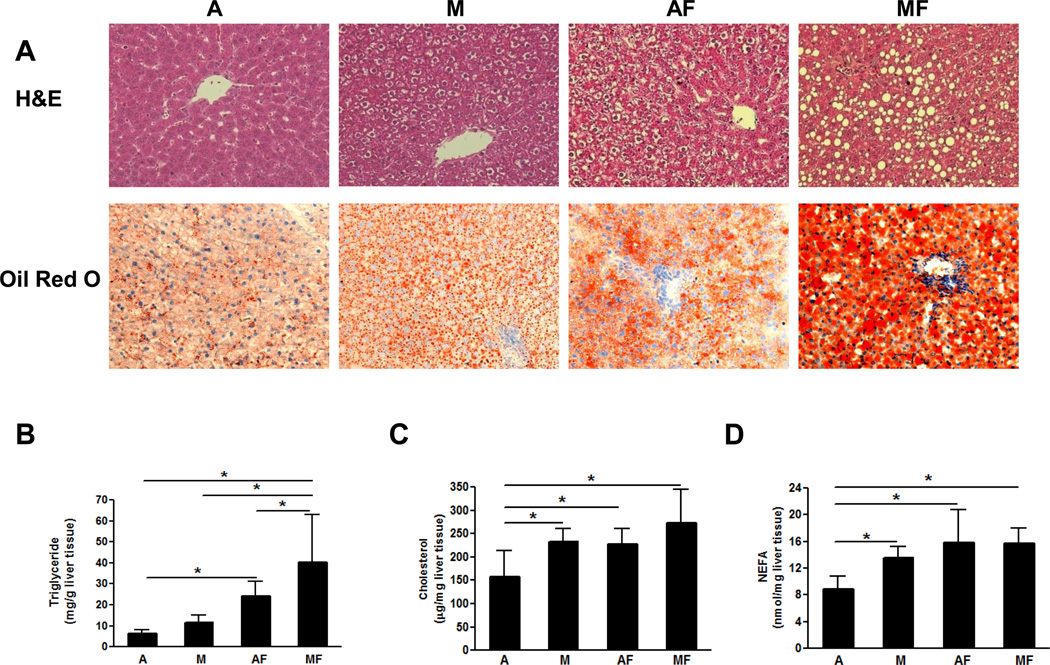
As expected, fructose feeding led to a significant increase in hepatic triglyceride content in both adequate copper and marginal copper deficient rats. However, marginal copper deficiency and fructose feeding addictively increased hepatic triglyceride accumulation. (Fig.4 B). These changes were also visible in histological slides with H&E staining (Fig.4 A). Interestingly, marginal copper deficiency-induced fat accumulation was characterized mainly by microvesicular steatosis as shown by Oil Red O staining, suggesting impaired mitochondrial function [20]. However, macrovesicular steatosis was evident in marginal copper animals fed with fructose (Fig.4 A). In rats fed adequate copper with fructose, hepatic steatosis was both macro- and microvesicular. Hepatic cholesterol was significantly increased in marginal copper deficient rats, as well as in adequate copper and marginal copper deficient rats fed with fructose (Fig.4 C). Marginal copper deficiency as well as fructose feeding led to a significant increase in hepatic NEFAs compared to controls (Fig.4 D).
Fig. 4. Effect of marginal copper deficiency and fructose feeding on the hepatic lipid accumulation.
(A) Representative photomicrographs of the H&E and Oil Red O staining of liver section (200×). (B) Hepatic triglyceride. (C) Hepatic cholesterol. (D) Hepatic NEFA. Data represent means ± SD (n=5–9). *p<0.05. A, adequate copper diet; M, marginal copper deficient diet; AF, adequate copper diet+30% fructose drinking; MF, marginal copper deficient diet+30% fructose drinking.
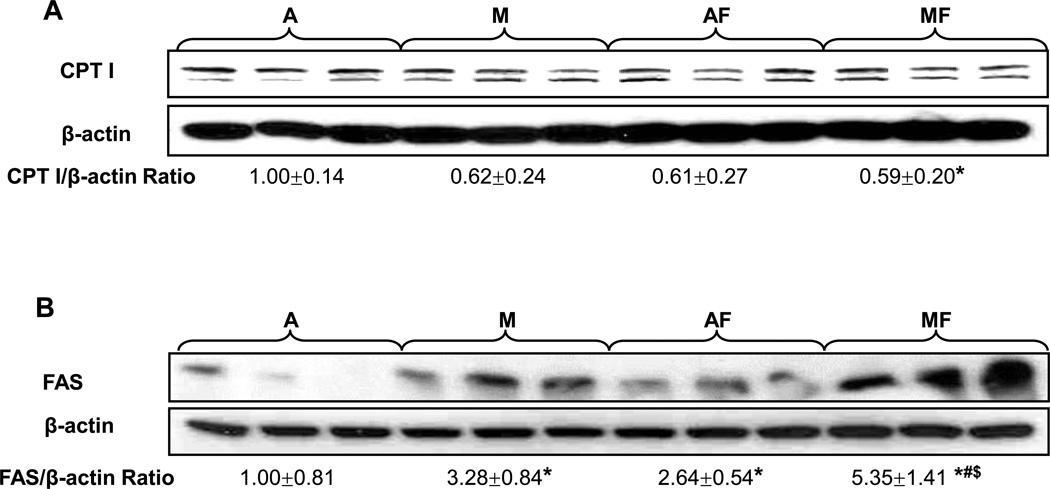
Copper Deficiency Impaired Mitochondria Fatty Acid β-Oxidation and Increased de novo Lipogenesis
CPT I is the rate limiting enzyme of mitochondrial fatty acid β-oxidation. As shown by Western Blot (Fig.5 A), there are two bands, with the top one being the liver isoform. CPT I protein expression was significantly decreased in the livers of marginal copper deficient rats fed with fructose, which further supports histological findings. To further examine the mechanism(s) of fat accumulation, FAS, a critical enzyme in the terminal step of fatty acid synthesis, and an important marker of de novo lipogenesis, was analyzed by Western Blot (Fig.5 B). Hepatic FAS expression was increased by fructose feeding in adequate copper rats, whereas it was prominently enhanced in response to marginal copper deficiency (especially with fructose feeding), suggesting that copper deficiency induces fat accumulation, likely by accelerating fatty acid synthesis.
Fig. 5. Effect of marginal copper deficiency and fructose feeding on the expression of hepatic lipid metabolic enzymes.
(A) Hepatic CPT I and (B) FAS expression was determined by Western Blots. There are two bands in CPT I, with the top one being the liver isoform. Optical density of band was quantified by ImageJ software. The ratio to β-actin was calculated by assigning the value from adequate copper diet controls as one. Data represent means ± SD (n=3). *p<0.05 versus A; #p<0.05 versus M; $p<0.05 versus AF. A, adequate copper diet; M, marginal copper deficient diet; AF, adequate copper diet+30% fructose drinking; MF, marginal copper deficient diet+30% fructose drinking.
Decreased Antioxidant Defense System and Increased Oxidative Stress by Dietary Marginal Copper Deficiency and Fructose Feeding
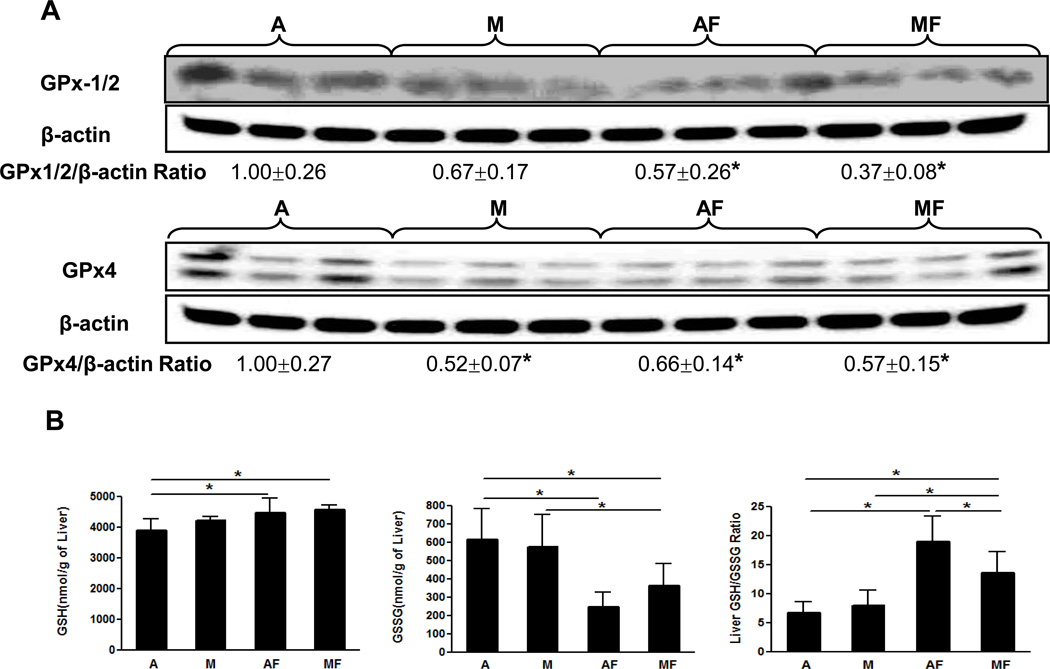
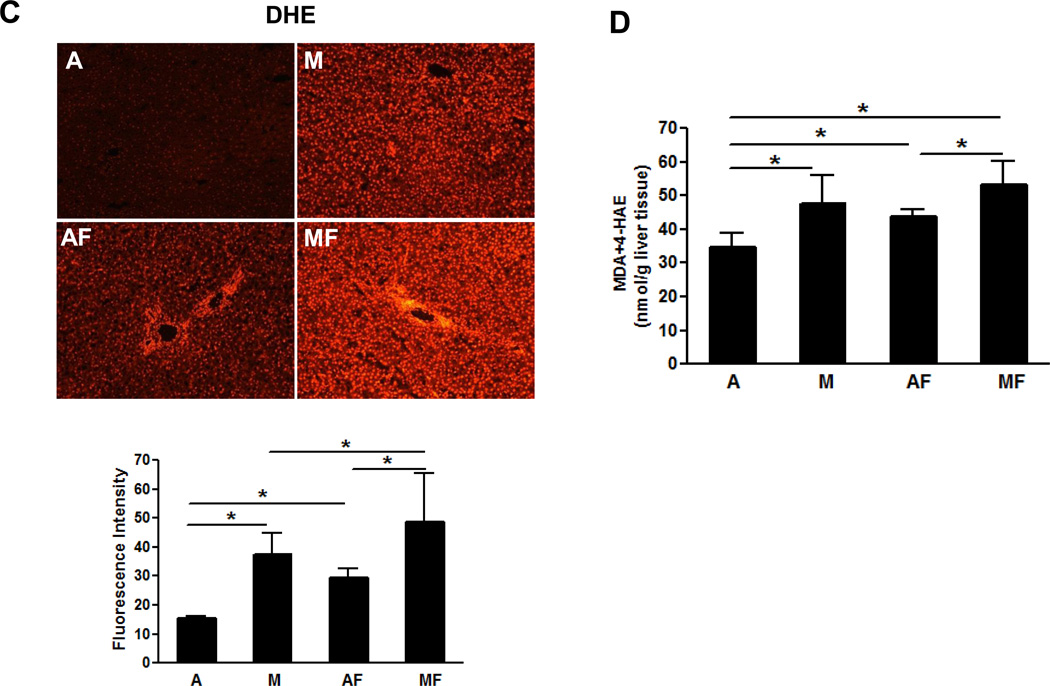
We next examined the effect of marginal copper deficiency and fructose feeding on the hepatic antioxidant defense system, including SOD1, GPx, GSH and GSSG. Hepatic SOD1 expression, as mentioned above, paralleled the copper status (Fig.2 C). The expression of GPx1/2 was markedly inhibited in both adequate and marginal copper deficient rats when fed with fructose, and GPx-4 was significantly inhibited by either marginal copper deficiency or fructose feeding (Fig.6 A). Unlike SOD1 (Fig 2C), it appeared that GPx expression was not correlated to copper status. Hepatic GSH showed a significant increase in both adequate and marginal copper deficient rats when fed fructose (Fig.6 B). GSSG level was significantly decreased by fructose feeding in either marginal copper deficiency or adequate copper groups. GSH/GSSG ratio, which is an indicator of oxidative stress, significantly increased in either adequate or marginal copper deficient rats when fed with fructose (Fig.6 B). Either dietary marginal copper deficiency or fructose feeding increased hepatic superoxide production, marginal copper deficiency and fructose feeding additively increased the superoxide production as shown by DHE staining and image quantification (Fig.6 C). The end products of lipid peroxidation, MDA and 4-HAE were significantly increased in the livers of marginal copper deficient rats compared with controls, and fructose feeding also led to significantly increased hepatic MDA and 4-HAE in adequate copper rats (Fig.6 D).
Fig. 6. Effect of marginal copper deficiency and fructose feeding on hepatic antioxidant defense system.
(A) Hepatic GPx1/2 and GPx4 expression were determined by Western Blots. Optical density of band was quantified by ImageJ software. The ratio to β-actin was calculated by assigning the value from adequate copper diet controls as one. Data represent means ± SD (n=3). *p<0.05 versus A. (B) Hepatic GSH, GSSG and GSH/GSSG ratio measured by HPLC. (C) Representative photomicrographs of the dihydroethidium staining of liver section (100×) and quantification (bottom). Dihydroethidium is oxidized by superoxide to yield red fluorescence that binds to the nucleic acids, staining the nucleus a bright fluorescent red. The bottom figure showed the mean of red fluorescence density quantified by Image J. Data represent means ± SD (n=4–9). *p<0.05. (D) Lipid peroxidation was assessed by MDA+4-HAE in liver homogenate. Data represent means ± SD (n=5–9). *p<0.05. A, adequate copper diet; M, marginal copper deficient diet; AF, adequate copper diet+30% fructose drinking; MF, marginal copper deficient diet+30% fructose drinking.
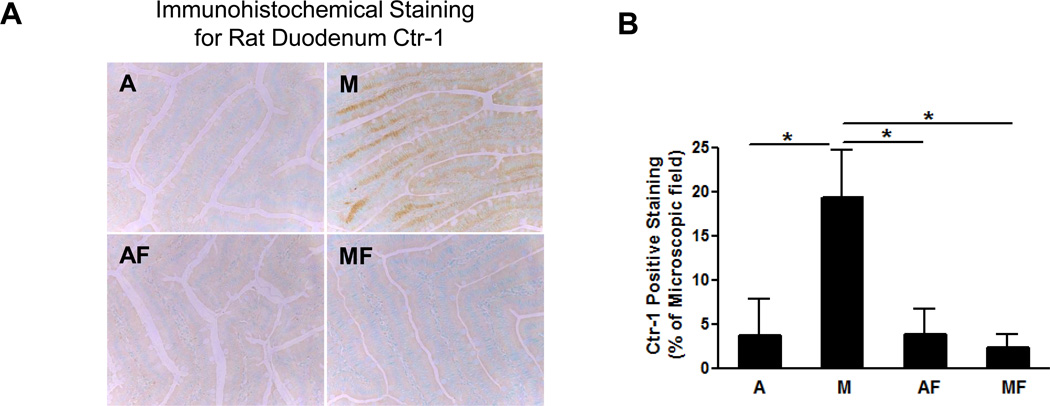
Dietary Fructose Feeding Negated Marginal Copper Deficiency Induced Ctr-1 Accumulation in the Duodenum Epithelium
Dietary copper is mainly absorbed in the duodenum and early jejunum by Ctr-1, a copper specific transporter, which is located at the apical membrane of enterocytes [21]. To further investigate the mechanisms by which fructose feeding impairs copper status, Ctr-1 was determined by immunohistochemical staining. We found that Ctr-1 expression was significantly increased by marginal copper deficiency, and accumulated extensively on the basolateral membrane of duodenum epithelium; however, this phenomenon was negated with fructose feeding (Fig.7 A, B).
Fig. 7. Effect of marginal copper deficiency and fructose feeding on duodenal epithelium Ctr-1.
(A) Representative photomicrographs of immunohistochemical staining for rat duodenum Ctr-1 (200×). (B) Image quantification of Ctr-1 positive staining. Data represent means ± SD (n=5–6). *p<0.05. A, adequate copper diet; M, marginal copper deficient diet; AF, adequate copper diet+30% fructose drinking; MF, marginal copper deficient diet+30% fructose drinking.
Discussion
Because of its metabolic pathways, fructose rapidly induces hepatic de novo lipogenesis. Moreover, increased intestinal permeability associated with high fructose feeding may lead to endotoxemia and increase the secretion of inflammatory cytokines, which, in turn, can lead to insulin resistance and fatty liver [22]. However, even with increased de novo lipogenesis, steatosis should only occur when lipid export or fatty acid β-oxidation is decreased, suggesting that other mechanisms may be involved in the deleterious effects of fructose on the liver.
A very recent study has shown that NAFLD patients were associated with lower hepatic copper, and hepatic copper level correlated with the severity of hepatic steatosis [9, 36]. In addition, it has been demonstrated that copper deficient diet induced hepatic steatosis in rats [36], suggesting copper deficiency might be an important risk factor in NAFLD. Our present study demonstrated that fructose consumption further impaired marginal copper status and precipitated copper deficiency, possibly by inhibiting Ctr-1 mediated copper absorption through the intestinal epithelium. Moreover, marginal copper deficiency and fructose appeared to act together to exacerbate liver injury and accelerate hepatic fat accumulation. Copper deficiency-associated mitochondrial dysfunction and antioxidant defense system suppression primed the liver to fructose toxicity, possibly through the hepatic iron overload secondary to copper deficiency. Decreased mitochondrial fatty acid β-oxidation caused by copper deficiency represents a likely important mechanism underlying fructose induced fatty liver in this study. In addition, copper deficiency may induce insulin resistance and increase de novo lipogenesis which both contribute to hepatic lipid accumulation through multiple pathways.
Liver injury induced by fructose feeding and marginal copper deficient diet is unique in that it caused robust increased AST and moderately increased ALT. Serum AST is comprised of two distinct isoenzymes, one located in cytoplasm, and the other in mitochondria. In the liver, 60 to 80% of the total AST activity is of mitochondrial origin [23]. We also found that either marginal copper deficiency or fructose alone did not significantly increase ALT or AST levels. Marginal copper plus fructose caused a significant increase in both ALT and AST, with the AST being the most prominent (up to 5 fold), suggesting that fructose and copper deficiency act synergistically to cause liver injury. In fact, previous work has shown that both acute and chronic severe copper deficiency led to abnormal mitochondria [24].
SODs and GPxs represent the major defenses against oxidative stress. The distribution of SOD1 is mainly in the cytosol, and recently it was also found in the mitochondria [25]. Loss of SOD1 led to severe mitochondrial damage in neuroblastoma cells and enhanced oxidative damage in the livers of alcohol fed animals [26, 27]. Furthermore, mutant SOD1 localized in the inter-membrane space of mitochondria caused mitochondrial dysfunction, suggesting the important role of this enzyme in the preservation of mitochondria homeostasis and function [28]. In this study, the total hepatic SOD1 expression was significantly reduced in marginally copper deficient rats and aggravated by fructose feeding. Therefore, it is likely that inhibition of SOD1 may partially contribute to copper deficiency-induced mitochondrial dysfunction. GPx-1/2 and GPx-4 were markedly down-regulated by marginal copper deficiency when fed with fructose. Despite increased GSH synthesis, GSH cannot be fully oxidized to remove hydrogen peroxide due to the lack of GPx. Decreased antioxidant defense as a result of suppressed SOD1 and GPx led to increased accumulation of superoxide and hydrogen peroxide. Our data showed that hepatic iron was significantly increased in marginal copper deficiency rats fed with fructose, and iron is a catalyst to convert hydrogen peroxide to hydroxyl radicals through Haber-Weiss and Fenton reactions. This may, in turn, impair mitochondria, leading to mitochondrial dysfunction and cell death. Thus, copper deficiency induced liver injury may be mediated, in part, through increased iron accumulation.
The accumulation of lipids in the liver results from an imbalance among the uptake, synthesis, export, and oxidation of fatty acids. In this study, marginal copper deficiency-induced fatty liver is characterized by microvesicular steatosis, which is the histological hallmark of impairment of mitochondrial function [20]. Consistent with the histological findings, CPT I, the rate limiting enzyme involved in fatty acid β-oxidation, was markedly suppressed by copper deficiency. We also showed that hepatic FAS expression was up-regulated by copper deficiency, suggesting enhanced de novo lipogenesis which may further contribute to lipid accumulation. However, de novo lipogenesis only accounts for a small part of hepatic free fatty acids [29], therefore, it appears that impaired mitochondrial fatty acid β-oxidation likely plays an important role in fructose induced NAFLD. It has been demonstrated that both increased fatty acids and cholesterol synthesis are associated with GSH synthesis, and the induction of FAS and 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase are completely prevented when GSH synthesis is blocked by L-buthionine sulfoximine (BSO) in copper deficient rats [30, 31]. Consistent with these findings, our data showed increased hepatic GSH synthesis with copper deficiency, suggesting GSH synthesis could be a mechanism by which copper deficiency induces fatty liver. A very recent study suggested that iron overload was associated with increased cholesterol synthesis [32]. Moreover, restricting iron intake in copper deficient animals improved fatty liver [9, 10, 33]. Our data clearly showed that hepatic and whole body iron levels moved in the opposite direction of copper levels, suggesting that iron overload secondary to copper deficiency is another likely mechanism by which copper deficiency induces fatty liver.
Previous studies have already shown that impaired gut absorption of copper resulting from dietary fructose may account for copper deficiency [14, 15]. Copper is mainly absorbed in the duodenum and small intestine, via Ctr-1, divalent metal transpoter1 and diffusion. Ctr-1 is believed to be the primary protein responsible for the import of dietary copper across the apical membrane. Intestinal epithelial cell conditional Ctr-1 knockout mice showed a very high copper level in intestinal epithelial cells, while copper was deficient in the liver, suggesting a critical role for Ctr-1 in dietary Cu absorption [34]. In the present study, we found Ctr-1 expression was significantly increased by marginal copper deficiency, and extensive Ctr-1 accumulated in the basolateral membrane side, possibly an adaptive compensation in response to dietary copper deficiency, which is an effort to transport copper out of the epithelium into the blood. This phenomenon was abrogated by dietary fructose, suggesting fructose may block copper transportation by targeting intestinal Ctr1.
In conclusion, our data demonstrated that fructose feeding further impaired copper status and led to iron overload in marginal copper deficient rats. Dietary marginal copper deficiency and fructose feeding work together to exacerbate liver injury and accelerate hepatic fat accumulation, possibly mediated through increased iron accumulation. Therefore, high fructose-induced NAFLD may be due, in part, to copper deficiency. Decreased mitochondrial fatty acid β-oxidation caused by copper deficiency plays an important role in fructose induced fatty liver. Furthermore, impairment of intestinal epithelium Ctr1 by dietary fructose may lead to decreased copper absorption, and thereby, copper deficiency. Our current study provides new insights into mechanisms of fructose induced fatty liver and the complex interactions of dietary components in the development of fatty liver.
Acknowledgment
This study was supported in part by National Institute on Alcohol Abuse and Alcoholism Grants RO1AA015970, PO1AA017103, R37AA010762, RO1AA018869, RC2 AA019385 (C.J.M.), RO1AA014623, RO1AA016013, RO1AA018844 (Z.Z.) and the Veterans Administration (C.J.M.) and by National Institute of Diabetes and Digestive and Kidney Diseases DK-055030 (D.A.S.), RO1DK071765 (C.J.M.). We thank Xingguo Sun for technical support on histology, Irina A. Kirpich, Jingwen Zhang and Leila Gobejishvili for assistance in animal experiments. We thank Marion McClain for careful reading of this manuscript.
List of abbreviations
- Ctr1
copper transporter 1
- AST
aspartate aminotransferase
- CPT I
carnitine palmitoyl-CoA transferase I
- FAS
fatty acid synthase
- NAFLD
nonalcoholic fatty liver disease
- ICP-MS
inductively coupled plasma mass spectroscopy
- ALT
alanine aminotransferase
- NEFA
non-esterified fatty acids
- MCP-1
monocyte chemoattractant protein-1
- DHE
dihydroethidium
- MDA
malondialdehyde
- 4-HAE
4-hydroxyalkenals
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- H&E
hematoxylin and eosin
- TUNEL
Terminal Deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling
- SOD
superoxide dismutase
- GPx
glutathione peroxidase
- HMG-CoA
3-hydroxy-3-methyl-glutaryl-CoA
- BSO
buthionine sulfoximine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure: The authors have no financial and commercial conflicts of interest.
Contributor Information
Ming Song, Email: m0song03@louisville.edu.
Dale A Schuschke, Email: daschu01@louisville.edu.
Zhanxiang Zhou, Email: z_zhou@uncg.edu.
Theresa Chen, Email: tschen01@louisville.edu.
William M. Pierce, Jr., Email: wmpier01@louisville.edu.
Renwei Wang, Email: wangx945@umn.edu.
W. Thomas Johnson, Email: thomas.johnson@ars.usda.gov.
Craig J. McClain, Email: cjmccl01@louisville.edu.
References
- 1.Hallfrisch J. Metabolic effects of dietary fructose. FASEB J. 1990;4:2652–2660. doi: 10.1096/fasebj.4.9.2189777. [DOI] [PubMed] [Google Scholar]
- 2.Spruss A, Bergheim I. Dietary fructose and intestinal barrier: potential risk factor in the pathogenesis of nonalcoholic fatty liver disease. J Nutr Biochem. 2009;20:657–662. doi: 10.1016/j.jnutbio.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Assy N, Nasser G, Kamayse I, Nseir W, Beniashvili Z, Djibre A, et al. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol. 2008;22:811–816. doi: 10.1155/2008/810961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48:993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holden JM, Wolf WR, Mertz W. Zinc and copper in self-selected diets. J Am Diet Assoc. 1979;75:23–28. [PubMed] [Google Scholar]
- 6.Fields M, Ferretti RJ, Smith JC, Jr, Reiser S. The interaction of type of dietary carbohydrates with copper deficiency. Am J Clin Nutr. 1984;39:289–295. doi: 10.1093/ajcn/39.2.289. [DOI] [PubMed] [Google Scholar]
- 7.Fields M, Ferretti RJ, Smith JC, Jr, Reiser S. Effect of dietary carbohydrates and copper status on blood pressure of rats. Life Sci. 1984;34:763–769. doi: 10.1016/0024-3205(84)90384-9. [DOI] [PubMed] [Google Scholar]
- 8.Fields M, Lewis CG, Lure MD. Responses of insulin to oral glucose and fructose loads in marginally copper-deficient rats fed starch or fructose. Nutrition. 1996;12:524–528. doi: 10.1016/s0899-9007(96)91730-x. [DOI] [PubMed] [Google Scholar]
- 9.Aigner E, Theurl I, Haufe H, Seifert M, Hohla F, Scharinger L, et al. Copper availability contributes to iron perturbations in human nonalcoholic fatty liver disease. Gastroenterology. 2008;135:680–688. doi: 10.1053/j.gastro.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Fields M, Lewis CG. Hepatic iron overload may contribute to hypertriglyceridemia and hypercholesterolemia in copper-deficient rats. Metabolism. 1997;46:377–381. doi: 10.1016/s0026-0495(97)90051-2. [DOI] [PubMed] [Google Scholar]
- 11.Fields M, Ferretti RJ, Smith JC, Jr, Reiser S. Effect of copper deficiency on metabolism and mortality in rats fed sucrose or starch diets. J Nutr. 1983;113:1335–1345. doi: 10.1093/jn/113.7.1335. [DOI] [PubMed] [Google Scholar]
- 12.Fields M, Ferretti RJ, Reiser S, Smith JC., Jr The severity of copper deficiency in rats is determined by the type of dietary carbohydrate. Proc Soc Exp Biol Med. 1984;175:530–537. doi: 10.3181/00379727-175-41832. [DOI] [PubMed] [Google Scholar]
- 13.Fields M, Lewis CG. Dietary fructose but not starch is responsible for hyperlipidemia associated with copper deficiency in rats: effect of high-fat diet. J Am Coll Nutr. 1999;18:83–87. doi: 10.1080/07315724.1999.10718831. [DOI] [PubMed] [Google Scholar]
- 14.Holbrook J, Fields M, Smith JC, Jr, Reiser S. Tissue distribution and excretion of copper-67 intraperitoneally administered to rats fed fructose or starch. J Nutr. 1986;16:831–838. doi: 10.1093/jn/116.5.831. [DOI] [PubMed] [Google Scholar]
- 15.Fields M, Holbrook J, Scholfield D, Smith JC, Jr, Reiser S. Effect of fructose or starch on copper-67 absorption and excretion by the rat. J Nutr. 1986;116:625–632. doi: 10.1093/jn/116.4.625. [DOI] [PubMed] [Google Scholar]
- 16.Schosinsky KH, Lehmann HP, Beeler MF. Measurement of ceruloplasmin from its oxidase activity in serum by use of o-dianisidine dihydrochloride. Clin Chem. 1974;20:1556–1563. [PubMed] [Google Scholar]
- 17.Richie JP, Jr, Lang CA. The determination of glutathione, cyst(e)ine, and other thiols and disulfides in biological samples using high-performance liquid chromatography with dual electrochemical detection. Anal Biochem. 1987;163:9–15. doi: 10.1016/0003-2697(87)90085-6. [DOI] [PubMed] [Google Scholar]
- 18.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 19.Harvey LJ, McArdle HJ. Biomarkers of copper status: a brief update. Br J Nutr. 2008;99:S10–S13. doi: 10.1017/S0007114508006806. [DOI] [PubMed] [Google Scholar]
- 20.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 21.van den Berghe PV, Klomp LW. New developments in the regulation of intestinal copper absorption. Nutr Rev. 2009;67:658–672. doi: 10.1111/j.1753-4887.2009.00250.x. [DOI] [PubMed] [Google Scholar]
- 22.Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094–1104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 23.Panteghini M. Aspartate aminotransferase isoenzymes. Clin Biochem. 1990;23:311–319. doi: 10.1016/0009-9120(90)80062-n. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher CH, Reeve VE, Wright R. Copper deficiency in the rat. Effect on the ultrastructure of hepatocytes. Aust J Exp Biol Med Sci. 1973;51:181–189. doi: 10.1038/icb.1973.15. [DOI] [PubMed] [Google Scholar]
- 25.Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 26.Aquilano K, Vigilanza P, Rotilio G, Ciriolo MR. Mitochondrial damage due to SOD1 deficiency in SH-SY5Y neuroblastoma cells: a rationale for the redundancy of SOD1. FASEB J. 2006;20:E930–E941. doi: 10.1096/fj.05-5225fje. [DOI] [PubMed] [Google Scholar]
- 27.Kessova IG, Ho YS, Thung S, Cederbaum AI. Alcohol-induced liver injury in mice lacking Cu, Zn-superoxide dismutase. Hepatology. 2003;38:1136–1145. doi: 10.1053/jhep.2003.50450. [DOI] [PubMed] [Google Scholar]
- 28.Magrané J, Hervias I, Henning MS, Damiano M, Kawamata H, Manfredi G. Mutant SOD1 in neuronal mitochondria causes toxicity and mitochondrial dynamics abnormalities. Hum Mol Genet. 2009;18:4552–4564. doi: 10.1093/hmg/ddp421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson J, Kim S, Allen KG, Baillie R, Clarke SD. Hepatic fatty acid synthase gene transcription is induced by a dietary copper deficiency. Am J Physiol. 1997;272:E1124–E1129. doi: 10.1152/ajpendo.1997.272.6.E1124. [DOI] [PubMed] [Google Scholar]
- 31.Kim S, Chao PY, Allen KG. Inhibition of elevated hepatic glutathione abolishes copper deficiency cholesterolemia. FASEB J. 1992;6:2467–2471. doi: 10.1096/fasebj.6.7.1563598. [DOI] [PubMed] [Google Scholar]
- 32.Graham RM, Chua AC, Carter KW, Delima RD, Johnstone D, Herbison CE, et al. Hepatic iron loading in mice increases cholesterol biosynthesis. Hepatology. 2010;52:462–471. doi: 10.1002/hep.23712. [DOI] [PubMed] [Google Scholar]
- 33.Minamiyama Y, Takemura S, Kodai S, Shinkawa H, Tsukioka T, Ichikawa H, et al. Iron restriction improves type 2 diabetes mellitus in Otsuka Long-Evans Tokushima fatty rats. Am J Physiol Endocrinol Metab. 2010;298:E1140–E1149. doi: 10.1152/ajpendo.00620.2009. [DOI] [PubMed] [Google Scholar]
- 34.Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006;4:235–244. doi: 10.1016/j.cmet.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Day CP. From fat to inflammation. Gastroenterology. 2006;130:207–210. doi: 10.1053/j.gastro.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Aigner E, Strasser M, Haufe H, Sonnweber T, Hohla F, Stadlmayr A, Solioz M, Tilg H, Patsch W, Weiss G, Stickel F, Datz C. A role for low hepatic copper concentrations in nonalcoholic Fatty liver disease. Am J Gastroenterol. 2010;105(9):1978–1985. doi: 10.1038/ajg.2010.170. [DOI] [PubMed] [Google Scholar]